Abstract
Chronic transfusion therapy (CTT) for sickle cell disease (SCD) reduces disease morbidity by suppressing the amount of circulating hemoglobin S (HbS)-containing red blood cells (RBC). The effectiveness of CTT depends on the rate of RBC clearance. Glucose-6-phosphate dehydrogenase (G6PD) deficient donor RBC may exhibit increased hemolysis, but it is unknown if transfusion of these units results in less effective transfusion outcomes in SCD.
Children with SCD on CTT were followed prospectively for multiple transfusions. G6PD activity of transfused units was measured prior to expiration date. HbA clearance (∆HbA) was calculated as the difference of estimated post-transfusion HbA to the pre-transfusion HbA of the subsequent transfusion episode. Sixty-two patients received 388 transfusions. Of 755 RBC units, 687 (91%) had normal G6PD (>60% activity), 38 (5%) had moderately low G6PD (10 – 60% activity), and 30 (4%) had severely low G6PD (<10% activity). Of 358 evaluable transfusions, 54 (15%) included ≥1 G6PD deficient units, and 22 (6%) had ≥1 severely deficient units. The proportion of the transfusion episode consisting of G6PD deficient units was associated with increased ∆HbA for all G6PD deficient units (p=0.05) and for severely G6PD deficient units (p=0.0070). In multivariate mixed effects modeling, ∆HbA was positively associated with severely G6PD deficient units (p=0.0074) and RBC alloimmunization (p=0.03) and negatively associated with recipient splenectomy (p=0.015). Higher ∆HbA was associated with higher HbS and reticulocyte counts at the subsequent transfusion episode. In conclusion, G6PD deficient RBC transfusions may have shorter in vivo survival and adversely affect the suppression of sickle erythropoiesis.
Keywords: Sickle cell disease, glucose-6 phosphate dehydrogenase deficiency, chronic transfusion therapy
INTRODUCTION
Chronic transfusion therapy (CTT) for sickle cell disease (SCD) is an essential treatment for the prevention of severe sickle complications such as stroke. Red blood cell (RBC) transfusions to individuals with SCD have unique considerations, including the selection of units that are hemoglobin S (HbS) negative and RBC antigen matching for C/c, E/e, K, and potentially other antigens due to the high prevalence of alloimmunization in SCD.1 To meet these antigen matching requirements, donors who are phenotypically matched for SCD patients are disproportionately of African descent,2, 3 thus are more likely to have glucose-6-phosphate dehydrogenase (G6PD) deficiency or hemoglobin variants other than HbS.4, 5
G6PD deficiency is the most common enzyme deficiency worldwide, affecting an estimated 5% of the world population, or 400 million individuals.6 G6PD deficiency is an X-linked inherited disorder that includes a large number of allelic variants, such that variants may be classified as severely deficient (<10% enzyme activity) or moderately deficient (10 – 60% activity).7 The prevalence of G6PD deficiency is highest in sub-Saharan Africa and the Middle East, with male summary prevalence estimates of 8.5% and 7.2%, respectively.6 The vast majority of individuals with G6PD deficiency are asymptomatic, thus they may be unaware of their G6PD deficiency status and are as likely to be a blood donor as a G6PD replete individual. G6PD is the rate-limiting enzyme of the pentose phosphate pathway, generating reduced nicotinamide-adenine dinucleotide phosphate (NADPH), which is essential to protecting red blood cells (RBC) from oxidative stress. In G6PD deficiency, reactive oxygen species can accumulate, resulting in episodes of intravascular and extravascular hemolysis.
In vitro data of stored RBC units from G6PD deficient donors suggests that G6PD deficient RBC have increased reactive oxygen species accumulation and hemolysis when subjected to conditions simulating transfusion.8 To date, there remains limited evidence regarding the effects of transfusion of G6PD deficient blood; however studies indicate that G6PD deficient RBC transfusions may be associated with insufficient rise in hemoglobin post-transfusion in children and may promote hemolysis in neonatal recipients.9 The World Health Organization recommends that G6PD deficient donors are acceptable unless there is history of clinical hemolysis; in contrast, blood from these donors is considered not acceptable for intrauterine and neonatal exchange transfusion or for transfusion to recipients with G6PD deficiency.7 Despite this, blood donors are not routinely screened for G6PD deficiency, thus little is known about the effects of these units on transfusion outcomes in children with SCD.
The primary hematologic goal of CTT in SCD is the suppression of erythropoiesis, thus the effectiveness of CTT is monitored by pre-transfusion levels of HbS and reticulocytes. The survival of transfused RBC affects the suppression of erythropoiesis and thus sickling, hemolysis, and vaso-occlusion of recipient erythrocytes. In this study, we sought to examine the effect of donor G6PD deficiency on the clearance of HbA in children with SCD on CTT.
METHODS
A prospective observational study of children ages 3 – 20 years with SCA (HbSS or HbSβ0 thalassemia) on CTT for at least 6 months was conducted at Children’s Healthcare of Atlanta (CHOA) and Children’s National Medical Center (CNMC). Written, informed consent and assent were obtained, and this study was approved by the Institutional Review Boards of CHOA and CNMC. Participants who were receiving CTT were followed for multiple transfusions (up to 17 months), recording pre-transfusion hemoglobin (including HbA and HbS) and reticulocyte values, RBC antigen matching, and the age of the blood units. Electronic medical records were reviewed to identify history of splenectomy, splenomegaly present on physical exam at the time of transfusion, and RBC alloimmunization histories. All RBC units were HbS negative, pre-storage leukoreduced, and serologically matched for C/c, E/e, and K antigens (limited phenotype matching). For patients with RBC alloimmunization, RBC units had additional serological matching for Fya and Jkb in addition to other antigens to which they had alloantibodies (extended phenotype matching). Transfusion volume and frequency were determined by institutional protocol, in which patients receive 10 – 15 mL/kg of packed RBC (1 – 3 RBC units) per transfusion, based on patient body weight and pre-transfusion Hb.
G6PD testing
Segments of donor units, when available, were tested for G6PD activity level no later than their date of expiration. The quantitative G6PD assay (Trinity Biotech, Berkeley Heights, NJ) was performed as previously described.4 G6PD activity was expressed as units of enzyme activity per gram of hemoglobin (U/g Hb). G6PD deficiency was defined as activity <60% of the normal mean (<3.9 U/g Hb), while severe G6PD deficiency was defined as activity <10% of the normal mean for stored RBC units (<1.3 U/g Hb).4 The G6PD-deficient ratio for a transfusion episode was defined as the number of deficient units divided by the number of units in the transfusion (range 1 – 3 units per transfusion). Thus the G6PD-deficient ratio could be 0 (no deficient units), 0.33 (1 of 3 units deficient), 0.5 (1 of 2 units deficient), 0.67 (2 of 3 units deficient), or 1.0 (all units deficient) for a given transfusion episode. The G6PD-deficient ratio was evaluated for all (moderate and severe) G6PD deficient units and for exclusively severe G6PD deficient units.
Hemoglobin analysis of unit segments was done by alkaline hemoglobin electrophoresis. Hemoglobin variants were confirmed by high performance liquid chromatography (HPLC) using the Bio-Rad Variant II HPLC (Hercules, CA).
Hemoglobin A Clearance (∆HbA) calculation
The loss of HbA (∆HbA) from the end of a transfusion episode to the end of the transfusion cycle (i.e. immediately prior to the next scheduled transfusion) was calculated as using the following equations. The amount of donor Hb was expressed as “HbA” which is the sum of HbA1 and any donor variant Hb (HbV) (e.g. HbC). Pre-transfusion HbA was recorded 0 – 3 days prior to each transfusion. Post-transfusion HbA was calculated based on the volume of transfusion (VolT, mL), estimated unit hematocrit (unit Hct, %), body weight (Wt, kg), using the equation: Post HbA (g/dL) = Pre HbA (g/dL) + [(unit Hct) × VolT / 3 × Wt]. In a small number of transfusions in which pre-transfusion phlebotomy was performed for partial manual exchange, the amount of HbA removed by phlebotomy ((Pre HbA%/100) × [(Pre Hb (g/dL) × VolPh / 3 × TBV]) was subtracted from the estimated Post HbA equation, where VolPh = phlebotomy volume (mL) and TBV = total blood volume (mL).10, 11 Change in HbA following the transfusion (ΔHbA, g/dL/day) was calculated as: ΔHbA (g/dL/day) = (Post HbA – Pre HbA of the subsequent transfusion) / transfusion interval (days). The unit Hct was estimated based on quality analysis data from each blood supplier for the unit’s preservative solution (citrate-phosphate-dextrose-adenine (CPDA) or additive solution (AS)).
In a group of 217 CTT episodes (49 of which had unit G6PD measurements), immediate post-transfusion HbA was directly measured within 30 minutes of the transfusion completion. Measured post-HbA was compared to estimated post-HbA as follows: bias was the median difference between measured and estimated post-HbA; precision was the interquartile range of the bias; accuracy was the percentage of estimated post-HbA within 10% (P10) and 15% (P15) of measured post-HbA.
Statistical Analysis
The association of ∆HbA with G6PD deficient transfusion was assessed several ways, utilizing mixed modeling to account for repeated measures per patient. Least-square means (ls-means) were used to compare ∆HbA in transfusions with ≥1 G6PD-deficient unit vs. 0 G6PD-deficient units. Comparison of ΔHbA in a transfusion episode associated with new RBC antibody detection compared to other transfusions was done by Wilcoxon Rank Sum test. The univariate associations of ∆HbA with the G6PD-deficient ratio were determined by linear mixed modeling. Multivariate linear mixed effects modeling was used to examine the effect of patient characteristics (age, RBC alloimmunization, splenectomy, splenomegaly at time of transfusion) and transfusion characteristics (G6PD-deficient ratio, mean age of transfused units, and oldest age of a transfused unit) on ∆HbA. Mixed modeling was used to account for both the fixed effect of repeated transfusion measurements per patient and random effects that vary per transfusion. Univariate mixed effects analysis was also used to assess the association of ∆HbA with continuous measures of erythropoiesis (HbS, reticulocytes). In assessing RBC unit age, the normality of distribution was assessed by Kolmogorov-Smirnov goodness-of-fit test, and the age of G6PD-deficient and non-deficient units was compared by Wilcoxon Rank Sum test.
RESULTS
G6PD levels were measured in 755 RBC units received by 62 SCD patients over the course of 388 transfusion episodes. G6PD levels were normal in 687 (91%), moderately low in 38 (5%) and severely low in 30 (4%) of these units. There were 10 units (all with normal G6PD levels) that were heterozygous for HbA and variant Hb: 7 HbAC, 1 HbA/alpha chain variant (possibly HbO-Indonesia or Setif), 1 HbA/Hasharon, and 1 HbA/variant (possibly Hb J-Baltimore, N-Baltimore, or I-High Wycombe).
Of the 388 transfusion episodes, 6 were excluded because ≥1 unit in the transfusion lacked G6PD testing results, and 24 were excluded for lack of ∆HbA estimation (due to missing pre-transfusion HbA measurement). Therefore a total of 358 transfusion episodes (687 units) were analyzed to assess the association of G6PD status with ∆HbA. The median age of patients was 10.6 years (range 2.2 – 18.0 years). A history of RBC alloimmunization was present in 17 (27%) patients and 99 (28%) transfusion episodes; history of splenectomy in 17 (27%) patients and 72 (20%) transfusion episodes, and splenomegaly in 1 (1.6%) patient with 11 (3.1%) transfusion episodes. Among the transfusion episodes included, there was 1 new RBC antibody detected post-transfusion, an antibody of undetermined specificity in a patient without prior RBC alloimmunization. No delayed hemolytic transfusion reactions observed. There were no instances in which cross-match incompatible units were transfused during the study.
The median storage age of RBC units was 18 days (interquartile range 12 – 26 days) with only 5% of units between the ages of 36 – 42 days. The storage age of G6PD-deficient units was lower than non-deficient units (median 14.0 vs. 18.0 days, p=0.031). The frequency of units >21 days old was lower for G6PD deficient units as compared to non-deficient units (20.7% vs. 40.9% p=0.0026), Supplemental Figure)
Overall, 34 (55%) patients received ≥1 transfusion of a G6PD deficient unit, and 16 (26%) received ≥1 transfusion of a severely G6PD deficient unit. G6PD deficient units were more common in units with extended phenotype matching provided to patients with RBC alloimmunization than units with limited phenotype matching. G6PD deficient transfusions were administered to 13 (76%) patients with RBC alloimmunization vs. 21 (47%) patients without alloimmunization (p=0.035), and severe G6PD deficient transfusions were administered to 8 (47%) with RBC alloimmunization vs. 8 (18%) without alloimmunization (p=0.019).
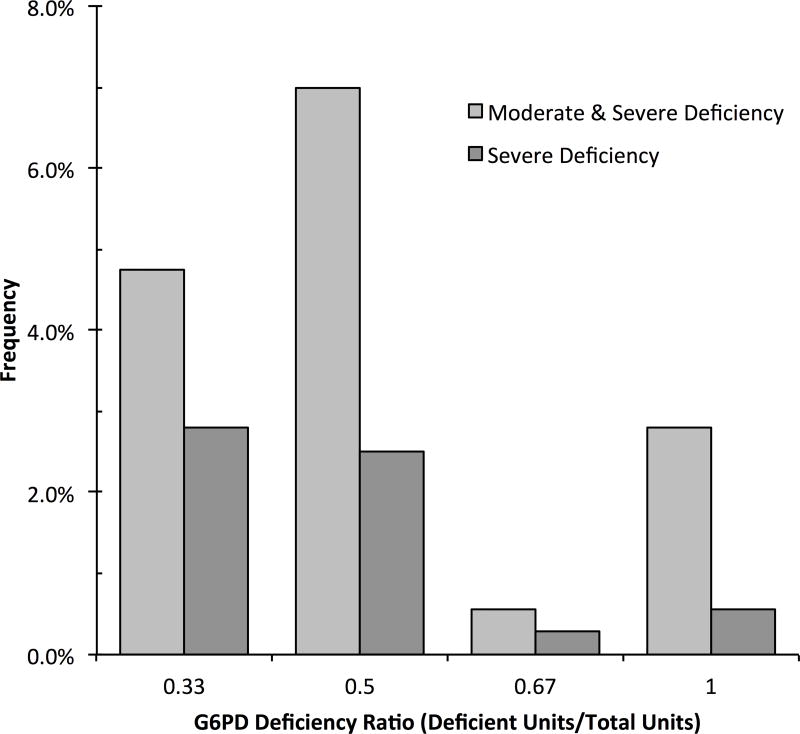
Figure 1 shows the proportion of transfusion episodes containing G6PD deficient units, categorized by the G6PD deficiency ratio. Overall 54 (15%) transfusions contained at least 1 unit with G6PD deficiency, and 22 (6%) contained at least 1 severely deficient unit.
Figure 1.
Frequency of transfusion episodes with G6PD deficient RBC units, categorized by the G6PD Deficiency ratio of the transfusion episode. The G6PD-deficiency ratio is the number of units in a transfusion with G6PD deficiency divided by the total number of units in the transfusion episode.
Post-Transfusion HbA Estimation
Comparison of estimated to measured post-HbA showed a negative bias of 0.32 g/dL (95% C.I. 0.23, 0.41) and precision of 0.81 g/dL (95% C.I. 0.61, 1.09). Accuracy of estimated post-HbA was 82.5% within 10% of measured post-HbA and 93.6% within 15% of measured post-HbA.
∆HbA Analysis
The mean loss of HbA (∆HbA) per transfusion was 0.086 g/dL/day (range 0.004 – 0.167 g/dL/day). The ∆HbA was greater among transfusions with ≥1 G6PD deficient unit than those with no G6PD deficient units (ls-means 0.094 vs. 0.085 g/dL/day, p=0.018). ∆HbA was also greater when comparing transfusions with ≥1 severely deficient units to transfusions with no severe G6PD deficient units (ls-means 0.098 vs. 0.086 g/dL/day, p=0.033). There was not a significant difference in ∆HbA when comparing transfusions with ≥1 moderate G6PD deficient unit to those with ≥1 severe G6PD deficient unit (ls-means 0.092 vs. 0.101 g/dL/day, p=0.27).
The ΔHbA following the 1 transfusion associated with new RBC antibody formation was 0.098 g/dL/day, not significantly different from other transfusion episodes (p=0.55)
In univariate mixed effects analysis of ∆HbA to the G6PD-deficient ratio, ∆HbA was greater in transfusions with higher overall G6PD-deficient ratios, including both moderate and severe G6PD deficient units (p=0.05). When exclusively comparing severe G6PD deficient transfusions to non-severe G6PD deficient transfusions, the positive association of ∆HbA with G6PD-deficient ratio was stronger (p=0.0070). In multivariate linear mixed effects modeling (Table I), greater ∆HbA was positively associated with severe G6PD-deficient ratio (p=0.007), patient RBC alloimmunization (p=0.029), and negatively associated with splenectomy (p=0.013). Other donor and unit characteristics examined (donor Hb variant, mean storage age of the units, and the storage age of the oldest unit per transfusion) demonstrated no association with ∆HbA.
Table I.
Multivariate Linear Mixed Model of ΔHbA
| Parameter Estimate | p | |
|---|---|---|
| Donor/Unit Characteristics | ||
| Severe G6PD Deficient ratio | 0.0323 | 0.007 |
| Donor Hb variant | 0.017 | 0.073 |
| Mean storage age of units per transfusion | 0.00057 | 0.12 |
| Storage age of oldest unit per transfusion | −0.00036 | 0.26 |
| Recipient Characteristics | ||
| Patient age | −0.00085 | 0.16 |
| RBC Alloimmunization | 0.0105 | 0.029 |
| Splenectomy | −0.0126 | 0.013 |
| Splenomegaly | 0.018 | 0.22 |
∆HbA was also found to have significant impact on measures of erythropoiesis prior to the subsequent transfusion. In mixed effects analysis, ∆HbA was positively associated with both HbS (p=0.038) and absolute reticulocyte count (p<0.0001) prior to the next transfusion episode.
DISCUSSION
The ability of CTT to prevent sickle cell-related complications is dependent upon maintaining a low percentage of circulating HbS-containing erythrocytes, which in turn is dependent on the rates of clearance of both HbS and HbA-containing RBC from circulation. As demonstrated within this study, the clearance of transfused RBC (reflected by ∆HbA) is variable, both among different patients but also among sequential transfusions within the same patient, suggesting that both recipient and donor unit characteristics influence transfused RBC survival. Elucidating the factors that influence clearance of transfused RBCs is important to ultimately determine if certain characteristics of RBC units, such as G6PD levels, can be selected to minimize HbA loss and therefore optimize transfusion therapy to SCD patients.
In this study we showed that G6PD deficiency in donor RBC units negatively affects the goals of CTT in children with SCD, namely the survival of HbA-containing RBCs and resultant suppression of erythropoiesis, as reflected by post-transfusion HbS and reticulocyte levels. Severe G6PD deficient units were shown to have a more significant effect on HbA clearance post transfusion than moderately deficient units. While no adverse outcomes of transfusion with G6PD deficient units were noted within the course of the study, the observed higher HbS and absolute reticulocyte counts following transfusion with these units is concerning, as pre-transfusion elevated reticulocyte counts and HbS levels >30% may be associated with progressive cerebrovascular disease and stroke despite CTT.12–14
This is the first study to examine the prevalence of G6PD deficient units provided to children with SCD during CTT. Previously, Francis et al. showed a low prevalence of G6PD deficiency among randomly selected RBC units (0.3%) but a much higher prevalence of 12.3% among D+C-E- RBC units, which is the most common Rh phenotype of SCD patients.15 Our study showed a 9% prevalence of G6PD deficiency among units provided to SCD patients; however given that most patients require more than 1 RBC unit to provide sufficient transfusion volume, the prevalence of G6PD deficient transfusion episodes was 15%. Thus, as G6PD deficient transfusions to SCD patients are common events, caution must be exercised in considering whether these RBC units are deleterious or benign in SCD. Individuals on CTT who have poor suppression of HbS levels may be candidates for whom G6PD deficient transfusions should be avoided, in order to maximize survival of transfused RBCs. Additionally, in keeping with the WHO recommendations for transfusion of G6PD deficient units, consideration should be given towards testing the G6PD status of all patients on CTT in order to avoid transfusion of G6PD deficient units to these patients.16
In this study, the storage age of RBC units was not associated with differences in HbA clearance post transfusion; however this study was not powered to examine the impact of older units on HbA decline. The majority of units in this study had a storage duration of less than 3 weeks, while only 5% of units had a storage duration of 5 weeks or longer, thus the impact of the extremes of storage duration cannot be evaluated by this study. Studies of RBC metabolomics have shown declines in glutathione and other metabolites involved in cellular energy and antioxidant defense pathways in RBC stored up to 42 days.17 Additionally, in a study of autologous transfusion to healthy adults, storage duration up to 6 weeks was associated with extravascular hemolysis and decreased post-transfusion RBC recovery18, events that could negatively impact CTT outcomes in SCD. Thus further study of the impact of transfusing RBC units near their maximum storage age to patients with SCD is needed.
A limitation of this study was the lack of direct measurement of transfused RBC recovery and survival by techniques such as biotin or chromium-51 labeling. While ∆HbA correlates with post-transfusion hemoglobin and reticulocyte counts as expected, it is a surrogate for the true outcome of interest which is the clearance of transfused RBC. Transfused RBCs remain detectable in vivo for up to 4 months post transfusion, thus the loss of HbA in a given time interval reflects the clearance of RBC from multiple recent transfusions, not solely the most recent one, which confounds the ability to assess the effects of a single transfusion episode. Direct measurement of RBC survival, with labeling of RBC units prior to transfusion, would provide a more accurate reflection of transfusion clearance.
In summary, the effectiveness of CTT in SCD in suppressing HbS-containing RBC is dependent upon the in vivo survival of transfused RBCs. Recipient characteristics such as RBC alloimmunization and splenectomy and the donor characteristic of G6PD status affect the clearance of HbA post-transfusion, which in turn is associated with post-transfusion HbS and reticulocyte values. Selection against G6PD deficient units may be a consideration for some SCD patients with poor suppression of erythropoiesis or other characteristics that might promote poor in vivo survival of transfused RBCs. Further studies of metabolic changes during RBC storage will help to optimize transfusion therapy to children with SCD.
Supplementary Material
Acknowledgments
This work was supported by Cooperative Agreement 5 NU58 DD001138-03, funded by the Centers for Disease Control and Prevention, The Harold Amos Medical Faculty Development Program (Robert Wood Johnson Foundation grant 71590), and the National Blood Foundation.. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the Department of Health and Human Services. Data management through REDCap was supported by grant UL1 TR000424.
References
- 1.Fasano RM, Chou ST. Red Blood Cell Antigen Genotyping for Sickle Cell Disease, Thalassemia, and Other Transfusion Complications. Transfus Med Rev. 2016 doi: 10.1016/j.tmrv.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Yazer MH, Anani WQ, Denomme GA, et al. Trends in antigen-negative red blood cell distributions by racial or ethnic groups in the United States. Transfusion. 2017 doi: 10.1111/trf.14376. [DOI] [PubMed] [Google Scholar]
- 3.Shaz BH, Hillyer CD. Minority donation in the United States: challenges and needs. Curr Opin Hematol. 2010;17:544–9. doi: 10.1097/MOH.0b013e32833e5ac7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis RO, Jhang J, Hendrickson JE, Zimring JC, Hod EA, Spitalnik SL. Frequency of glucose-6-phosphate dehydrogenase-deficient red blood cell units in a metropolitan transfusion service. Transfusion. 2013;53:606–11. doi: 10.1111/j.1537-2995.2012.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raciti PM, Francis RO, Spitalnik PF, Schwartz J, Jhang JS. Acquired hemoglobin variants and exposure to glucose-6-phosphate dehydrogenase deficient red blood cell units during exchange transfusion for sickle cell disease in a patient requiring antigen-matched blood. J Clin Apher. 2013;28:325–9. doi: 10.1002/jca.21255. [DOI] [PubMed] [Google Scholar]
- 6.Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis. 2009;42:267–78. doi: 10.1016/j.bcmd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bulletin of the World Health Organization. 1989;67:601–11. [PMC free article] [PubMed] [Google Scholar]
- 8.Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better "storers" than donors of red blood cells. Free Radic Biol Med. 2016;96:152–65. doi: 10.1016/j.freeradbiomed.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Renzaho AM, Husser E, Polonsky M. Should blood donors be routinely screened for glucose-6-phosphate dehydrogenase deficiency? A systematic review of clinical studies focusing on patients transfused with glucose-6-phosphate dehydrogenase-deficient red cells. Transfus Med Rev. 2014;28:7–17. doi: 10.1016/j.tmrv.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Davies P, Robertson S, Hegde S, Greenwood R, Massey E, Davis P. Calculating the required transfusion volume in children. Transfusion. 2007;47:212–6. doi: 10.1111/j.1537-2995.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 11.Neyrinck MM, Vrielink H Joint Task Force for E, Certification. Calculations in apheresis. J Clin Apher. 2015;30:38–42. doi: 10.1002/jca.21347. [DOI] [PubMed] [Google Scholar]
- 12.Kaushal M, Byrnes C, Khademian Z, et al. Examination of Reticulocytosis among Chronically Transfused Children with Sickle Cell Anemia. PLoS One. 2016;11:e0153244. doi: 10.1371/journal.pone.0153244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulbert ML, McKinstry RC, Lacey JL, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117:772–9. doi: 10.1182/blood-2010-01-261123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurlet-Jensen AM, Prohovnik I, Pavlakis SG, Piomelli S. Effects of total hemoglobin and hemoglobin S concentration on cerebral blood flow during transfusion therapy to prevent stroke in sickle cell disease. Stroke. 1994;25:1688–92. doi: 10.1161/01.str.25.8.1688. [DOI] [PubMed] [Google Scholar]
- 15.Yee MEM, Josephson CD, Winkler AM, et al. Red blood cell minor antigen mismatches during chronic transfusion therapy for sickle cell anemia. Transfusion. 2017 doi: 10.1111/trf.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blood donor selection: guidelines on assessing donor suitability for blood donation. Geneva, Switzerland: World Health Organization; 2012. [PubMed] [Google Scholar]
- 17.Roback JD, Josephson CD, Waller EK, et al. Metabolomics of ADSOL (AS-1) red blood cell storage. Transfus Med Rev. 2014;28:41–55. doi: 10.1016/j.tmrv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest. 2017;127:375–82. doi: 10.1172/JCI90837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.