Abstract
Circulating exosomes containing donor HLA and lung-associated self-antigens (SAg) are thought to play an important role in allograft rejection after human lung transplantation (LTx). We characterized exosomes isolated from serum of 10 lung transplant recipients (LTxR) diagnosed with bronchiolitis obliterans syndrome (BOS) and compared to exosomes isolated from serum of 10 stable LTxR. Lung-associated SAg (Kα1Tubulin (Kα1T) and Collagen V (Col-V)); major histocompatibility complex class II molecule; co-stimulatory molecules, CD40, CD80, and CD86; and transcription factors class II major histocompatibility complex trans-activator, nuclear factor-κB, hypoxia-inducible factor 1-alpha, interleukin-1 receptor-associated kinase 1, myeloid differentiation primary response gene 88, and 20S proteasome were detected in exosomes from BOS, but not stable LTxR. In contrast, adhesion molecules were present in both groups. C57BL/6 mice immunized with exosomes from BOS but not stable LTxR demonstrated Ab to SAg (Col-V: 33.5±15.7 vs 10.4±6.4, p=0.021; Kα1T: 925±403 vs 317±285, p=0.044) and HLA (mean fluorescence intensity: BOS: 8450, Stable: 632; p<0.05). Furthermore, splenic lymphocytes demonstrated increased frequency of lung SAg-specific IL-17 (Col-V: 128±46 vs 31±21, p=0.013; Kα1T: 194±47 vs 67±43, p=0.014) and interferon-γ (Col-V: 165±79 vs 38±40, p=0.042; Kα1T: 232±64 vs 118±39, p=0.012). Reduced levels of IL-10-producing cells were seen in BOS exosome immunized mice compared to mice immunized with stable exosomes (Col-V: 59±23 vs 211±85, p=0.016; Kα1T: 78±49 vs 295±104, p=0.017). Due to the unique immune-stimulating properties of exosomes induced during rejection, we propose that they play an important role in eliciting both allo- and SAg-specific immunity, leading to chronic rejection after LTx.
Introduction
Chronic rejection, clinically diagnosed as bronchiolitis obliterans syndrome (BOS), continues to be major complication of lung transplantation (LTx), affecting roughly 50% of lung transplant recipients (LTxR) within three years of transplant (1–3). Previous findings from our group demonstrated that LTxR with pre-transplant antibodies (Abs) to lung-associated self-antigens (SAg) had a higher incidence of primary graft dysfunction, donor-specific anti-HLA development, and more risk factors for BOS development (4–6). In addition, de novo developments of Ab to Collagen V (Col-V) and K-alpha 1-tubulin (Kα1T) have also been associated with BOS, resulting in poor allograft survival (7). Studies have demonstrated that, in addition to immune responses to HLA and SAg (7–9), respiratory viral infections (10–12) and acute rejection episodes (13, 14) are also risk factors for BOS.
Exosomes are nano-vesicles of an average size of 40–100nm. They contain membrane and cytosolic proteins as well as molecules essential for exosome biogenesis, which are secreted by various cells and released in biological fluids (15). The exosomes’ composition highly depends on the biological function of the parental cell. Therefore, exosomes contain specific microRNA (miRNA), mRNA, and proteins associated with specific cells types including epithelial cells, B cells, T cells and dendritic cells (15–17). Sigdel et al demonstrated that urinary exosomes isolated from kidney transplant recipients (KTxR) with acute rejection had proteins involved in inflammatory responses; urinary exosomes isolated from stable KTxR did not (18). A recent report by Dieudé et al also demonstrated that exosomes isolated from human umbilical vein endothelial cells and mouse endothelial cells contain active 20S proteasome that increases the immunogenicity of the exosomes, leading to the development of Ab to the kidney-associated SAg perlecan in a mouse model (19).
We recently demonstrated that exosomes isolated from sera and from bronchoalveolar lavage (BAL) fluid of LTxR diagnosed with BOS express mismatched donor HLA as well as the SAg, Col-V and Kα1T. These exosomes also contained immunomodulatory miRNA involved in endothelial activation, inflammation, and IL17 upregulation (20). Studies have also demonstrated that donor derived exosomes induces T cell mediated allo-response resulted in allograft rejection (21, 22). Therefore, we propose that circulating exosomes may play an important role in activating and perpetuating immune responses that lead to BOS after human LTx. In this communication, we demonstrate that exosomes isolated from the sera of LTxR diagnosed with BOS are markedly different from the exosomes isolated from stable LTxR. The exosomes differ in their antigenic properties, proteasome content, and in the presence of co-stimulatory molecules and transcription factors involved in immune activation. Furthermore, we will describe how exosomes from LTxR with BOS induced both cellular and humoral immune responses to SAg, but exosomes from stable LTxR did not. The exosomes isolated from these two groups were different in their ability to activate immune cells that secrete SAg-specific cytokines.
Materials and Methods
Patient population
Twenty patients undergoing bilateral LTx at Washington University Barnes-Jewish Hospital between 2012 and 2013 were enrolled in this study. Informed consent was obtained from all patients, and this study was approved by the Institutional Review Board at Washington University. Exosomes were isolated from the sera of 10 stable LTxR (24.4±10.7 months) and from 10 LTxR diagnosed with BOS (17.3±8.2 months). An equal volume of sera was taken from each patient and was pooled for immunoblot with specific Ab to SAg, Kα1T and Col-V. BOS and normal pulmonary function were defined according to the International Society for Heart and Lung Transplant guidelines (1). No significant differences were observed in the demographics of each group (Table 1). Immunosuppressive regimens consisted of cyclosporine, azathioprine, and prednisone. Sera were collected at the time of BOS diagnosis or at the time an LTxR was deemed stable, and these samples were stored at −80° C until analysis.
Exosome isolation from sera by ultracentrifugation
Exosomes were isolated from sera using the ultracentrifugation method. Exosome purity was validated by the sucrose cushion method (20, 23). Briefly, 1 mL of serum was centrifuged at 3,000g for 20 minutes at room temperature to remove cells, and again at 10,000g for 20 minutes to remove cell debris. Serum was diluted with 1X PBS and centrifuged at 100,000g for 70 minutes at 4°C. The exosome pellet was washed twice with 1X PBS and centrifuged at 100,000xg for 70 minutes. The purity of the exosomes was determined using sucrose cushion method (20, 23). Finally, the isolated exosomes were lysed with RIPA buffer; protein concentration was measured with the bicinchoninic acid method for immunoblot.
Immunoblot
Detection and semi-quantitation of costimulatory molecules
Exosomes isolated from pooled serum of LTxR diagnosed with BOS (n=10) and from stable LTxR (n=10) were analyzed to determine the presence of co-stimulatory molecules (CD-40, CD-80 and CD-86) by western blot. Briefly, 3μg of total exosome proteins were resolved in 12% Bis-Tris polyacrylamide gel (Thermo Fisher Scientific, Waltham, MA) and were transferred into a polyvinylidene fluoride membrane. The membrane was blocked with 5% nonfat milk prepared in 1X PBS. Abs specific to co-stimulatory molecules CD-40 (Abcam ab13545), CD-80 (Abcam ab134120), CD-86 (Abcam ab53004), and Alix (Bio Legend 634501) were used to detect the specific protein in the exosomes. Goat-anti-rabbit Ab conjugated with HRP was used as a secondary Ab. The blots were developed using chemiluminescent HRP substrate (Millipore WBKLS0500) and exposed using the Odyssey CLx Imaging System (LI-COR Biosciences, Lincoln, NE). The band intensity was quantified using ImageJ software and normalized with Alix.
Detection and semi-quantitation of Col-V, Kα1T, and MHC class II (MHC-II) molecules
Pooled exosomes isolated from stable LTxR and from those with BOS were analyzed for the presence of SAg (ie, Col-V, Kα1T, Col-IV and Fibronectin) and MHC-II molecules. Ab specific to MHC-II (Abcam ab157210), Col-I (Abcam ab34710), Col-V (Abcam ab7046), and Kα1T (sc-12462-R, Santa Cruz Biotechnology), were used per manufacturer’s suggestion.
Detection and semi-quantitation of transcription factors
In order to identify the transcription factors present in the exosomes, western blot was performed as described above. Specific Abs to NF-κB (Cell Signaling, C22B4), hypoxia-inducible factor 1-alpha (HIF-1α Abcam ab51608), (CIITA, Abcam ab49132), IRAK1 (Cell Signaling D51G7), and MyD88 (Cell Signaling D80F5) were used.
Detection and semi-quantitation of proteasome
In order to detect 20S proteasome by western blot, we targeted α3 subunit (Santa Cruz Biotechnology sc-58414) using specific Ab.
Detection and semi-quantitation of adhesion molecules
Cell adhesion molecules were detected using specific Ab to E-Selectin (Bio Legend 322602), E-cadherin (Santa Cruz Biotechnology sc-8426), ICAM (Bio Legend 353101), P-selectin (Bio Legend 304902), PECAM (Bio Legend 303101) and VCAM (Bio Legend 305802). The band intensity was quantified using ImageJ software and was normalized with Alix.
Immunogenicity of exosomes isolated from LTxR with BOS and stable LTxR
C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were immunized with pooled exosomes isolated from the sera of LTxR with BOS (n=10) or stable LTxR (n=10). 100μg of exosomes in 100μl emulsified in Freund’s incomplete adjuvant were injected subcutaneously in 6-week-old male C57BL/6 mice (n=5) on days 1, 13, and 20. Sera were collected on days 10, 20, and 30 for detection of development of Ab to Col-V and Ka1T. Samples were analyzed with ELISA. Animals were sacrificed on day 30, and spleens were harvested for isolation of lymphocytes for ELISpot assay against SAg. The frequency of cells secreting SAg-specific cytokines was enumerated.
Measurement of HLA class I Ab in mice immunized with exosomes from LTxR with BOS and stable LTxR
Sera collected from mice immunized (on day 30) with exosomes from LTxR with BOS and from stable LTxR were used to measure the Ab to human HLA I using LABScreen Mixed Ag kit (LSM12, One Lambda Inc, Canoga Park, CA) by Luminex assay, according to manufacturer’s protocol. Briefly, 5 μl of beads coated with mixed HLA I Ag were added to 20 μl of serum samples in 96-well plates and incubated for 30 minutes at room temperature. The plates were washed and 100 μl of 1X PE-conjugated goat-anti-mouse Ab were added as secondary Ab and incubated for 30 minutes. Plates were then washed and up to 100 beads were analyzed using the Bio-Plex 200 System (Bio-Rad, Hercules, CA). Sera from C57BL/6 mice were used as negative controls and for normalization. Sera samples were considered positive for Ab to HLA class I if the mean fluorescence intensity (MFI) was greater than 2287.
Measurement of circulating cytokines
Sera samples collected on day 30 were used to measure cytokines by 12-plex Luminex assay kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, 50 μl of serum samples (1:50 dilution) was added to the multiplex beads coated in the wells and incubated for 2 hours at room temperature. Cytokines with known concentrations were used as standards in the same plate. The cytokines bound in the beads were detected using biotinylated anti-human multi-cytokine reporter and streptavidin-phycoerythrin detection. The plate was washed and read using Bio-PlexLuminex100, and cytokine concentrations were measured using a standard curve obtained in the same plate and demonstrated as MFI.
Detection of Ab to lung SAg, Kα1T and Col-V
Sera collected on days 10, 20, and 30 were used to measure Ab to the lung SAg, Col-V and Kα1T, by ELISA developed in our laboratory and detailed in earlier publications (8). Briefly, 1μg/ml Col-V (Sigma C3657) and Kα1T (prepared in the laboratory) were dissolved in 1X PBS and were coated on 96-well ELISA plates (Corning #3369) and stored at 4°C overnight. Sera were diluted (1:100) and were added to the wells and incubated at room temperature for 2 hours. Plates were then washed with PBS-tween (PBST 20), and 100 μl of goat-anti-mouse conjugated HRP (1:10000) was added to the wells. Thereafter, plates were developed with substrate tetra methyl benzidinec (Millipore ES022), and enzymatic reaction was stopped with 1N HCL. The colorimetric reading was obtained with ELISA reader at a 450nm wavelength. Finally, the Ab concentration of the sera was calculated with known concentrations of Ab to Col-V (Abcam ab7046) and Ka1T (Santa Cruz Biotechnology sc-12462-R) performed as standards in the same plates. Sera collected from naive C57BL/6 mice were used as negative control.
Detection of cellular immune responses to lung SAg and enumeration of SAg-specific cytokine-secreting cells
Splenocytes isolated by Ficoll-Hypaque gradient centrifugation after sacrifice of exosome-immunized animals were used in ELISpot analysis as described earlier (24). Briefly, 96-well plates were coated with Ab against IFN-γ, IL-10, and IL-17 and were incubated overnight at 4°C. The plates were washed with PBS and blocked with 5% BSA for 2 hours at room temperature. Splenocytes (3×105 cells) were seeded in the wells as responders, and Col-V (5μg/ml) or Kα1T (5μg/ml) were subsequently added as stimulators. The plates were then incubated in a humidified CO2 incubator for 48 hours. The plates were washed with PBS (3 times) and PBST (3 times). Subsequently, biotinylated cytokine-specific Ab was added for 3 hours and the plates were washed with PBST. HRP-labeled streptavidin was then added in the plates, incubated for 2 hours, and the plates were then developed with substrate (3-amino-9-ethylcarbazole). Finally, the plates were washed with milliQ and spots were captured with ImmunoSpot analyzer (Cellular Technology). FN-γ), IL-10, and IL-17 cytokines spots detected in the control wells were subtracted from the spots enumerated in the sample wells to determine spots per million cells. Splenocytes treated with concanavalin A served as positive control.
Statistical analysis
Ab development and levels were compared between BOS and stable exosome-immunized mice using Mann-Whitney two-tailed Student’s t-test. Statistical analysis for Ab development, SAg-specific cytokines, and serum cytokines was performed using GraphPad software (GraphPad Prism, La Jolla, CA). Statistical data in each cohort were expressed as mean±standard deviation. P-values less than 0.05 were considered statistically significant in each comparative analysis. Optical density of the immunoblot was quantified using ImageJ software.
Results
Increased levels of lung SAg (Col-V and Kα1T) and presence of MHC-II in exosomes isolated from BOS
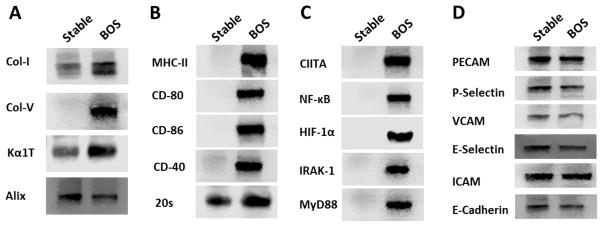
Exosomes isolated from LTxR diagnosed with BOS showed higher concentrations of the lung SAg, Col-V and Kα1T (2.5 fold), compared with stable LTxR. Even Col-I was elevated in the LTxR diagnosed with BOS (2.2 fold). Further, MHC-II was present only in the exosomes isolated from LTxR diagnosed with BOS, not in those isolated from stable LTxR (Figure 1A, B).
Figure 1. Immunoregulatory pathway-associated proteins in exosomes derived from LTxR diagnosed with BOS and stable LTxR.
Exosomes isolated from the sera of LTxR with BOS show significantly higher lung-associated SAg, Col-V and Kα1T, and 20S proteasome α3 subunit in LTxR diagnosed with BOS than in stable LTxR (A, B). Exosomes isolated from BOS demonstrated presence of co-stimulatory molecules CD-80, CD-86, CD-40 and MHC class II molecules (B) Transcription factors NF-kB, HIF-1A, MHC class II transactivator, IRAK-1 and MyD88 (C) but not in the exosomes of stable LTxR. Exosomes isolated from both LTxR with BOS and stable LTxR demonstrated the presence of the cell adhesion molecules PECAM, P-selectin, VCAM, E-selectin, ICAM, and E-cadherin (D). Alix served as loading control and exosome-specific marker.
Costimulatory molecules (CD40, CD80, and CD86) were present only in exosomes isolated from LTxR diagnosed with BOS
Exosomes isolated from LTxR diagnosed with BOS demonstrated the presence of costimulatory molecules (ie, CD40, CD80 and CD86). These costimulatory molecules were not detected in the exosomes isolated from sera of stable LTxR (Figure 1B). This demonstrates that exosomes from LTxR diagnosed with BOS contain costimulatory molecules and therefore can activate immune responses to allo- and SAg expressed on the exosomes isolated from LTxR diagnosed with BOS.
Higher levels of 20S proteasome in exosomes from LTxR diagnosed with BOS
Exosomes isolated from KTxR and from mice with endothelial cell activation (19) contain 20S proteasome, which has been proposed to activate immune responses. Based on this, we analyzed exosomes for the presence of 20S proteasomes, α3 subunit using specific Ab (sc-58414). Immunoblot results showed that exosomes isolated from LTxR diagnosed with BOS have increased levels of 20S proteasome α3 subunit compared with exosomes isolated from stable LTxR (Figure 1B).
Exosomes from LTxR diagnosed with BOS demonstrate unique transcription factors
We analyzed for selected transcription factors involved in immune activation including CIITA, NF-kB, HIF1-α and molecules involves in TLR-4 activation. The results showed the presence of transcription factors CIITA, NF-kB, HIF1-α and TLR-4 pathway-associated molecules IRAK1 and MyD88 in the exosomes isolated from LTxR diagnosed with BOS but not in exosomes isolated from stable LTxR (Figure 1C). These results also support the theory that circulating exosomes can be potent immunogens.
Cell adhesion molecules (eg, PECAM, VCAM, ICAM, E-Cadherin, P-selectin and E-selectin) were present in exosomes isolated from both LTxR diagnosed with BOS and from stable LTxR
Using specific Ab in western blot, we demonstrated that exosomes isolated from both LTxR diagnosed with BOS and from stable LTxR had similar quantities of various cell adhesion molecules, including PECAM, VCAM, ICAM, E-Cadherin, P-selectin, and E-selectin (Figure 1D).
Development of Ab to human HLA class I and lung SAg (Col-V and Kα1T) after immunization of mice with exosomes isolated from LTxR diagnosed with BOS
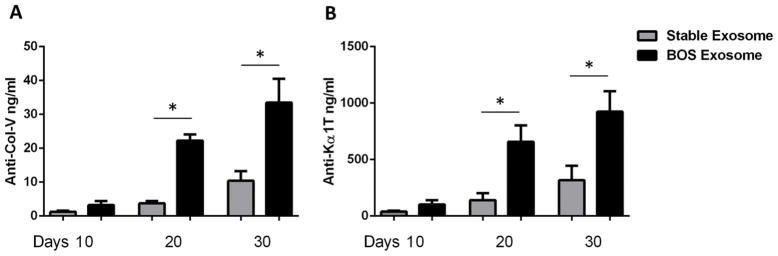
Mice injected with exosomes from LTxR diagnosed with BOS showed significantly increased levels of Ab to HLA class I compared to mice immunized with exosomes from stable LTxR (MFI; BOS: 8450 vs Stable: 632; p=0.0001) (Figure 2). Sera collected from mice immunized with exosomes from LTxR diagnosed with BOS also demonstrated significantly increased levels of Ab to Col-V and Kα1T but not to kidney SAg, Col-IV or Fibronectin, compared with mice immunized with exosomes isolated from stable LTxR on day 20 (Col-V: 22.3±4.1 vs 3.7±1.6, p=0.001; Kα1T: 658±325 vs 141±139, p=0.023) and on day 30 (Col-V: 33.5±15.7 vs 10.4±6.4, p=0.021; Kα1T: 925±403 vs 317±285, p=0.044) (Figure 3). These results demonstrate that surface expression of both donor HLA and lung SAg, Col-V and Kα1T, along with costimulatory molecules, transcription factors, and proteasome, induces strong humoral immune responses.
Figure 2. Detection of Ab to HLA class I in mice immunized with exosomes from LTxR diagnosed with BOS and from stable LTxR.
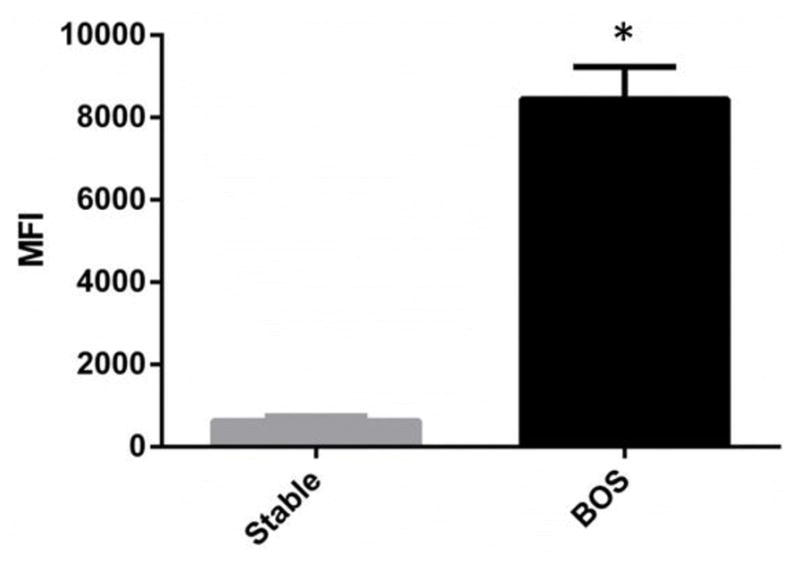
Serum samples collected from mice that were subcutaneously immunized with exosomes of LTxR diagnosed with BOS and stable LTxR were used to measure Ab to HLA class I Ag by Luminex Assay. Mice injected with exosomes from LTxR diagnosed with BOS showed significantly increased Ab to HLA class I compared with mice injected with exosomes from stable LTxR (MFI; BOS: 8450 vs stable: 632, p=0.0001). The positive cutoff value obtained from MFI value from normal C57BL/6J mice and Ab to HLA class I was considered positive if the MFI was greater than 2287.
Figure 3. Development of Ab to lung-associated SAg in mice injected with exosomes derived from LTxR with BOS and from stable LTxR.
Ab to lung-associated SAg was quantified in mice that were subcutaneously injected with exosomes from LTxR with BOS and stable LTxR using serum samples collected on days 10, 20 and 30. Ab to Col-V were compared in mice injected with stable LTx exosomes and BOS patients derived exosomes (A). Serum Ab concentrations against Kα1T were measured in mice injected with exosomes isolated from LTxR with BOS, stable LTxR, and adult C57BL/6 mice (B).
Increased frequency of SAg-specific inflammatory cytokine-producing cells after immunization of mice with exosomes from LTxR diagnosed with BOS
Mice immunized with exosomes from LTxR diagnosed with BOS or stable LTxR were used to quantify the cytokine-producing cells using splenocytes isolated on day 30. Mice immunized with BOS exosomes demonstrated increased frequency of SAg, Col-V and Kα1T, specific pro-inflammatory cytokines IFN-γ (Col-V: 165±79 vs 38±40, p=0.042; Kα1T: 232±64 vs 118±39, p=0.012) and IL-17 (Col-V: 128±46 vs 31±21, p=0.013; Kα1T: 194±47 vs 67±43, p=0.014) compared with mice immunized with exosomes from stable LTxR (Figure 4).
Figure 4. T cell response to lung-associated SAg in mice that were subcutaneously injected with exosomes derived from LTxR with BOS and from stable LTxR.
Splenocytes isolated from mice on day 30 were used to enumerate pro-inflammatory cytokines (ie, IFN-γ, IL-17) by ELISpot. We observed increased frequency of lung-associated SAg Col-V-specific IFN-γ and IL-17 cytokines (A) and Kα1T-specific IFN-γ and IL-17 cytokines (B) in mice that were injected with exosomes isolated from patients with BOS compared with mice injected with exosomes from stable LTxR.
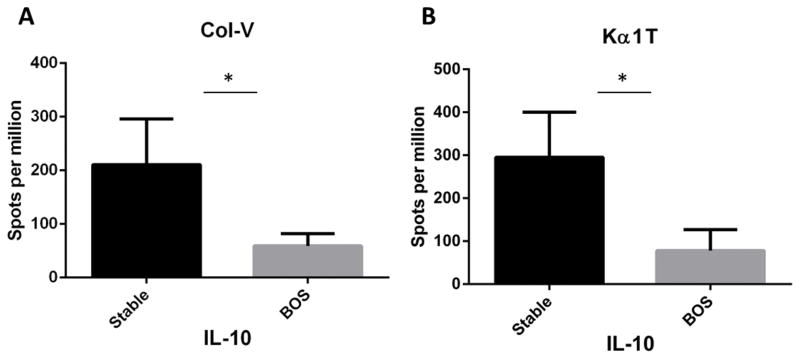
Decreased frequency of SAg-specific IL-10 responses in the spleen of mice immunized with exosomes from LTxR diagnosed with BOS
It is of interest that mice immunized with exosomes from LTxR diagnosed with BOS also demonstrated a significantly reduced frequency of cytokine IL-10 compared with mice immunized with exosomes from stable LTxR (Col-V: 59±23 vs 211±85, p=0.016; Kα1T: 78±49 vs 295±104, p=0.017) (Figure 5). These results suggest that the differences in the exosome cargo (ie, costimulatory molecules, transcription factors, miRNA etc) can influence immune response to SAg that could increase the risk of BOS.
Figure 5. Frequency of anti-inflammatory cytokine IL-10 producing cells in mice that were subcutaneously injected with exosomes derived from LTxR diagnosed with BOS and stable LTxR.
Mice immunized with exosomes from LTxR diagnosed with BOS and stable LTxR were used to enumerate anti-inflammatory cytokines by ELISpot. We noted reduced frequency of lung-associated SAg Col-V-specific IL-10 cytokines (A) and Kα1T-specific IL-10 cytokines (B) in mice injected with exosomes isolated from patients with BOS compared with mice injected with exosomes from stable LTxR.
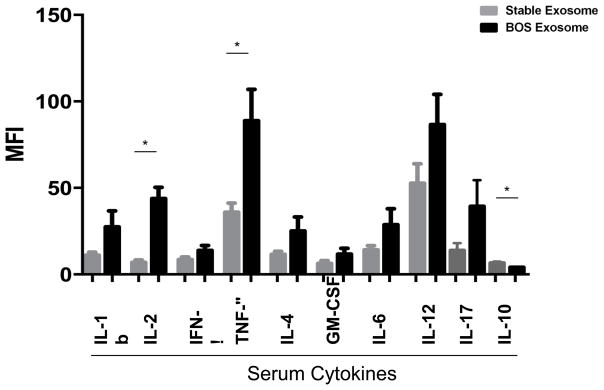
Circulating cytokines are also induced in mice immunized with exosomes isolated from LTxR diagnosed with BOS
Sera collected from mice immunized with exosomes isolated from LTxR diagnosed with BOS or from stable LTxR were used to measure cytokines with Luminex assay. Mice immunized with exosomes isolated from LTxR diagnosed with BOS demonstrated significantly increased levels of IL-2 (43.8±13.2 vs 6.9±2.8, p=0.001) and TNF-α (88.8±36.3 vs 35.9±10.4, p=0.031) and decreased IL-10 (4.06±0.15 vs 6.4±0.8, p=0.036) than mice immunized with stable LTxR exosomes. Levels of other pro-inflammatory cytokines were also increased in the circulation, such as IL-1b (27.4±18.6 vs 11.0±3.7), IFN-γ (13.8±5.7 vs 8.5±3), IL-4 (25±16.3 vs 11.6±3.6), GM-CSF (11.6±7 vs 6.3±3.3), IL-6 (28.6±18.6 vs 14.2±4.6), IL-12 (86.6±35.1 vs 52.6±22.6) and IL-17 (39.2±15.1 vs 13.7±4.3) although none of these levels reached statistical significance (Figure 6).
Figure 6. Serum inflammatory cytokine levels in that were mice immunized with exosomes derived from LTxR diagnosed with BOS and stable LTxR.
Sera collected on day 30 from mice immunized with exosomes derived from LTxR with BOS shown increased inflammatory cytokines IL-1b, IL-2 (p=0.0016), IFN-γ, TNF-α (p=0.031), IL-4, GM-CSF, IL-6, IL-12, IL-17 and decreased IL-10 (p=0.036) compared with stable LTxR recipients analyzed by Luminex assay.
Discussion
Immune responses to donor-mismatched HLA and non-HLA (ie, tissue-associated SAg) are considered major risk factors for rejection after human LTx (4, 7, 9). Recent studies from our laboratory demonstrated that exosomes isolated from sera and BAL fluid of LTxR diagnosed with BOS have surface expression of donor-mismatched HLA and lung SAg, Col-V and Kα1T, none of which was demonstrable on exosomes isolated from stable LTxR and from sera collected pre-transplant from the patients tested in this report who subsequently developed BOS. Furthermore, exosomes isolated from LTxR diagnosed with BOS also contain several immunoregulatory miRNAs known to be involved in endothelial activation and Th17 upregulation (20).
In this study, we demonstrate that exosomes isolated from LTxR diagnosed with BOS have significantly different levels of costimulatory molecules CD40, CD80, and CD86; transcription factors NF-kB and HIF-1α; MHC-II transactivator; MHC-II molecules; and 20S proteasomes compared with exosomes isolated from stable LTxR. This supports the proposition that continued release of exosomes from LTxR diagnosed with BOS can lead to perpetuation of immune responses, causing BOS after human LTx. Various costimulatory molecules (ie, CD40, CD80, and CD86) present only in the exosomes isolated from LTxR with BOS supports the theory that exosomes can stimulate T-cell responses by direct, semi-direct and indirect pathways of Ag presentation resulting in immune responses to both allo and lung SAg. Reports from Dieude’s group have shown that mice immunized with exosomes isolated from serum-starved HUVEC and mouse endothelial cells produced Ab to the kidney-associated SAg perlecan in non-grafted and aorta-transplanted mouse models (19). In addition, significant increased humoral responses to perlecan were demonstrated in the exosomes that contained active 20S proteasome compared with mice immunized with 20S-proteasome-inhibited exosomes (19). In our study, mice immunized with exosomes derived from LTxR diagnosed with BOS showed significantly increased levels of Ab to the lung SAg, Col-V and Kα1T (Figure 3). We also showed that exosomes isolated from LTxR diagnosed with BOS have increased levels of 20S proteasome compared with stable LTxR. Given the surface expression of lung SAg along with the presence of costimulatory molecules, MHC-II, transcription factors, and 20S proteasome in the exosomes of LTxR diagnosed with BOS, it is unsurprising that these exosomes can induce Ab to lung SAg.
Previous findings from our group have demonstrated that exosomes isolated from LTxR diagnosed with BOS expressed donor-mismatched HLA class I Ag (ie, HLA-A2) which were undetectable on the exosomes isolated from stable LTxR (20). Here we demonstrate that surface expression of HLA also induces humoral immune responses in mice. These results confirm that exosomes containing donor-mismatched HLA as well as SAg can induce immune responses to both, which we consider to be a factor in increasing the risk for the development of BOS after human LTx. We performed luminex assay to measure the Ab development to MHC-class II in sera collected from mice immunized with BOS and stable exosomes. The results did not show significant differences between BOS and stable exosomes immunized animals (data not shown).
Evidence by Burlingham et al and others have shown that CD4+ T cell- and monocyte-dependent cellular immunity induces the pathogenies of BOS, in particular Col-V-specific Th17 responses, which are involved in airway injury and ultimately, BOS (25, 26). A previous report from our lab demonstrated that IL-17 neutralization can prevent the development anti-MHC-induced obliterative airway disease in a mouse model (8). Recently, it has been demonstrated that obliterative bronchiolitis in a C57BL/6 orthotropic LTx mouse model (27) can also be prevented by IL-17 neutralization. We demonstrate here that splenocytes isolated from mice immunized with BOS exosomes resulted in a significant increase in Col-V and Kα1T-specific IFN-γ and IL-17 producing cells compared with mice immunized with exosomes from stable LTxR. These results strongly suggest that exosomes containing allo- and lung SAg, along with cargos discussed above, can be involved in the induction and perpetuation of Th-17 responses that contribute to BOS development after human LTx. More significant is our finding that immunization of mice with exosomes from stable LTxR resulted in higher levels of SAg-specific IL10-secreting cells and reduced levels of IL17 and IFN-γ cells in contrast to exosomes isolated from patients diagnosed with BOS (Figure 4). This also supports that exosomes affect regulation of immune responses not only in mice, but also in human LTxR at risk for BOS. We performed H&E and trichrome staining to determine the cellular infiltration and fibrosis. Mice immunized with exosomes from BOS as well as stable did not result in pathology to the native lungs of mice (data not shown). We propose that this is due to the absence of primary injury to the lungs due to our earlier findings that when Ab to lung SAg were administered following orthotopic single LTx, only the transplanted lung, but not the native lung, demonstrated obliterative airway disease lesions (28).
Previous findings from our group defined that ligation of Ab to Kα1T in airway epithelial cells increases HIF-1α expression, leading to upregulation of fibrogenic growth factors that may be involved in the development of fibrosis in LTxR with BOS (29). Here we demonstrated that BOS-derived exosomes contained NF-kB and HIF1-α, which can activate the inflammatory cascade and lead to the production of pro-inflammatory cytokines. Furthermore, we demonstrated that immunization of mice with exosomes containing transcription factors from BOS patients showed increased serum inflammatory cytokines IL-1b, IL-2, IFN-γ, TNF-α, IL-4, GM-CSF, IL-6, IL-12 and IL-17.
In conclusion, our results demonstrated that exosomes isolated from LTxR diagnosed with BOS contain surface expression of donor HLA, lung SAg, MHC-II, costimulatory molecules, cell adhesion molecules, and various transcription factors. We also used a murine immunization model to show that exosomes isolated from patients diagnosed with BOS are immunogenic, resulting in the development of humoral and cellular immune responses to the lung-associated SAg, Col-V and Kα1T. Furthermore, we showed the importance of exosome composition in eliciting different immune responses with distinct cytokine induction. It is likely that this may involve alteration of the peripheral regulatory mechanisms by exosomes. Finally, we propose that induction and continuous release of exosomes from the transplanted organ will play an important role in the pathogenesis of chronic rejection following human organ transplantation, and we suggest that blocking exosome production and release may be a viable strategy for prevention of chronic rejection.
Table I.
Demographics of 20 lung transplant recipients
| Clinical features | BOS (n=10) | Stable (n=10) |
|---|---|---|
| Mean recipient age at transplant, years | 49±12 | 54±8 |
| Male sex | 5(50%) | 5(50%) |
| Race | ||
| White | 10(100) | 9(90) |
| African-American | 0(0) | 1(10) |
| End-stage lung disease | ||
| Cystic fibrosis | 5(50) | 1(10) |
| Idiopathic pulmonary fibrosis | 3(30) | 0(0) |
| Chronic obstructive pulmonary disease | 1(10) | 7(70) |
| Other | 1(10) | 2(20) |
| HLA mismatch | ||
| A | 1.5±0.5 | 1.4±0.5 |
| B | 1.8±0.4 | 1.6±0.5 |
| DR | 1.9±0.3 | 1.3±0.9 |
| Bilateral LTx | 10(100) | 10(100) |
| Mean time from LTx to sampling, months | 17.3±8.2 | 24.4±10.7 |
| Mean time from LTx to BOS onset, months | 16.2±8.9 | N/A |
Values presented as n (%), unless otherwise noted.
Abbreviations: BOS: bronchiolitis obliterans syndrome, HLA, human leukocyte antigen; LTx, lung transplantation; N/A, not applicable.
Acknowledgments
The authors would like to acknowledge Billie Glasscock and Clare Prendergast for their assistance in preparing and submitting this manuscript.
Abbreviations
- BAL
bronchoalveolar lavage
- BOS
bronchiolitis obliterans syndrome
- Col-I
collagen I
- Col-V
collagen V
- HIF-1α
hypoxia-inducible factor 1-alpha
- Ka1T
K-alpha-1-tubulin
- LTx
lung transplantation
- KTxR
kidney transplant recipient
- LTxR
lung transplant recipient
- MFI
mean fluorescence intensity
- MHC-II
major histocompatibility complex class II
- miRNA
microRNA
- PBST
phosphate-buffered saline with tween 20
- SAgs
self-antigens
Footnotes
This work was supported by National Institutes of Health Grants AI123034, HL092514, HL056643 (TM).
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report--2011. J Heart Lung Transplant. 2011;30:1104–1122. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Al-Githmi I, Batawil N, Shigemura N, Hsin M, Lee TW, He GW, Yim A. Bronchiolitis obliterans following lung transplantation. Eur J Cardiothorac Surg. 2006;30:846–851. doi: 10.1016/j.ejcts.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Sundaresan S, Trulock EP, Mohanakumar T, Cooper JD, Patterson GA. Prevalence and outcome of bronchiolitis obliterans syndrome after lung transplantation. Washington University Lung Transplant Group. The Annals of thoracic surgery. 1995;60:1341–1346. doi: 10.1016/0003-4975(95)00751-6. discussion 1346–1347. [DOI] [PubMed] [Google Scholar]
- 4.Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kauke T, Kneidinger N, Martin B, Dick A, Schneider C, Schramm R, Meimarakis G, Preissler G, Eickelberg O, von Dossow V, Behr J, Hatz R, Neurohr C, Winter H. Bronchiolitis obliterans syndrome due to donor-specific HLA-antibodies. Tissue antigens. 2015;86:178–185. doi: 10.1111/tan.12626. [DOI] [PubMed] [Google Scholar]
- 6.Kuo E, Maruyama T, Fernandez F, Mohanakumar T. Molecular mechanisms of chronic rejection following transplantation. Immunol Res. 2005;32:179–185. doi: 10.1385/IR:32:1-3:179. [DOI] [PubMed] [Google Scholar]
- 7.Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T. Antibodies to K-alpha 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12:2164–2171. doi: 10.1111/j.1600-6143.2012.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, Steward N, Aloush A, Hachem R, Trulock E, Meyers B, Patterson GA, Mohanakumar T. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624–631. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharat A, Kuo E, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Respiratory virus-induced dysregulation of T-regulatory cells leads to chronic rejection. Ann Thorac Surg. 2010;90:1637–1644. doi: 10.1016/j.athoracsur.2010.06.048. discussion 1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almaghrabi RS, Omrani AS, Memish ZA. Cytomegalovirus infection in lung transplant recipients. Expert Rev Respir Med. 2017;11:377–383. doi: 10.1080/17476348.2017.1317596. [DOI] [PubMed] [Google Scholar]
- 12.Fisher CE, Mohanakumar T, Limaye AP. Respiratory virus infections and chronic lung allograft dysfunction: Assessment of virology determinants. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35:946–947. doi: 10.1016/j.healun.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Aguilar PR, Carpenter D, Ritter J, Yusen RD, Witt CA, Byers DE, Mohanakumar T, Kreisel D, Trulock EP, Hachem RR. The Role of C4d Deposition in the Diagnosis of Antibody-Mediated Rejection after Lung Transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017 doi: 10.1111/ajt.14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z, Yang W, Steward N, Sweet SC, Danziger-Isakov L, Heeger PS, Mohanakumar T. Role of Circulating MicroRNAs in the Immunopathogenesis of Rejection After Pediatric Lung Transplantation. Transplantation. 2017;101:2461–2468. doi: 10.1097/TP.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 16.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 17.Levanen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL, Skold CM, Svartengren M, Grunewald J, Gabrielsson S, Eklund A, Larsson BM, Woodruff PG, Erle DJ, Wheelock AM. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. The Journal of allergy and clinical immunology. 2013;131:894–903. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigdel TK, Ng YW, Lee S, Nicora CD, Qian WJ, Smith RD, Camp DG, 2nd, Sarwal MM. Perturbations in the urinary exosome in transplant rejection. Frontiers in medicine. 2014;1:57. doi: 10.3389/fmed.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieude M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, Hamelin K, Qi S, Pallet N, Beland C, Dhahri W, Cailhier JF, Rousseau M, Duchez AC, Levesque T, Lau A, Rondeau C, Gingras D, Muruve D, Rivard A, Cardinal H, Perreault C, Desjardins M, Boilard E, Thibault P, Hebert MJ. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Science translational medicine. 2015;7:318ra200. doi: 10.1126/scitranslmed.aac9816. [DOI] [PubMed] [Google Scholar]
- 20.Gunasekaran M, Xu Z, Nayak DK, Sharma M, Hachem R, Walia R, Bremner RM, Smith MA, Mohanakumar T. Donor-Derived Exosomes With Lung Self-Antigens in Human Lung Allograft Rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17:474–484. doi: 10.1111/ajt.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan ML, Gibson GA, Watkins SC, Larregina AT, Morelli AE. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest. 2016;126:2805–2820. doi: 10.1172/JCI84577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, Abdi R, Uehara M, Kim JI, Markmann JF, Tocco G, Benichou G. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol. 2016;1:aaf8759. doi: 10.1126/sciimmunol.aaf8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology. 2006;Chapter 3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 24.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6:1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 25.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Cummings OW, Heidler KM, Blum JS, Wilkes DS. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 27.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, Fisher AJ, Cummings OW, Heidler KM, Keller MR, Burlingham WJ, Wilkes DS. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–922. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian V, Ramachandran S, Banan B, Bharat A, Wang X, Benshoff N, Kreisel D, Gelman AE, Mohanakumar T. Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am J Transplant. 2014;14:2359–2366. doi: 10.1111/ajt.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiriveedhi V, Gelman AE, Mohanakumar T. HIF-1alpha signaling by airway epithelial cell K-alpha1-tubulin: role in fibrosis and chronic rejection of human lung allografts. Cell Immunol. 2012;273:59–66. doi: 10.1016/j.cellimm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]