Summary
Background
Atrial fibrillation (AF) and venous thromboembolism (VTE) frequently co-occur. These conditions have shared risk factors and are accompanied by coagulation abnormalities. Furthermore, mechanistic pathways may directly link the disorders.
Objectives
To test the hypothesis that individuals with incident AF are at greater risk of developing VTE, and those with VTE are at elevated risk of AF. We also tested whether associations were stronger in the first 6 months after the initial diagnosis, and explored race differences.
Patients/Methods
15,129 ARIC study participants (45-64 years, 55% female, 26% black) were followed from 1987- 2011 for incident AF and VTE (median follow-up 19.8 years). Multivariable-adjusted Cox regression was used, with AF and VTE modeled as time-dependent exposures.
Results
Incident AF was associated with greater risk of subsequent incident VTE [HR (95%CI): 1.71 (1.32-2.22)]; the association was stronger in blacks [2.30 (1.48-3.58)] and during the first 6 months after AF diagnosis [5.08 (3.08-8.38)]. Similarly, incident VTE was associated with increased risk of incident AF [1.73 (1.34-2.24)], especially in blacks [2.40 (1.55-3.74)] and in the first 6 months after VTE diagnosis [4.50 (2.61-7.77)].
Conclusions
The occurrence of AF was associated with increased risk of incident VTE, and occurrence of VTE was associated with greater risk of incident AF. Associations were particularly strong among blacks, and during the first 6 months after the initial diagnosis, though they remained elevated even after 6 months. These findings highlight patient populations that may be at increased risk of AF and VTE, and perhaps should be targeted with preventive strategies.
Keywords: atrial fibrillation, epidemiology, venous thromboembolism, racial disparities, Atherosclerosis Risk in Communities (ARIC) Study
Introduction
Atrial fibrillation (AF) and venous thromboembolism (VTE) are common conditions, particularly among the elderly [1]. The lifetime risk for AF is 1 in 4 [2], while the lifetime risk for VTE is 1 in 8 [3]. Both AF and VTE have substantial morbidity and mortality [1], thus necessitating further investigation of predisposing factors. As recently reviewed [4], AF and VTE frequently co-exist, and it has been suggested that each disorder is a risk factor for the other. This hypothesis warrants further study.
Conventionally, deep vein thrombosis (DVT) and pulmonary embolism (PE) are viewed as different clinical manifestations of the same disease. Thrombi typically originate in the deep veins (usually of the legs) and can embolize to the pulmonary arteries, transiting through the right heart. However, about one-half of patients with PE have no evidence of DVT by compression ultrasonography [5-7] or magnetic resonance imaging [8], suggesting that some PE events may arise in situ or from sites other than the deep veins of the legs. It is well-established that AF can promote thrombus formation in the left atrium, which can lead to ischemic stroke [1] and possibly myocardial infarction [9]. Case reports have shown that thrombus formation can occur in the right atrium of AF patients [4], but less is known about how frequently this occurs and whether it leads to PE. VTE risk may be particularly elevated in the time-period shortly after AF diagnosis as it may take time for patients to return to sinus rhythm and for anticoagulant control to stabilize.
PE can also lead to AF through increasing pulmonary vascular resistance and right ventricular afterload by obstructing the pulmonary arteries, and via the release of vasoconstrictive mediators and inflammatory cytokines [4, 10, 11]. Resultant increased right atrial pressure and strain may trigger AF. Patients presenting with massive or submassive PE have elevated right ventricular systolic pressure [12]. While it is established that PE can cause AF acutely and risk of AF may be highest shortly after diagnosis with PE, there is evidence to support the notion that adverse effects of PE on cardiac function may be long-term [13].
In sum, a bidirectional relationship may be present between AF and VTE. Yet, the associations may not be causal in many cases, but rather due to the coincidence of two chronic conditions in patients with poor health. PE and AF have several shared risk factors, such as older age, obesity, heart failure and inflammatory states [4], and both conditions are associated with a procoagulant state [14, 15]. Using longitudinal data from the Atherosclerosis Risk in Communities (ARIC) study, we tested the hypotheses that a) individuals with incident AF are at elevated risk of developing VTE, and particularly for VTE presenting as PE without DVT, and b) individuals with incident VTE are at greater risk of developing AF, especially when the VTE presents with PE (regardless of DVT status). For both hypotheses we speculated that associations would be stronger in the first 6 months after an AF or VTE diagnosis, respectively. If a bidirectional relationship does exist between AF and VTE, findings from this research may help to identify patients who should be targeted for preventive strategies, given their elevated risk.
Methods
The ARIC study is a longitudinal cohort which began in 1987-1989 when 15,792 individuals, aged 45-64 years and predominantly black or white, were recruited from 4 U.S. communities: suburbs of Minneapolis, Minnesota; Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland [16]. Participants have been followed continuously for hospitalizations and deaths, and have taken part in numerous follow-up clinical visits. Relevant to this analysis, visit 2 took place in 1990-1992, visit 3 in 1993-1995, and visit 4 in 1996-1998. For the present analysis follow-up ended December 31, 2011, since this is the last date for which validated VTE events are available. The ARIC study protocol has had continuous approval from local institutional review boards, and all participants gave written informed consent.
For the present analysis we excluded individuals who were not black or white and blacks from the Minnesota and Maryland centers due to small numbers (n = 103), as well as those who at baseline had a missing or unreadable electrocardiogram (n = 242), prevalent AF by ARIC administered electrocardiograms (n = 37), prevalent VTE (n = 76), or were missing key covariates (n = 205). For both hypotheses, the final analytic sample size was 15,129.
Incident AF and VTE ascertainment
Participants were followed for hospitalizations and deaths through 1) annual telephone calls to participants or proxies (>90% participation), 2) active surveillance of local hospital discharge indexes, 3) searches of state death records and 4) linkage to the National Death Index. Trained abstractors collect information from all hospitalizations, including International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes for discharge diagnoses and procedures associated with each hospitalization.
Potential VTE cases were identified from hospital records with ICD-9-CM codes indicating possible VTE (415.1×, 451, 451.1×, 451.2, 451.8×, 451.9, 453.0, 453.1, 453.2, 453.8, 453.9, 996.7×, 997.2, and 999.2, and procedure code 38.7). Records of potential cases were then extracted, and independently reviewed by two physicians (ARF and MC) [17]. DVT was defined on the basis of duplex ultrasound or venogram or, in rare cases, by impedance plethysmography, computed tomography, or autopsy. Definite PE required ventilation/perfusion scanning showing multiple segmental or subsegmental mismatched perfusion defects, or a positive pulmonary angiogram, computed tomography, or autopsy [17]. Incident VTE was defined as the first occurrence of validated DVT or PE from baseline through the end of follow-up.
Incident AF was identified through electrocardiograms performed at study visits, and through diagnostic codes from hospitalization discharge summaries [18]. At each visit a standard supine 12-lead resting electrocardiogram was recorded after a 12-hour fast, and at least one hour after smoking tobacco or ingestion of caffeine. All electrocardiogram recordings were done with MAC PC Personal Cardiographs (Marquette Electronics, Inc., Milwaukee, WI), and were transmitted by telephone to the ARIC Central Electrocardiogram Reading Center for coding, interpretation and storage. Electrocardiogram recordings automatically coded as AF were visually re-checked by a trained cardiologist to confirm the diagnosis [19]. AF was also considered present if the ICD‐9‐CM codes 427.31 or 427.32 were listed in any hospitalization. AF events associated with open cardiac surgery were not included. In a validation study conducted within ARIC, the positive predictive value of this case definition was very good at ≈90% and the sensitivity was >80% [18]. The AF incidence date was defined as the earliest date in which an AF diagnosis was made during follow‐up.
Other variables of interest
The ARIC study protocol was similar (typically identical) across study visits. Participants self-reported age, sex, race, cigarette smoking status, physical activity (modified Baecke questionnaire)[20] and prevalent coronary heart disease (CHD) and heart failure. As has been done previously in ARIC,[21] prevalent HF at baseline was defined as the following: 1) an affirmative response to “Were any of the medications you took during the last 2 weeks for heart failure?” or 2) stage 3 or “manifest heart failure” by Gothenburg criteria. Prevalent coronary heart disease (CHD) was defined by self-reported previous physician diagnosis of MI or coronary revascularization, or prevalent MI by 12-lead electrocardiogram.
Trained staff measured height, waist circumference, and blood pressure using standardized protocols. Fasting (12-hour) blood samples were drawn, and plasma and serum were frozen at −70°C until analyzed, as previously described in detail [16]. Participants were also asked to bring their current medications to the clinic visits; medication names and dosages were recorded. Prevalent diabetes was defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, self-reported physician diagnosis of diabetes, or being on diabetes medication. Estimated glomerular filtration rate (eGFR) was calculated using the 2012 CKD EPI equation, which incorporates both cystatin C and creatinine [22].
During annual follow-up phone calls participants reported information about all medications currently being taken (including oral anticoagulants). At the time the study was conducted, warfarin was the only anticoagulant with widespread usage in the United States. Other antithrombotic drugs like aspirin and clopidogrel were not considered anticoagulants in the present analysis. Incident stroke was identified in a manner similar to incident AF and VTE; records of all possible strokes were adjudicated.
Statistical analysis
Characteristics of the final analytic sample at visit 1 are reported, for the overall sample and according to whether the participant developed incident AF or VTE during follow-up.
To test hypothesis #1, which evaluated whether individuals with incident AF were at elevated risk of developing VTE, Cox proportional hazards regression was used, with person-time accruing from the visit 1 date until the date of the incident VTE event, death, loss-to-follow-up, or December 31, 2011 (end of study). Incident AF status was included in the models as a time-dependent exposure; participants contributed person-time to the “no AF” category until the time of incident AF, when they began contributing person-time toward the “AF” category.
Nested models were used; in general, covariate information came from the clinic visit preceding development of AF. Model 1 adjusted for age, sex and race-site (5-level variable). Model 2 further adjusted for income, education, physical activity, smoking, waist circumference, height, systolic blood pressure, anti-hypertensive medication, diabetes, prevalent CHD, prevalent heart failure, eGFR and anticoagulant use. Model 3 additionally adjusted for anticoagulant use as time-varying covariate using information from annual follow-up phone calls. In addition to VTE overall as the outcome, we also analyzed separately PE events without DVT, and events that included evidence of DVT (participants were censored at first VTE event). Further, we conducted analyses modeling incident AF status as a 3-level time-dependent exposure (i.e. no AF, <6 months since AF diagnosis, ≥6 months since AF diagnosis). Using this modeling, it is possible for a person with AF to contribute person-time to all 3 categories. Kaplan-Meier curves were also constructed to examine the cumulative incidence of VTE longitudinally by AF status.
Analyses for hypothesis #2, which tested whether individuals with incident VTE are at elevated risk of developing AF, followed a similar pattern to the hypothesis #1 analyses. Differences were that a) person-time accrued from baseline until the date of the incident AF event, death, loss-to-follow-up, or December 31, 2011 (end of study), b) covariate information came from the clinic visit preceding development of VTE, c) incident VTE status was included as a time-dependent exposure, and d) in secondary analyses we evaluated as exposures events with evidence of PE, and those that only presented with DVT.
For both hypotheses, in the 48 instances when VTE and AF occurred during the same hospitalization, we censored the participant at this time so that neither the VTE nor the AF event was counted in the analysis. Interactions by age, race and sex were evaluated by including cross-product terms in the models. In sensitivity analyses we also censored upon cancer diagnosis and additionally adjusted for number of hospitalizations as a time-varying covariate, and additionally adjusted for incident stroke.
Results
At baseline, the 15,129 individuals in our analytic sample were 55.0% female, 25.8% black and on average (± SD) 54.2 ± 5.8 years old. Descriptive characteristics of the participants at baseline, overall and stratified according to whether they developed AF or VTE during follow-up, are presented in Table 1. The overall study sample was followed for a mean of 19.8 ± 6.0 years (median 22.5 years) with a maximum follow-up of 25.1 years. The number of incident AF and VTE events by calendar year are presented in Supplemental Figure 1.
Table 1.
Characteristics of participants* overall, and by incident af and incident VTE status, respectively: The Atherosclerosis Risk in Communities Study
| All | Incident AF | Incident VTE | |||
|---|---|---|---|---|---|
|
| |||||
| No | Yes | No | Yes | ||
| N | 15,129 | 13,081 | 2,048 | 14,516 | 613 |
|
Baseline Characteristics (1987-1989) |
|||||
| Age (years) | 54.2 ± 5.8 | 53.8 ± 5.7 | 56.7 ± 5.4 | 54.1 ± 5.8 | 55.1 ± 5.7 |
| Male, % | 45.5 | 43.5 | 54.4 | 45.1 | 42.6 |
| African American, % | 25.9 | 27.2 | 17.8 | 25.5 | 34.2 |
| Cigarette smoking status, % | |||||
| Current | 26.1 | 25.5 | 29.9 | 26.2 | 23.8 |
| Former | 32.4 | 31.8 | 36.1 | 32.3 | 35.1 |
| Never | 41.5 | 42.7 | 34.0 | 41.5 | 41.1 |
| Physical activity score (range 1-5) |
2.4 ± 0.8 | 2.4 ± 0.8 | 2.5 ± 0.8 | 2.4 ± 0.8 | 2.4 ± 0.8 |
| Waist circumference, cm | 97 ± 14 | 96 ± 14 | 102 ± 14 | 97 ± 14 | 101 ± 14 |
| Height, cm | 169 ± 9 | 168 ± 9 | 170 ± 10 | 169 ± 9 | 169 ± 9 |
| eGFR, mL/min/1.73m2 | 102 ± 16 | 103 ± 16 | 99 ± 16 | 103 ± 16 | 101 ± 18 |
| Systolic blood pressure (mmHg) |
121 ± 19 | 121 ± 19 | 126 ± 20 | 121 ± 19 | 122 ± 19 |
| Hypertensive medication, % | 30.3 | 28.2 | 43.4 | 30.0 | 36.4 |
| Diabetes mellitus, % | 11.7 | 10.9 | 16.9 | 11.5 | 14.5 |
| Prevalent CHD, % | 4.8 | 4.1 | 9.3 | 4.8 | 4.9 |
| Prevalent heart failure, % | 4.6 | 4.0 | 8.1 | 4.5 | 5.7 |
| Use of oral anticoagulants, % | 0.4 | 0.3 | 1.0 | 0.4 | 0.5 |
|
Follow-Up Characteristics (1987-2011) |
|||||
| Anticoagulant use, % | 5.7 | 2.9 | 23.5 | 5.0 | 20.7 |
Values correspond to mean ± SD or percentage.
AF, atrial fibrillation; VTE, venous thromboembolism; eGFR, estimated glomerular filtration rate; CHD, coronary heart disease.
Association of AF with incident VTE
A total of 2,048 participants developed AF (prior to or without VTE) during follow-up. The average age at the time of developing AF was 74.1 ± 7.7 years old. After developing AF, these individuals contributed 10,759 years of person-time to the analysis. After an AF diagnosis there were 68 VTE events, yielding a VTE incidence rate of 6.3 per 1000 person-years (Table 2). By contrast, in the absence of AF there were 613 VTE events, for a VTE incidence rate of 2.4 per 1000 person-years. In multivariable-adjusted models, having AF (versus no AF) was associated with greater risk of incident VTE; the HR (95% CI) was 2.02 (1.56-2.61) after adjustment for demographics (model 1), 1.71 (1.32-2.22) additionally accounting for risk factors (model 2), and 1.88 (1.44-2.45) after further adjusting for anticoagulant use information from annual follow-up phone calls (model 3). In a sensitivity analysis additionally adjusting for stroke as a time-dependent covariate, the HR was 1.73 (1.32-2.25).
Table 2.
Hazard ratios (95% CIs) of incident VTE after AF, for overall VTE and by VTE type: The Atherosclerosis Risk in Communities Study, 1987-2011
| No AF (n=13,081) | AF (n=2048) | |
|---|---|---|
| VTE (n = 681) | ||
| # Events | 613 | 68 |
| Person-years | 259,659 | 10,759 |
| Incidence Rate (95% CI)* | 2.4 (2.2-2.6) | 6.3 (4.9-8.0) |
| Hazard ratio (95% CI) | ||
| Model 1 | 1 (REF) | 2.02 (1.56-2.61) |
| Model 2 | 1 (REF) | 1.71 (1.32-2.22) |
| Model 3 | 1 (REF) | 1.88 (1.44-2.45) |
| PE (without DVT) (n = 190) | ||
| # Events | 173 | 17 |
| Incidence Rate (95% CI)* | 0.7 (0.6-0.8) | 1.6 (0.96-2.5) |
| Hazard ratio (95% CI) | ||
| Model 1 | 1 (REF) | 1.59 (0.96-2.64) |
| Model 2 | 1 (REF) | 1.41 (0.85-2.36) |
| Model 3 | 1 (REF) | 1.47 (0.86-2.52) |
| Any DVT† (n = 491) | ||
| # Events | 440 | 51 |
| Incidence Rate (95% CI)* | 1.7 (1.5-1.9) | 4.7 (3.6-6.2) |
| Hazard ratio (95% CI) | ||
| Model 1 | 1 (REF) | 2.23 (1.65-3.00) |
| Model 2 | 1 (REF) | 1.86 (1.37-2.51) |
| Model 3 | 1 (REF) | 2.07 (1.52-2.81) |
Incidence rate is per 1000 person-years.
Includes events presenting as DVT alone, and those presenting as both DVT and PE.
CI, confidence interval; VTE, venous thromboembolism; AF, atrial fibrillation; DVT, deep vein thrombosis.
Model 1 is adjusted for age, sex and race-field center.
Model 2: Model 1+ income, education, physical activity, smoking, waist circumference, height, systolic blood pressure, anti-hypertensive medication, diabetes, prevalent coronary heart disease, prevalent heart failure, prevalent anticoagulant use and eGFR.
Model 3: Model 2 + time-dependent anticoagulant use.
When subgroups were evaluated, the association was stronger in blacks [2.30 (1.48-3.58)] than in whites [1.56 (1.14-2.15)], p-interaction 0.02 (model 2 results shown). This interaction is depicted visually using Kaplan Meyer curves (Figure 1). There was no evidence of interaction by sex. In sensitivity analyses where we censored upon cancer diagnosis and additionally adjusted for number of hospitalizations the results were similar [HR: 1.98 (1.44-2.73)].
Figure 1.
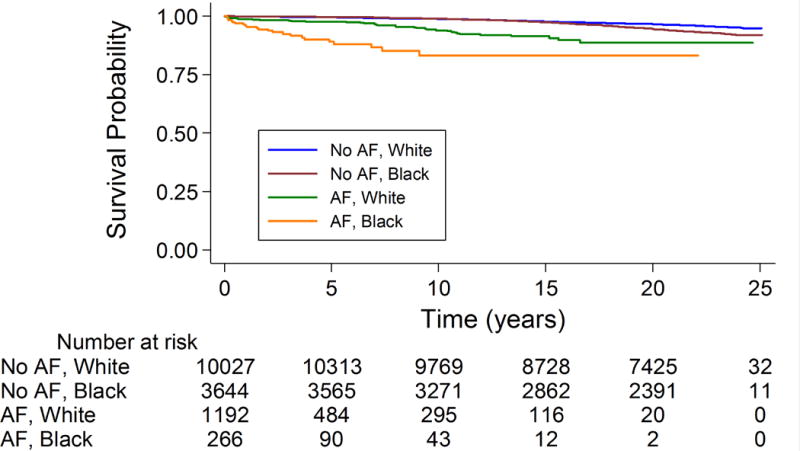
Kaplan-Meier curve* of incident VTE stratified by race (white or black) and AF status: The Atherosclerosis Risk in Communities Study, 1987-2011
*Participants contribute to the strata without AF until the incident AF event. Log-rank test p<0.0001.
Of the 68 VTE events that occurred after AF, 17 were classified as PE only, and 51 had evidence of DVT (with or without PE). Hazard ratios (model 2 adjustments) associated with having AF (versus no AF) were 1.41 (0.85-2.36) for events presenting as PE without evidence of DVT, and 1.86 (1.37-2.51) for VTE events with evidence of DVT. Results when the outcomes were DVT only and DVT plus PE are shown in Supplemental Table 1.
When AF status was modeled as a time-dependent 3-level exposure (Table 3), the HR (model 2) for incident VTE during the 1st 6 months after an AF diagnosis was 5.08 (3.08-8.38), whereas for ≥6 months since the AF diagnosis the HR was 1.42 (1.06-1.90), as compared to in the absence of AF. The HR’s were similar with additional adjustment for time-dependent anticoagulation (model 3).
Table 3.
Hazard ratios (95% CIs) of incident VTE by time since AF diagnosis: The Atherosclerosis Risk in Communities Study, 1987-2011
| No AF | Time since AF diagnosis | ||
|---|---|---|---|
|
| |||
| ≤ 6 months | > 6 months | ||
|
|
|||
| N | 13,081 | 429 | 1619 |
| # VTE Events | 613 | 16 | 52 |
| Person-years | 259,659 | 859 | 9,900 |
| Hazard ratio (95% CI) | |||
| Model 1 | 1 (REF) | 6.23 (3.78-10.26) | 1.67 (1.25-2.23) |
| Model 2 | 1 (REF) | 5.08 (3.08-8.38) | 1.42 (1.06-1.90) |
| Model 3 | 1 (REF) | 5.24 (3.17-8.65) | 1.55 (1.15-2.09) |
CI, confidence interval; VTE, venous thromboembolism; AF, atrial fibrillation.
Model 1 is adjusted for age, sex and race-field center.
Model 2: Model 1+ income, education, physical activity, smoking, waist circumference, height, systolic blood pressure, anti-hypertensive medication, diabetes, prevalent coronary heart disease, prevalent heart failure, prevalent anticoagulant use and eGFR.
Model 3: Model 2 + time-dependent anticoagulant use.
Association of VTE with incident AF
A total of 613 individuals developed incident VTE prior to or without AF. The average age at VTE was 69.9 ± 7.9 years old, and after their VTE event they contributed 2,990 person-years to the analysis and experienced 62 AF events, with an incidence rate of 20.7 per 1,000 person-years (Table 4). In those without incident VTE there were 2,048 AF events, for an incidence rate of 7.3 per 1,000 person years. Incident VTE was associated with elevated risk of AF after adjusting for demographics [model 1: 1.94 (1.50-2.50)] and cardiovascular risk factors [model 2: 1.73 (1.34-2.24)]. However, the association was attenuated with additional adjustment for time-dependent anticoagulant use [model 3: 1.20 (0.91-1.57)]. In a sensitivity analysis additionally adjusting for stroke as a time-dependent covariate, the HR was 1.16 (0.89-1.53).
Table 4.
Hazard ratios (95% CI) of incident AF after VTE, overall and by type of VTE: The Atherosclerosis Risk in Communities Study, 1987-2011
| No VTE (n=14,516) | VTE (n=613) | |
|---|---|---|
| # AF Events | 2,048 | 62 |
| Person-years | 279,785 | 2,990 |
| Incidence Rate (95% CI)* | 7.3 (7.0-7.6) | 20.7 (16.0-26.4) |
| Hazard ratio (95% CI) | ||
| Model 1 | 1 (REF) | 1.94 (1.50-2.50) |
| Model 2 | 1 (REF) | 1.73 (1.34-2.24) |
| Model 3 | 1 (REF) | 1.20 (0.91-1.57) |
| No VTE (n=14,858) | Any PE† (n=271) | |
| # AF Events | 2,090 | 20 |
| Incidence Rate (95% CI)* | 7.4 (7.1-7.7) | 16.4 (10.4-24.9) |
| Hazard ratio (95% CI) | ||
| Model 1 | 1 (REF) | 1.44 (0.93-2.24) |
| Model 2 | 1 (REF) | 1.29 (0.83-2.01) |
| Model 3 | 1 (REF) | 0.73 (0.46-1.16) |
| No VTE (n=14,787) | DVT only (n=342) | |
| # AF Events | 2,068 | 42 |
| Incidence Rate (95% CI)* | 7.4 (7.0-7.7) | 23.7 (17.3-31.7) |
| Hazard ratio (95% CI) | ||
| Model 1 | 1 (REF) | 2.28 (1.68-3.10) |
| Model 2 | 1 (REF) | 2.05 (1.50-2.78) |
| Model 3 | 1 (REF) | 1.61 (1.18-2.21) |
Incidence rate is per 1000 person-years.
Includes events presenting as PE alone, and those presenting as both DVT and PE.
CI, confidence interval; AF, atrial fibrillation; VTE, venous thromboembolism.
Model 1 is adjusted for age, sex and race-field center.
Model 2: Model 1+ income, education, physical activity, smoking, waist circumference, height, systolic blood pressure, anti-hypertensive medication, diabetes, prevalent coronary heart disease, prevalent heart failure, prevalent anticoagulant use and eGFR.
Model 3: Model 2 + time-dependent anticoagulant use.
When subgroups were evaluated, the association of incident VTE with AF was stronger in blacks [2.40 (1.55-3.74)] than whites [1.46 (1.07-2.01)] (p interaction = 0.04); see also Figure 2. There was no interaction by sex. In sensitivity analyses where we censored upon cancer diagnosis and additionally adjusted for number of hospitalizations the HR was 2.14 (1.57-2.91).
Figure 2.
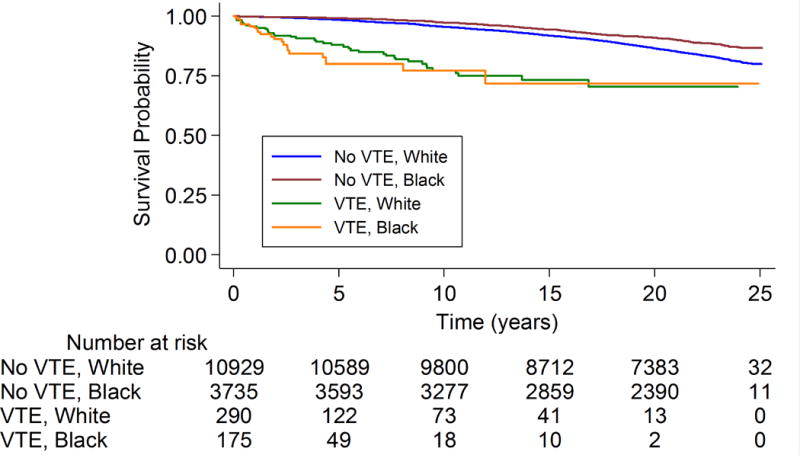
Kaplan-Meier curve* of incident AF stratified by race (white or black) and VTE status: The Atherosclerosis Risk in Communities Study, 1987-2011
*Participants contribute to the strata without VTE until the incident VTE event. Log-rank test p<0.0001.
After model 2 adjustments, the HR (95% CI) for incident AF associated with any PE (versus no VTE) was 1.29 (0.83-2.01), whereas when the exposure was events presenting as DVT only the HR for incident AF was 2.05 (1.50-2.78). Supplemental Table 2 presents results when the exposures were PE without DVT, and VTE presenting as both PE and DVT.
When VTE was modeled as a 3-level exposure (Table 5), with absence of AF as the reference group, within the first 6 months after incident VTE the HR for incident AF was 4.50 (2.61-7.77), whereas beyond 6 months after an incident VTE event the HR was 1.49 (1.12-1.98) (model 2 adjustments).
Table 5.
Hazard ratios (95% CIs) of incident AF by time since VTE diagnosis: The Atherosclerosis Risk in Communities Study, 1987-2011
| No VTE | Time since VTE diagnosis | ||
|---|---|---|---|
|
| |||
| ≤ 6 months | > 6 months | ||
|
|
|||
| N | 14,516 | 175 | 438 |
| # AF Events | 2,048 | 13 | 49 |
| Person-years | 279,785 | 244 | 2746 |
| Hazard ratio (95% CI) | |||
| Model 1 | 1 (REF) | 5.14 (2.98-8.88) | 1.66 (1.25-2.21) |
| Model 2 | 1 (REF) | 4.50 (2.61-7.77) | 1.49 (1.12-1.98) |
| Model 3 | 1 (REF) | 4.15 (2.40-7.17) | 0.99 (0.73-1.34) |
CI, confidence interval; AF, atrial fibrillation; VTE, venous thromboembolism.
Model 1 is adjusted for age, sex and race-field center.
Model 2: Model 1+ income, education, physical activity, smoking, waist circumference, height, systolic blood pressure, anti-hypertensive medication, diabetes, prevalent coronary heart disease, prevalent heart failure, prevalent anticoagulant use and eGFR.
Model 3: Model 2 + time-dependent anticoagulant use.
Discussion
In this community-based longitudinal study of over 15,000 older adults, there was evidence of a bidirectional association between incident AF and incident VTE. Incident AF was associated with a 2-fold increased risk of future VTE. Similarly, occurrence of VTE was associated with an almost 2-fold higher risk of subsequent AF. These associations were only modestly attenuated after accounting for additional cardiovascular risk factors. Emphasizing the strength of the association of these two diseases, risk of developing the subsequent condition was approximately 5-fold higher in the first 6 months after diagnosis with the first condition. Furthermore, there were important differences by race; as compared to whites, blacks were at greater risk of incident VTE subsequent to AF, and of incident AF subsequent to VTE.
Our finding that incident AF is associated with greater risk of incident VTE, and that this association is particularly pronounced in the first 6 months after AF diagnosis, is consistent with findings recently reported by the Tromsø study [23]. AF has also been linked to prior VTE via several other lines of evidence [4], including a longitudinal analysis of the Longitudinal Health Insurance Database 2000 [24], two case-control studies [25, 26], registry studies of PE patients [4], small clinical studies [10, 11], and autopsy studies [27]. In the present paper we conducted analyses by VTE classification (i.e. PE without VTE, DVT (with or without PE)) in order to provide information about whether AF may be an independent risk factor for PE through the pathway of right atrial thrombi formation. If, in fact, AF leads to VTE through right atrial thrombus formation and subsequent PE, the association between AF and risk of PE without evidence of DVT should be stronger than associations between AF and risk of events including DVT. In our analyses, the risk of PE without DVT was not especially elevated. In contrast, in the Tromsø study the magnitude of the association for incident PE after AF was stronger than for incident DVT after AF [23]. However, their confidence intervals were wide and overlapping, it is not clear how they analyzed cases presenting with both DVT and PE, and it is unclear the extent to which patients presenting with PE also received leg imaging. The latter is also a limitation of the present ARIC analyses. It is possible AF risk may be elevated in the context of DVT due to either a procoagulant state or shared risk factors.
VTE was likewise associated with approximately 2-fold higher incidence of AF in the present analysis. Similar findings were reported in the Tromsø study [28], and in a longitudinal registry based analysis of hospital admissions data [29]. Numerous registries of PE patients and small clinical studies, dating back to 1943, have reported AF becoming apparent shortly after the development of PE [4]. Since the proposed mechanism linking VTE to greater AF risk acts through elevated right ventricular systolic pressure in the context of PE, we also evaluated as exposures incident PE and incident DVT, separately. Contrary to our hypothesis, the association with AF was not stronger when the exposure was incident PE. Also in our analysis, risk of AF following PE was attenuated when we adjusted for anticoagulant therapy as a time-varying covariate. Novel research in animal models has suggested that inhibition of coagulation can prevent the development of atrial fibrosis [30], and therefore AF. Accordingly, anticoagulation prescribed for PE may also lower risk of developing AF.
Race differences were present in the risk of VTE subsequent to AF, and of AF subsequent to VTE, with associations being stronger in blacks than whites. While biological racial differences may underlie these observations, we speculate that these findings are due to racial disparities in access to care, likelihood of seeking treatment for more mild disease, treatment intensity and anticoagulation control [31-33]. Using ARIC study data, it has been previously reported that, relative to white individuals with AF, black individuals with AF are at greater risk of stroke, heart failure, coronary heart disease and mortality [34]. The ARIC findings presented both herein and previously underscore the need to improve management of chronic health conditions among black patients in order to mitigate health disparities. Notably, blacks have a lower overall incidence of AF relative to whites [18], but a higher incidence of VTE [3].
The bidirectional association between the occurrence AF and VTE, particularly in the first 6 months after diagnosis, highlights population groups that may be at particularly elevated risk for developing the other condition subsequently. According to present guidelines not all AF patients are recommended anticoagulation [35], and not all VTE patients receive anticoagulation for secondary prevention [36]. For patients with co-occurring VTE and AF, but for which neither condition when considered in isolation meets the criterion for requiring anticoagulation, physicians should carefully weigh the advantages versus the bleeding risks of oral anticoagulation [4].
This study has several strengths including the longitudinal design, well-enumerated population with excellent retention, careful event surveillance, assessment of potential confounders, and validation of VTE events. There are also limitations of our study. In regards to AF ascertainment, AF events were not validated. Also, asymptomatic [37] AF and AF managed exclusively in an outpatient facility could not be identified, because the large majority of our incident AF cases were ascertained from the hospitalization discharge records. We have found, however, adequate validity of these codes for the identification of AF events.[18] Furthermore, it can be difficult to accurately identify the exact date of AF incidence. Misclassification was also almost certainly present in the categorizations of VTE subtypes, since not everyone with PE undergoes leg imaging, and lung imaging is not always conducted among individuals with DVT but no symptoms of PE. Furthermore, major changes in the diagnosis of AF and VTE have taken place over the course of this study, particularly with small PEs being more likely to be identified later in follow-up. However, the etiologic association should be constant over time, and misclassification in earlier years as a result of less sensitive diagnostic testing would likely bias our results toward the null. Additionally, information about use of oral anticoagulants was collected annually; ideally surveillance for oral anticoagulant use would have been continuous. Other limitations of the present analysis are a) the limited numbers of dual events, b) the inability to ascertain health care context, access, attention, and treatment, c) residual confounding from the multiple morbidities that may predispose to both AF and VTE, and c) for some analyses there were few events and therefore precision was low.
In conclusion, there appears to be a bidirectional association between VTE and AF, whereby individuals with AF are at approximately 2-fold greater risk of being diagnosed with incident VTE, and individuals with VTE are at about 2-fold higher risk of being diagnosed with AF. The risk of subsequent disease was particularly elevated in the initial 6 months after diagnosis with the first condition, and the associations were stronger for blacks than for whites. Clinicians should be cognizant that these conditions are frequently comorbid. Guidelines promoting oral anticoagulation to prevent ischemic stroke in AF patients [35] may have the added benefit of also providing protection against VTE, and anticoagulation to prevent VTE recurrence [36] may additionally protect against AF [4].
Supplementary Material
Essentials.
Atrial fibrillation (AF) may increase risk of venous thromboembolism (VTE), and vice versa.
Bidirectionality was assessed prospectively via data from 15 129 black and white individuals.
AF was associated with greater risk of developing VTE, and VTE with greater risk of AF.
Associations were strongest among blacks and in the first 6 months after initial diagnosis.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This work was also supported by the National Heart Lung and Blood Institute grants R01HL59367 (A. R. Folsom), R01HL126637 (L. Y. Chen), and American Heart Association grant 16EIA26410001 (A. Alonso).
Footnotes
DR MARY CUSHMAN (Orcid ID : 0000-0002-7871-6143)
Addendum
P. L. Lutsey, A. Alonso, and A. R. Folsom were responsible for the concept and design.
P. L. Lutsey, F. L. Norby, A. Alonso, M. Cushman, L. Y. Chen, E. D. Michos, and A. R. Folsom were responsible for acquisition, analysis, or interpretation of data.
P. L. Lutsey drafted the manuscript.
P. L. Lutsey, F. L. Norby, A. Alonso, M. Cushman, L. Y. Chen, E. D. Michos, and A. R. Folsom critically revised the manuscript for important intellectual content.
F. L. Norby performed the statistical analysis.
A. R. Folsom obtained funding.
Disclosure of Conflict of Interests
E. D. Michos reports an honorarium from Siemens Diagnostics, unrelated to the submitted work. The other authors state that they have no conflict of interest.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C, Stroke Statistics S Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 3.Bell EJ, Lutsey PL, Basu S, Cushman M, Heckbert SR, Lloyd-Jones DM, Folsom AR. Lifetime Risk of Venous Thromboembolism in Two Cohort Studies. Am J Med. 2016;129:339e19–26. doi: 10.1016/j.amjmed.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bikdeli B, Abou Ziki MD, Lip GY. Pulmonary Embolism and Atrial Fibrillation: Two Sides of the Same Coin? A Systematic Review. Semin Thromb Hemost. 2017 doi: 10.1055/s-0036-1598005. [DOI] [PubMed] [Google Scholar]
- 5.Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Presence of lower limb deep vein thrombosis and prognosis in patients with symptomatic pulmonary embolism: preliminary report. Eur J Vasc Endovasc Surg. 2009;37:225–31. doi: 10.1016/j.ejvs.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez D, Aujesky D, Diaz G, Monreal M, Otero R, Marti D, Marin E, Aracil E, Sueiro A, Yusen RD, Investigators R Prognostic significance of deep vein thrombosis in patients presenting with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2010;181:983–91. doi: 10.1164/rccm.200908-1204OC. [DOI] [PubMed] [Google Scholar]
- 7.Girard P, Sanchez O, Leroyer C, Musset D, Meyer G, Stern JB, Parent F, Evaulation du Scanner Spirale dans l’Embolie Pulmonaire Study G Deep venous thrombosis in patients with acute pulmonary embolism: prevalence, risk factors, and clinical significance. Chest. 2005;128:1593–600. doi: 10.1378/chest.128.3.1593. [DOI] [PubMed] [Google Scholar]
- 8.van Langevelde K, Sramek A, Vincken PW, van Rooden JK, Rosendaal FR, Cannegieter SC. Finding the origin of pulmonary emboli with a total-body magnetic resonance direct thrombus imaging technique. Haematologica. 2013;98:309–15. doi: 10.3324/haematol.2012.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–14. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gex G, Gerstel E, Righini M, G LEG, Aujesky D, Roy PM, Sanchez O, Verschuren F, Rutschmann OT, Perneger T, Perrier A. Is atrial fibrillation associated with pulmonary embolism? J Thromb Haemost. 2012;10:347–51. doi: 10.1111/j.1538-7836.2011.04608.x. [DOI] [PubMed] [Google Scholar]
- 11.Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008;4:49–59. doi: 10.2174/157340308783565384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest. 2009;136:1202–10. doi: 10.1378/chest.08-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevinson BG, Hernandez-Nino J, Rose G, Kline JA. Echocardiographic and functional cardiopulmonary problems 6 months after first-time pulmonary embolism in previously healthy patients. Eur Heart J. 2007;28:2517–24. doi: 10.1093/eurheartj/ehm295. [DOI] [PubMed] [Google Scholar]
- 14.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009;373:155–66. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–74. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 16.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: Design and Objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 17.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–7. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–11. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical Activity and Cardiovascular Disease in African Americans in ARIC. Medicine and science in sports and exercise. 2013;45:901–7. doi: 10.1249/MSS.0b013e31827d87ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart Failure Incidence and Survival (from the Atherosclerosis Risk in Communities Study) American Journal of Cardiology. 101:1016–22. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enga KF, Rye-Holmboe I, Hald EM, Lochen ML, Mathiesen EB, Njolstad I, Wilsgaard T, Braekkan SK, Hansen JB. Atrial fibrillation and future risk of venous thromboembolism:the Tromso study. J Thromb Haemost. 2015;13:10–6. doi: 10.1111/jth.12762. [DOI] [PubMed] [Google Scholar]
- 24.Wang CC, Lin CL, Wang GJ, Chang CT, Sung FC, Kao CH. Atrial fibrillation associated with increased risk of venous thromboembolism. A population-based cohort study. Thromb Haemost. 2015;113:185–92. doi: 10.1160/TH14-05-0405. [DOI] [PubMed] [Google Scholar]
- 25.Sørensen HT, Horvath-Puho E, Lash TL, Christiansen CF, Pesavento R, Pedersen L, Baron JA, Prandoni P. Heart Disease May Be a Risk Factor for Pulmonary Embolism Without Peripheral Deep Venous Thrombosis. Circulation. 2011;124:1435–41. doi: 10.1161/circulationaha.111.025627. [DOI] [PubMed] [Google Scholar]
- 26.Pesavento R, Piovella C, Prandoni P. Heart disease in patients with pulmonary embolism. Curr Opin Pulm Med. 2010;16:415–8. doi: 10.1097/MCP.0b013e32833b6581. [DOI] [PubMed] [Google Scholar]
- 27.Aberg H. Atrial fibrillation. I. A study of atrial thrombosis and systemic embolism in a necropsy material. Acta Med Scand. 1969;185:373–9. [PubMed] [Google Scholar]
- 28.Hald EM, Enga KF, Lochen ML, Mathiesen EB, Njolstad I, Wilsgaard T, Braekkan SK, Hansen JB. Venous thromboembolism increases the risk of atrial fibrillation: the Tromso study. J Am Heart Assoc. 2014;3:e000483. doi: 10.1161/JAHA.113.000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng AC, Adikari D, Yuan D, Lau JK, Yong AS, Chow V, Kritharides L. The Prevalence and Incidence of Atrial Fibrillation in Patients with Acute Pulmonary Embolism. PLoS One. 2016;11:e0150448. doi: 10.1371/journal.pone.0150448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spronk HM, De Jong AM, Verheule S, De Boer HC, Maass AH, Lau DH, Rienstra M, van Hunnik A, Kuiper M, Lumeij S, Zeemering S, Linz D, Kamphuisen PW, Ten Cate H, Crijns HJ, Van Gelder IC, van Zonneveld AJ, Schotten U. Hypercoagulability causes atrial fibrosis and promotes atrial fibrillation. Eur Heart J. 2017;38:38–50. doi: 10.1093/eurheartj/ehw119. [DOI] [PubMed] [Google Scholar]
- 31.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M, Howard G. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41:581–7. doi: 10.1161/STROKEAHA.109.573907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhave PD, Lu X, Girotra S, Kamel H, Vaughan Sarrazin MS. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015;12:1406–12. doi: 10.1016/j.hrthm.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao SR, Reisman JI, Kressin NR, Berlowitz DR, Ash AS, Ozonoff A, Miller DR, Hylek EM, Zhao S, Rose AJ. Explaining Racial Disparities in Anticoagulation Control. American Journal of Medical Quality. 2014;30:214–22. doi: 10.1177/1062860614526282. [DOI] [PubMed] [Google Scholar]
- 34.Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR, Alonso A. Racial Differences in Atrial Fibrillation-Related Cardiovascular Disease and Mortality: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2016;1:433–41. doi: 10.1001/jamacardio.2016.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice G 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JR, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315–52. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation. 2013;127:930–7. doi: 10.1161/CIRCULATIONAHA.112.126656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


