Abstract
Co-inhibitory receptors, such as CTLA-4 and PD-1, play a critical role in maintaining immune homeostasis by dampening T cell responses. Recently, they have gained attention as therapeutic targets in chronic disease settings where their dysregulated expression contributes to suppressed immune responses. The novel co-inhibitory receptor TIGIT (T cell immunoglobulin and ITIM domain) has been shown to play an important role in modulating immune responses in the context of autoimmunity and cancer. However, the molecular mechanisms by which TIGIT modulates immune responses are still insufficiently understood. We have generated a panel of monoclonal anti-mouse TIGIT antibodies that show functional properties in mice in vivo and can serve as important tools to study the underlying mechanisms of TIGIT function. We have identified agonistic as well as blocking anti-TIGIT antibody clones that are capable of modulating T cell responses in vivo. Administration of either agonist or blocking anti-TIGIT antibodies modulated autoimmune disease severity while administration of blocking anti-TIGIT antibody synergized with anti-PD-1 antibody to affect partial or even complete tumor regression. The antibodies presented in this study can thus serve as important tools for detailed analysis of TIGIT function in different disease settings and the knowledge gained will provide valuable insight for the development of novel therapeutic approaches targeting TIGIT.
Keywords: TIGIT, co-stimulation, agonistic antibodies, blocking antibodies
Introduction
Co-inhibitory receptors play an essential role in maintaining immune homeostasis. Loss of co-inhibitory receptor expression can lead to autoimmunity while persistent expression can suppress effective immunity in the setting of chronic viral infection or cancer. Blocking antibodies to co-inhibitory receptors PD-1 and CTLA-4 are being harnessed clinically to improve anti-tumor T cell responses. In addition to these well-studied co-inhibitory molecules, we have been studying a number of novel co-inhibitory molecules that regulate autoimmunity and anti-tumor immunity. TIGIT (T cell immunoglobulin and ITIM domain; also called Vsig9, Vstm3, or WUCAM), one of these novel co-inhibitory receptors, is expressed on NK cells, activated T cells, memory T cells, and a subset of regulatory T cells (Tregs), and was shown to inhibit immune responses by affecting T cells and antigen presenting cells (1–3). We (1, 2, 4) and others (5, 6) have recently shown that TIGIT also plays an important role in modulating immune responses in the context of autoimmunity and cancer. TIGIT shares its two ligands, CD155 (PVR) and CD112 (PVRL2, nectin-2), with the co-stimulatory molecule CD226 and together they comprise a pathway that strongly resembles the well-known B7/CD28/CTLA-4 pathway (3, 6, 7). Similar to CTLA-4, TIGIT can inhibit immune responses by delivering inhibitory signals to effector T and NK cells, by inducing tolerogenic dendritic cells (DCs), and by enhancing the suppressive capacity of Tregs (1–3).
The TIGIT/CD226 pathway has been linked to multiple autoimmune diseases in humans including type 1 diabetes, multiple sclerosis (MS), and rheumatoid arthritis (8, 9). Indeed, data from experimental models have confirmed the protective role of TIGIT in autoimmune settings. TIGIT−/− mice show increased susceptibility to experimental autoimmune encephalomyelitis (EAE), a mouse model of the human disease MS (1, 6). Furthermore, TIGIT blockade leads to exacerbation of collagen-induced arthritis and graft versus host disease (6). In contrast, TIGIT has been shown to suppress anti-tumor immunity because loss of TIGIT enhances anti-tumor immunity and results in improved tumor control (4, 5). In this regard, a number of recent publications indicate that TIGIT synergizes with the PD-1/PD-L1 pathway in promoting CD8+ T cell dysfunction, thereby directly impairing protective anti-tumor T cell responses (5, 10). In addition, TIGIT can further suppress anti-tumor immunity indirectly through promotion of Treg function in tumor-infiltrating lymphocytes (4).
Therapeutic targeting of the CTLA-4 and PD-1 pathways is able to improve CD8+ T cell function and has shown remarkable efficacy in treating several human cancers. However, anti-CTLA4 and PD-1 antibody blockade results in stable responses in only about 10–30% patients, and a number of human cancers are refractory to treatment with these two antibodies. Due to its expression on tumor-infiltrating lymphocytes and its ability to synergize with the PD-1/PD-L1 pathway, TIGIT has recently received much attention as a potential therapeutic target for promoting anti-tumor immunity and inducing tumor regression (4, 5, 10, 11). However, the molecular mechanisms by which TIGIT modulates immune responses are still insufficiently understood and tools to modulate the TIGIT pathway in vivo have been lacking.
We have generated a panel of functional anti-TIGIT antibodies that can serve as a tool to study the underlying mechanisms of TIGIT function in detail. We have identified and characterized different clones of antibody producing hybridomas (agonistic and blocking) that are able to modulate T cell responses in vivo. Importantly, functional modulation of immune responses by these anti-TIGIT antibodies translates into differences in disease severity and progression in models of autoimmunity and cancer. The monoclonal anti-TIGIT antibodies we have generated thus represent a valuable set of tools that allows us to deepen our understanding of the underlying mechanisms by which TIGIT modulates immune responses in vivo in the context of disease.
Materials and Methods
Animals
TIGIT−/− and TIGIT-Tg mice were obtained from ZymoGenetics, Inc. (Seattle, WA). C57BL/6 (B6) and Balb/c mice were purchased from the Jackson Laboratories, Janvier, or were bred at the Cox-7 gnotobiotic animal facility operated by the Edwin L. Steele Laboratory at MGH. Armenian Hamsters were purchased from Harlan (Indianapolis, IN). Animals were kept in a conventional, pathogen-free facility at the Harvard Institutes of Medicine (Boston, MA), MGH, or at the Laboratory Animal Services Center (LASC Zurich, Switzerland) and all experiments were carried out in accordance with guidelines prescribed by the Institutional Animal Care and Use Committee (IACUC) at Harvard Medical School, MGH, or institutional policies at LASC and have been reviewed by the Cantonal veterinary office.
Generation of anti-TIGIT antibodies
Armenian hamsters and TIGIT−/− mice were immunized with recombinant mouse TIGIT tetramers (Zymogenetics, Inc.) by a combination of s.c. and foot pad injections as described previously (1). Briefly, animals were immunized with 50 µg of TIGIT tetramers in CFA on day 0, followed by booster injections of 25 µg protein on days 3, 10, and 18 and 25 µg protein in IFA on days 7 and 14. Draining lymph nodes were collected on day 21 and cells were fused with Sp2/0-Ag14 cells using polyethylene glycol 1450 and selected in HAT (hypoxanthine/aminopterin/thymidine) medium. The supernatants were screened for specific binding by anti-TIGIT ELISA and by flow cytometry using P815 cells transfected with mouse TIGIT and primary mouse splenocytes activated with plate bound anti-CD3 (2 µg/ml) for 48h. All hybridoma wells that showed TIGIT-specific binding were expanded and subcloned four times by limiting dilution and single colonies were sorted by flow cytometry. Binding specificity of the antibody producing hybridomas was confirmed by staining of activated primary TIGIT-expressing wild type T cells and TIGIT−/− T cells as controls. Anti-TIGIT clone 4D4 is an Armenian hamster IgG antibody, anti-TIGIT clones 1G9 and 1B4 are mouse IgG1 antibodies.
Blocking of CD155 binding to TIGIT was determined on P815 cells transfected with mouse TIGIT (Zymogenetics). P815-TIGIT cells were incubated with anti-TIGIT or isotype control antibody (Armenian hamster IgG for clone 4D4 or mouse IgG1 for 1G9, 1B4, and 2F6) for 30 min, followed by incubation with recombinant mouse CD155 for 30 min, staining with anti-CD155, and analysis by flow cytometry. Inhibition of CD155 binding was calculated as 100-(%CD155+ in sample/%CD155+ in ‘no Ab’ sample) x100.
In vitro stimulations. CD8+, CD4+Foxp3−, and CD4+Foxp3+ cells were sorted from Foxp3-GFP reporter mice (12) and 4×104 cells/well (CD8+and CD4+) or 2×104 cells/well (CD4+Foxp3+) were seeded into U-bottom 96-well plates and treated with 25µg/ml anti-TIGIT (clones 4D4, 1G9 and 1B4) or isotype control (mouse IgG1, armenian hamster IgG) antibody together with 4×104 mouse T activator CD3/CD28 Dynabeads™ (Gibco) per well. For the culture of CD4+Foxp3+ cells, 3 ng/ml TGF-β (RayBiotech) and 20 U/ml IL-2 (BioLegend) were added to the culture medium. After 48 hours, 3H-thymidine (PerkinElmer) was added for the last 18–22 hours before [3H]thymidine incorporation was analyzed to assess proliferation. Where indicated, cells were harvested after 48 hours, RNA was extracted with the ReliaPrep RNA Tissue Miniprep System (Promega), cDNA was prepared using the GoScript Reverse Transcriptase kit (Promega), and expression was analyzed by real-time PCR (RT-PCR) using a CFX384 Cycler (Bio-Rad Laboratories): Il10 was analyzed by Taqman (Applied Biosystems; Il10: Mm01288386_m1 and Actb: Mm00446968_m1), and Ifng was analyzed with SYBR Green assay (Primer sets used were: Ifng 5’-GCATTC ATGAGTATTGCCAAG-3’ and 5’-GGTGGACCACTCGGATGA-3’, PolII 5’- CTGGTCCTTCGAATCCGCATC-3’ and 5’-GCTCGATACCCTGCAGGGTCA-3’.). Ifng expression was normalized to PolII, Il10 expression to beta-actin. Relative expression was calculated as 2-dCT × 104.
Immunizations
Mice were immunized s.c. into the flanks with 200 µl of an emulsion containing 100 µg of myelin oligodendrocyte glycoprotein (MOG)35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) and 250 µg of M. tuberculosis extract H37 Ra (Difco) in adjuvant oil (CFA) on day 0 and treated i.p. with 100 µg of anti–TIGIT (clone 4D4, 1G9, or 1B4) or isotype control (armenian hamster IgG or mouse IgG1) on days 0, 2, and 4. For antigen-specific proliferation assays, spleens and lymph nodes were collected on day 10 and 2.5 × 106 cells/ml were re-stimulated in the presence of a range of concentrations of MOG35–55 peptide (0.01 µg/ml - 50 µg/ml). After 48h, culture supernatants were collected for the determination of secreted cytokines and cells were pulsed with [3H]thymidine for an additional 18h before [3H]thymidine incorporation was analyzed. Cytokine concentrations in culture supernatants were determined by cytometric bead array (BD Biosciences) according to the manufacturer’s instructions.
Experimental autoimmune encephalomyelitis (EAE)
AE was induced by s.c. immunization of mice into the flanks with 200 µl of an emulsion containing either 100 µg of MOG35–55 peptide in CFA (optimal conditions) or 20 µg of MOG35–55 peptide in CFA. In addition, the mice received 100ng of pertussis toxin (List Biological Laboratories) i.v. on days 0 and 2 and were treated i.p. with 100 µg of anti–TIGIT (clone 4D4, 1G9, or 1B4) or isotype control (armenian hamster IgG or mouse IgG1) on days 0, 2, and 4, 10 and 17. Clinical signs of EAE were assessed according to the following scores: 0, no signs of disease; 1, loss of tone in the tail; 2, hind limb paresis; 3, hind limb paralysis; 4, tetraplegia; 5, moribund. Mice were sacrificed for flow cytometric and histopathological analysis at day 14 (4D4, 1G9, and respective isotype controls) or day 17 (1B4 and isotype control) after disease induction.
For histopathology, brains and spinal cords were fixed in 10% neutral buffered formalin and processed routinely for paraffin embedment. Slides were stained with Luxol fast blue-hematoxylin and eosin stains. Inflammatory foci (>10 mononuclear cells) were counted in leptomeninges and parenchyma in a blinded fashion in that the pathologist was unaware of the clinical status and treatment that the mice had received. For flow cytometric analysis, brain and spinal cord of perfused mice were cut into pieces, digested for 30 min at 37°C with collagenase D (2.5 mg/ml, Roche) and DNase I (1 mg/ml, Sigma-Aldrich), passed trough a 70-µm filter, and mononuclear cells were isolated over a 37%/70% Percoll gradient (GE Healthcare) as described previously(13).
Flow cytometry
For intracellular cytokine staining, single cell suspensions from the indicated organs were restimulated using PMA (50 ng/ml, Sigma-Aldrich), ionomycin (1 µM, Sigma-Aldrich), and monensin (GolgiStop, BD Biosciences) for 4 h at 37°C, before staining and fixation/permeabilization was performed using the BD Fixation/Permeabilization Solution kit (BD Bioscience). In the glioblastoma model, single cell suspensions of the cervical Lymph nodes were stimulated with Leukocyte Activation Cocktail (BD Bioscience) for 4 h at 37°C and then stained for intracellular cytokines. Fluorescently labeled antibodies were from BioLegend, or eBioscience (anti-Foxp3). Samples were acquired on a FACSCalibur, LSR II, or Fortessa flow cytometer (BD Biosciences) or sorted on BD FACS Aria III 5L (BD Bioscience) and analyzed using the Flowjo software (Flowjo, LLC).
Tumor Experiments
1 × 106 MC38 cells were implanted subcutaneously into the right flank of C57BL/6 mice. When tumor size reached approximately 25 mm2, mice were randomized and treated as follows: anti-PD-1 (RMP1–14) and its isotype control (ratIgG) were given as a single administration of 100 µg i.p. 200 µg of anti-TIGIT (1B4) or isotype control (msIgG1) were given i.p. every third day for a total of four shots. Tumor size was measured in two dimensions by caliper and is expressed as the product of two perpendicular diameters. 105 Gl261-GFP-Gluc cells were stereotactically implanted in the brains of 6–8 week old male or female C57BL/6 mice. Tumor size was assessed by measuring circulating Gaussia Luciferase (Gluc) activity in the blood (14). Treatment was initiated in size matched tumors post achieving sizes of ~ 1 mm3, and tumor burden was measured over time by serial blood Gluc measurements. Animals were treated with IgG (n=14), anti-TIGIT (1B4, n=12), anti-PD1 (RMP1–14, n=12), or anti-TIGIT + anti-PD-1 (n=12 mice) every 3 days. Anti-TIGIT antibody was administered 4 times at 200 µg/mouse; anti-PD1 was given as one dose of 500 µg/mouse followed by 5 injections of 250 µg /mice. Animals were sacrificed when clinical symptoms developed or mice lost more than 20% of their initial body weight.
In re-challenge experiment, mice treated with anti-TIGIT + anti-PD-1 that initially rejected tumors or naïve mice as controls were implanted with 105 Gl261-GFP-Gluc cells in the contralateral hemisphere. 100 days post re-challenge mice initially treated with mice treated with anti-TIGIT + anti-PD-1 were sacrificed and splenocytes and cervical lymph node cells were analyzed by flow cytometry. Moribund tumor bearing mice that had been naïve served as controls for comparison.
Results
Generation and properties of anti-TIGIT antibodies
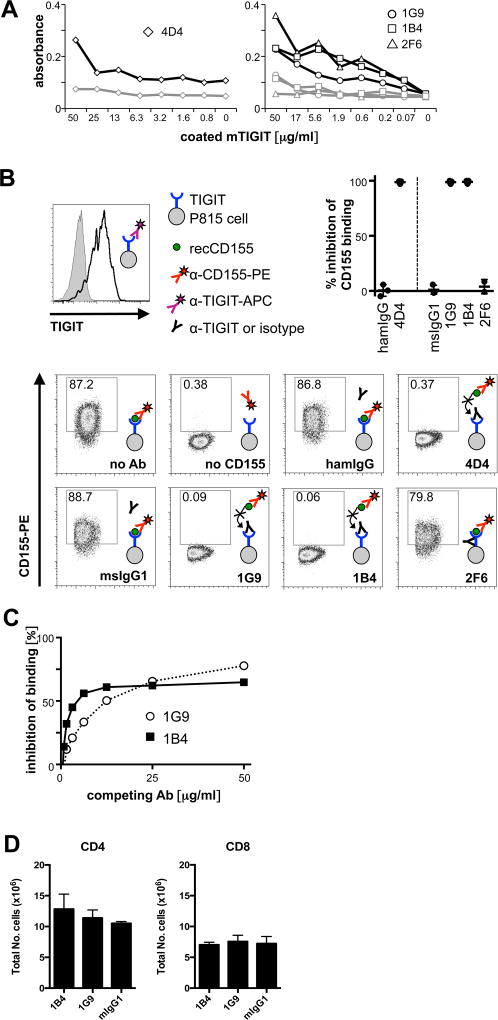
To be able to modulate TIGIT function in multiple experimental models in vivo, we set out to generate a set of monoclonal anti-TIGIT antibodies that would have agonistic or antagonistic (blocking) properties. We had previously reported the generation of an agonistic anti-TIGIT antibody in Armenian Hamster that inhibits T cell proliferation in vitro (clone 4D4, Armenian hamster IgG) (1). However, due to the species difference, this clone is not suitable for prolonged in vivo treatment of mice. We thus generated a second panel of monoclonal mouse anti-TIGIT antibodies by immunizing TIGIT−/− mice with recombinant mouse TIGIT tetramers. Positive clones were selected by specific binding in ELISA (Fig. 1A) as well as by specific staining of activated primary T cells. We then tested the ability of positive clones to block ligand binding in a competition assay with recombinant CD155, the high affinity ligand of TIGIT. Two clones (1G9 (1) and 1B4, both mouse IgG1) were able to fully block CD155 binding, as did the previously reported clone 4D4 (1) (Fig. 1B). The fact that the 1G9 and 1B4 antibody clones both block CD155 binding indicated that they recognize similar epitopes on TIGIT. Indeed, antibody competition experiments support that these antibodies interfere with each other’s binding and thus bind to the same or overlapping epitopes on TIGIT (Fig. 1C). In addition, their ability to compete with CD155 for TIGIT binding suggests that they might show functional properties in vivo.
Figure 1. Anti-TIGIT antibodies compete with CD155.
TIGIT-specific antibodies were generated in armenian hamster (clone 4D4) or TIGIT−/− mice (clones 1G9, 1B4, and 2F6). (A) anti-TIGIT antibodies were titrated in an ELISA against recombinant mouse TIGIT (black) or a control (grey) protein. (B) P815 cells transfected with mouse TIGIT that uniformly express TIGIT (top left) were incubated with anti-TIGIT or isotype control antibody (Armenian hamster IgG for clone 4D4 or mouse IgG1 for 1G9, 1B4, and 2F6). Samples were then incubated with recombinant mouse CD155, then stained with anti-CD155 and analyzed by flow cytometry. A sample not incubated with CD155 was used as a positive control for blocking of CD155 binding. Representative FACS plots and summary data of inhibition of CD155 binding of 3–5 independent experiments are shown. (C) TIGIT-expressing splenocytes from TIGIT-Tg mice were incubated with purified 1G9 or 1B4 at the concentrations indicated for 15 min, then washed, and stained with 1G9-PE. The inhibition of binding of 1G9-PE is shown. (D) 200 µg of purified 1G9, 1B4, or mouse IgG1 were administered i.p. to TIGIT-transgenic mice. 48 hrs later spleens were harvested and the presence of CD4+ and CD8+ T cells analyzed by flow cytometry. The average of two independent experiments is shown (n=2/group).
We next addressed whether the 1G9 and 1B4 antibody clones could deplete TIGIT+ T cells in vivo under steady state conditions. For this we took advantage of TIGIT-Tg mice that constitutively express high levels of TIGIT on peripheral CD4+ and CD8+ T cells (6). We administered 1G9, 1B4, or mouse IgG1 isotype control antibody to TIGIT-Tg mice and assessed the number of CD4+ and CD8+ T cells 48 hours later. We found that administration of 1G9 and 1B4, had no effect on the total number of CD4+ or CD8+ T cells (Figure 1D). This is in line with the inability of mouse IgG1 to promote antibody-dependent cell-mediated cytotoxicity. Thus, the anti-TIGIT antibody clones 1G9 and 1B4 block CD155 binding but do not deplete TIGIT+ cells in vivo under steady state conditions.
Anti-TIGIT antibodies modulate T cell responses in vivo
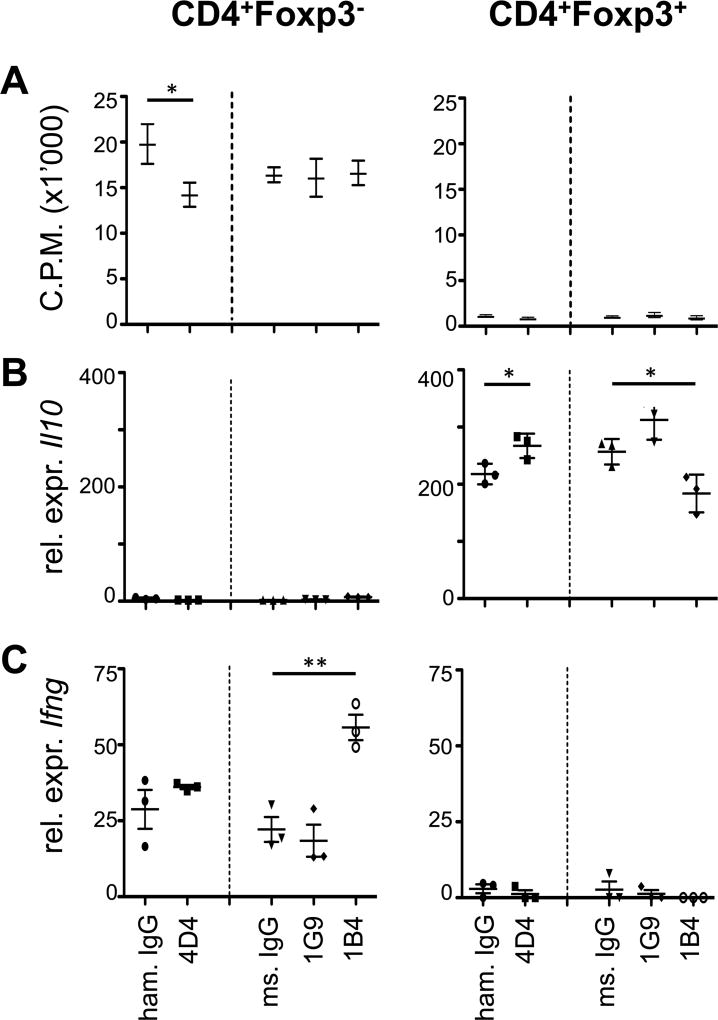
Next we addressed whether the hamster anti-mouse TIGIT Ab clone 4D4 and the two mouse anti-mouse clones 1G9 and 1B4 might have functional properties in vitro and in vivo. TIGIT has T cell intrinsic inhibitory properties in that it inhibits T cell proliferation and production of pro-inflammatory cytokines, such as IFN-γ, while enhancing IL-10 production (1, 2). So to test the functional properties of our anti-TIGIT antibodies in vitro, we sorted CD4+Foxp3− effector T cells and CD4+Foxp3+ regulatory T cells, stimulated them with anti-CD3/anti-CD28 beads in vitro, and tested how the anti-TIGIT antibodies affected cell proliferation and cytokine production. As described previously (1), we could observe decreased proliferation in the presence of clone 4D4, suggesting that it acts as an agonistic anti-TIGIT antibody. However, clones 1G9 and 1B4 did not affect T cell proliferation in vitro (Fig. 2A). Anti-TIGIT clone 4D4 also enhanced Il10 transcription in CD4+Foxp3+ regulatory T cells but had no effect on Ifng transcription in effector T cells (Fig. 2B, C). While anti-TIGIT clones 1G9 and 1B4 did not affect T cell proliferation in vitro, the presence of clone 1B4 resulted in increased Ifng transcription in CD4+Foxp3− effector T cells and decreased Il10 levels in CD4+Foxp3+ regulatory T cells (Fig. 2B, C), indicating that it might act as a TIGIT blocking antibody. Anti-TIGIT clone 1G9 had no effect on Ifng expression but slightly enhanced Il10 transcription in CD4+Foxp3+ regulatory T cells, although this increase did not reach significance (Fig. 2B, C).
Figure 2. Anti-TIGIT antibodies show limited functional properties in vitro.
CD8+, CD4+ and CD4+Foxp3+ cells were sorted from Foxp3-GFP reporter mice, 4×104 cells/well (CD8+and CD4+) or 2×104 cells/well (CD4+Foxp3+) were seeded into 96 well plates and stimulated with 4×104 CD3/CD28 Dynabeads/ in the presence of with 25 µg/ml of anti-TIGIT (clones 4D4, 1G9 and 1B4) or isotype control (armenian hamster IgG, mouse IgG1) antibodies. (A) Proliferation was measured after 48h based on 3H-thymidine incorporation (pooled data of 2–3 independent experiments is shown, mean ± SEM (CD8+and CD4+ n=9; CD4+Foxp3+ n=6)). (B, C) After 48 hours, cells were harvested and expression of (B) Il10 and (C) Ifng mRNA was determined by quantitative RT-PCR (representative plot, n=3, mean ± SD). *, P < 0.05; **, P < 0.01 (Student t test).
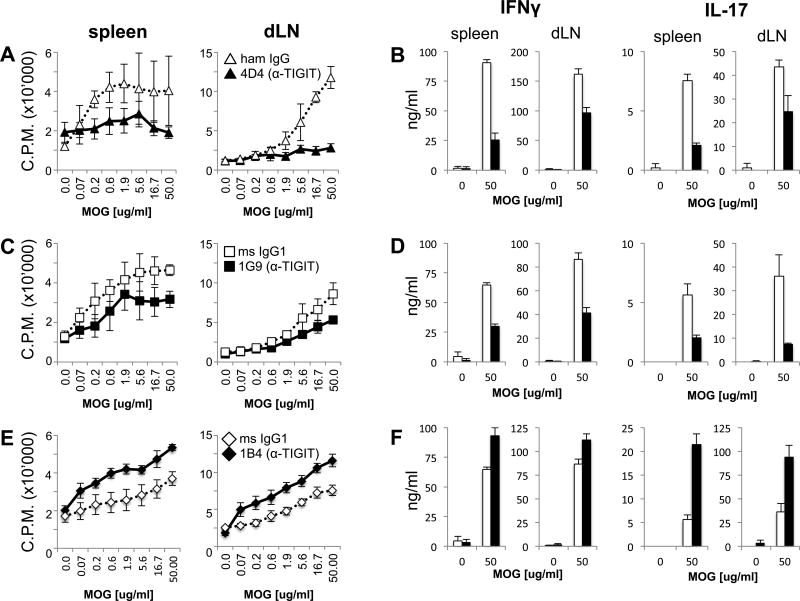
As functional properties of antibodies might differ in vitro and in vivo, we next tested how our anti-TIGIT clones affected T cell function in vivo. We immunized mice with MOG35–55 peptide in CFA and treated them with the anti-TIGIT antibodies or the appropriate isotype control on days 0, 2, and 4. After 10 days, cells from spleens and draining lymph nodes (dLNs) were isolated and re-stimulated with MOG35–55 peptide in vitro. Treatment with clone 4D4 strongly reduced dose-dependent proliferation upon re-stimulation with antigenic peptide (Fig. 3A). In line with this finding, MOG35–55 re-stimulated splenocytes and LN cells from 4D4-treated mice produced lower levels of the pro-inflammatory cytokines IFN-γ and IL-17 (Fig. 3B). Similarly, mice treated with clone 1G9 also displayed reduced antigen-specific proliferation upon re-stimulation, although to a lesser degree than mice treated with clone 4D4 (Fig. 3C). Nevertheless, a similar reduction in the secretion of IFN-γ and IL-17 could be observed in 1G9 treated mice (Fig. 3D). In contrast, treatment with clone 1B4 resulted in enhanced antigen-specific proliferation concomitant with increased secretion of IFN-γ and IL-17 (Fig. 3E and F). Importantly, for all three clones, antibody treatment had no effect on cell composition as frequencies and total numbers of lymphocytes in the anti-TIGIT or isotype control treated animals were comparable (Suppl. Fig. 1). Taken together, these results indicate that all three anti-TIGIT clones show functional properties in vivo and are capable of modulating T cell responses by affecting their magnitude and the cytokine profile. While clones 4D4 and 1G9 appear to have agonistic anti-TIGIT activity, clone 1B4 acts as a TIGIT blocking antibody in vivo.
Figure 3. Anti-TIGIT antibodies modulate T cell responses in vivo.
Wild type B6 mice were immunized s.c. with 100 µg MOG35–55 peptide in CFA and received 100 µg anti-TIGIT (or isotype control: Armenian hamster IgG for clone 4D4 or mouse IgG1 for 1G9 and 1B4) antibody i.p on days 0, 2, and 4. On day 10 spleens and lymph nodes were harvested and cells were re-stimulated with MOG35–55 peptide. (dLN: draining lymph node). (A, C, E) After 48 hours, 3H-thymidine was added for the last 18–22 hours before [3H]thymidine incorporation was analyzed to assess proliferation (mean ± SD of triplicate wells from 3–4 animals/group, 3–6 independent experiments). (B, D, F) IFN-γ and IL-17 were measured in the supernatants derived from the same cultures at 48 hours using cytometric bead array. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student t test).
Functional anti-TIGIT antibodies modulate disease severity in EAE
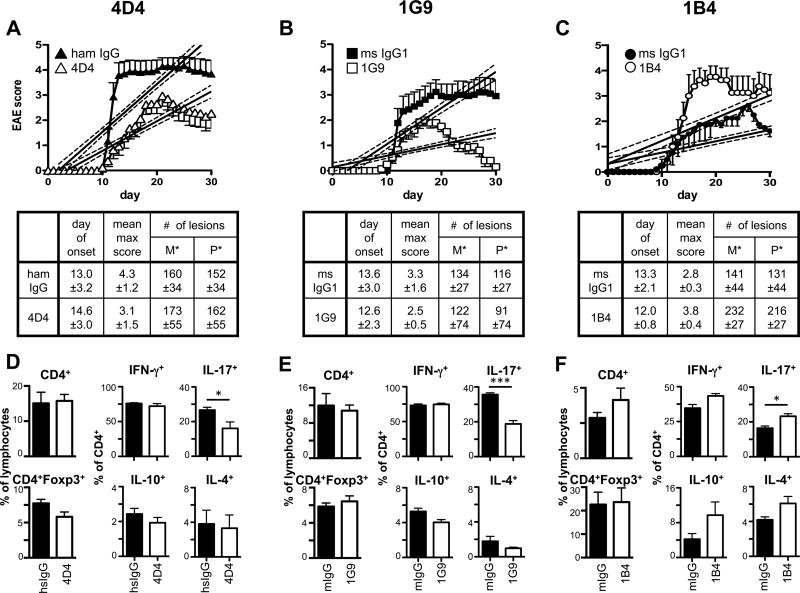
As all three anti-TIGIT antibody clones were able to modulate T cell responses in vivo, they were next tested for their ability to affect the development of T cell driven autoimmunity. Mice were immunized with MOG35–55 peptide in CFA to induce experimental autoimmune encephalomyelitis (EAE) and treated with anti-TIGIT antibody (or the appropriate isotype control). In line with the observed reduction in antigen-specific T cell responses, treatment with the agonistic clones 4D4 and 1G9 was able to reduce EAE severity (Figure 4A and B). Interestingly, clone 1G9 had a similar, if not stronger beneficial effect in this setting despite its slightly reduced potency in modulating the recall T cell responses (Fig. 3). This is most likely due to decreasing activity of the hamster antibody 4D4 in this prolonged experimental setting, highlighting the importance of species-specific tools for studies in long-term chronic disease models. We reasoned that treatment with the blocking antibody clone 1B4 would result in a similar phenotype as observed in TIGIT−/− mice, where, in contrast to wild type mice, suboptimal doses of MOG35–55 are sufficient to induce full-blown EAE (1). Indeed, 1B4-treated mice developed exacerbated disease in this experimental setting, confirming that clone 1B4 acts as a blocking anti-TIGIT antibody in vivo (Fig. 4C). Interestingly, treatment with both agonistic anti-TIGIT antibodies (clones 4D4 and 1G9) did not result in significant differences in the number of lesions or CNS infiltrating cells but significantly reduced the frequencies of IL-17+ Th17 cells (Fig. 4) infiltrating the CNS. In contrast, blocking of TIGIT with clone 1B4 significantly increased the number of lesions in both the meninges (p=0.029) and the parenchyma (p=0.045) and also led to increased frequencies of Th17 cells in the CNS (Fig. 4C and F). Collectively, these results show that the functional modulation of T cell responses with respect to magnitude and cytokine profile through the agonistic and blocking anti-TIGIT clones 4D4, 1G9, and 1B4 also translates into differences in disease severity during EAE.
Figure 4. Functional anti-TIGIT antibodies modulate disease severity in EAE.
Wild type B6 mice were immunized s.c. with 100 µg (A, B; D, E) or 10–15µg (C; F) MOG35–55 peptide in CFA, followed by injection of 100 ng pertussis toxin i.v. on day 0 and day 2. Mice also received 100 µg anti-TIGIT (or isotype control: Armenian hamster IgG for clone 4D4 or mouse IgG1 for 1G9 and 1B4) antibody i.p on days 0, 2, 4, 10, and 17 and were monitored daily for EAE. Mean clinical score ± SEM is shown and linear regression curves of the disease for each group is depicted (the 95% confidence intervals are represented with dashed lines; combined data of 2-3 independent experiments). The number of lesions in the meninges (M*) and parenchyma (P*) were determined by histopathology when control mice were at the peak of disease (A, B: day 14; C: day 17). (D–F) At the peak of disease, CNS infiltrating cells were isolated, re-stimulated with PMA/ionomycin for 4h and analyzed for the production of IFN-γ, IL-17A, IL-10, and IL-4 by intracellular cytokine staining. *, P < 0.05; ***, P < 0.001.
Blocking anti-TIGIT antibodies have synergistic effects with PD-1 blockade in cancer
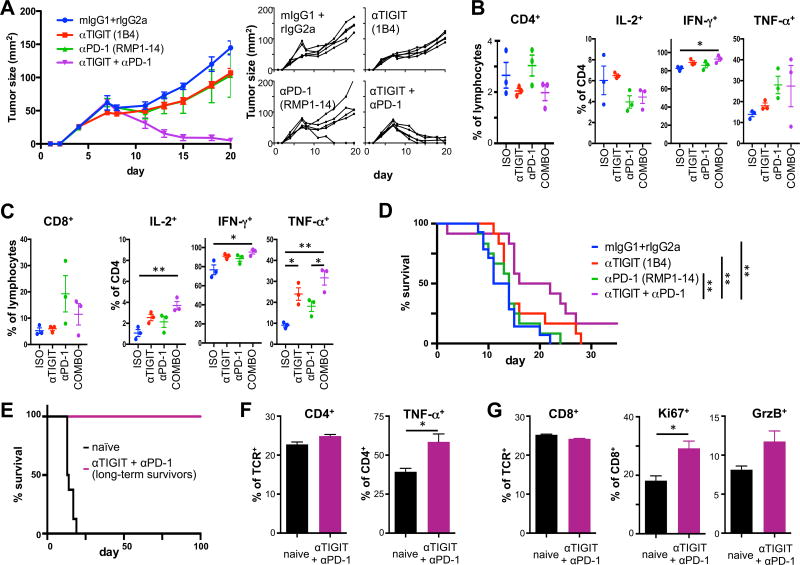
The blockade of co-inhibitory receptors is highly relevant in cancer where therapeutic effects are being exploited clinically. Anti-PD-1 therapy has shown great success but resistance to this therapy is increasing and some tumors (like colorectal carcinomas) are resistant anti-PD-1 or anti-CTLA-4 immunotherapy. Accordingly combination therapies are currently the most promising avenue for achieving durable responses. We therefore tested the efficacy of the anti-TIGIT blocking antibody 1B4 that we have generated in the setting wherein mice fail to respond to anti-PD-1 therapy. We found that anti-TIGIT 1B4 treatment alone led to a small but uniform retardation of established MC38 colon carcinoma growth. As expected, anti-PD-1 treatment alone resulted in a variable response with most mice showing initial tumor regression followed by escape and only one mouse showing complete tumor regression. However, we observed complete tumor regression in all mice when they were additionally treated with anti-TIGIT (Fig. 5A). Analysis of tumor-infiltrating lymphocytes (TILs) showed no consistent effects on the frequencies of CD4+ and CD8+ T cells across groups. Importantly, only the anti-TIGIT/anti-PD-1 co-blockade group showed increased IFN-γ secretion by CD4+ TILs (Fig. 5B) and increased production IFN-γ, TNF-α, and IL-2 in CD8+ TILs (Fig. 5C). Combined blockade of PD-1 and TIGIT thus markedly enhanced functionality of tumor infiltrating T cells, resulting in improved tumor control. To test whether these findings could be extended to other tumor models, we tested the efficacy of anti-TIGIT alone or in combination with anti-PD-1 in established GL261 glioblastoma. As observed for MC38 colon carcinomas, treatment with anti-TIGIT alone lead to a small, but consistent improvement of tumor control and a modest survival benefit (Fig. 5D). However, combination of anti-PD1 and anti-TIGIT markedly increased survival, with 17% of mice even showing long-term survival. Importantly, these mice had a durable anti-tumor response, as they remained tumor free for >100 days when re-challenged by with GL261 implanted in the contralateral hemisphere (Fig. 5E). Functional analysis on T cells in the cervical lymph nodes again revealed improved functionality in that TNF-α production in CD4+ T cells was increased (Fig. 5F) and CD8+ T cells showed enhanced proliferation and contained elevated levels of granzyme B (Fig. 5G). These data support the combination of TIGIT and PD-1 blockade in cancer therapy. Collectively, our data demonstrate that the monoclonal anti-TIGIT antibodies we have generated represent a valuable set of tools that can be used to modulate TIGIT function in different disease models in vivo.
Figure 5. Blocking anti-TIGIT antibody has synergistic effects with blockade of other co-inhibitory receptors in cancer.
(A–D) Wild type C57BL/6 mice were implanted with MC38 colon carcinoma. Mice with established tumors were treated with anti-PD-1, anti-TIGIT, anti-PD-1 + anti-TIGIT (COMBO), or isotype controls (ISO) and monitored for tumor growth. (A) Mean (left) and individual (right) tumor growth curves are shown (n=5/group). Similar results were obtained in an independent experiment. (B, C) TILs were harvested from tumor bearing WT mice (n=3) 9 days after tumor implantation when tumor sizes measured between 33 and 60 mm2. Frequency (± SEM) of CD4+ (B) and CD8+ (C) TILs was determined by flow cytometry. Cells were stimulated with PMA/ionomycin and frequency (± SEM) of (B) CD4+ and (C) CD8+ TILs producing IL-2, TNF-α, and IFN-γ were determined by intracellular cytokine staining. (D) Wild type C57BL/6 mice were orthotopically implanted with GL261-GFP-gluc cells. Mice with established tumors were size matched and treated with anti-PD-1, anti-TIGIT, anti-PD-1 + anti-TIGIT, or isotype controls (n=12/group) and monitored for survival. (E) Long term survivors (n=2) and naïve control mice (n=3) were re-challenged with GL261 tumor cells in the contralateral brain hemisphere and survival was monitored for >100 days. Frequency of CD4+ (F) and CD8+ (G) in cervical lymph nodes and their expression of (F) TNF-α or (G) Ki67 and Granzyme B (GrzB) were determined by flow cytometry. *, P < 0.05; **, P < 0.01.
Discussion
Co-inhibitory receptor pathways have gained much attention as potential targets for immune modulation. While a broad spectrum of pathologies that are caused or aggravated by dysregulated immune responses, such as cancer, autoimmunity, and chronic infection, can potentially be modulated by targeting co-inhibitory receptor pathways, the development of such therapies is spearheaded by cancer therapy, where blockade of the co-inhibitory receptors CTLA-4 and PD-1 has shown remarkable success (15). Despite these promising developments, low response rates, adverse autoimmune-like toxicity, and resistance highlight the need to identify additional pathways to broaden the therapeutic repertoire of co-inhibitory receptors and overcome these hurdles. TIGIT represents such a potential therapeutic target, however, its mechanism of action has not been fully elucidated. In this study we have identified two monoclonal anti-TIGIT antibodies that can be used to manipulate TIGIT function in vivo and investigate its function in detail.
While antibodies targeting co-inhibitory receptors have successfully been used for the treatment of cancer, our studies show that they could also be exploited to treat chronic autoimmune diseases. One of the identified clones (1G9) acts agonistically in vivo and leads to a reduction in the overall T cell expansion and pro-inflammatory cytokine production. This also translated into beneficial effects in the EAE model of CNS autoimmunity, which is driven by pathogenic IFN-γ and IL-17 producing T cells. Allelic variants in the TIGIT/CD226 pathway have been associated with susceptibility to autoimmune diseases in humans (8) and we, and others, have previously shown that TIGIT acts as a negative regulator in a number of autoimmune diseases (1, 6). Importantly, TIGIT is expressed at normal levels in MS patients and as such could be targeted therapeutically to dampen autoreactive T cell responses (16). Indeed, in vitro treatment of CD4+ T cells from MS patients with agonistic anti-TIGIT antibody results in decreased T cell proliferation and cytokine production (16). Our results expand this finding and demonstrate that treatment with agonistic anti-TIGIT antibody also dampens autoimmune T cell responses in vivo and the reduction of T cell expansion and pro-inflammatory cytokines leads to amelioration of autoimmune disease. Although further studies are needed to test the therapeutic potential of targeting TIGIT in human autoimmune diseases, we now have the tools at hand for a detailed analysis of the mechanistic action of agonistic anti-TIGIT treatment that forms the basis for such interventions. In this context, the role that co-inhibitory molecules may play in regulating progression in autoimmune diseases in humans has been indicated in a large cohort of patients suffering with multiple autoimmune diseases (17).
While the blocking anti-TIGIT antibody clone (1B4) had a small effect as a single agent on established colon carcinoma or glioblastoma, combined blockade of TGIT and PD-1 resulted in complete tumor regression in colon carcinoma and could induce long-term survival and a durable, protective anti-tumor response in the glioblastoma model. This is in keeping with a recent study where TIGIT blockade synergized with PD-L1 blockade in a model of established colon carcinoma (5). Furthermore, combined treatment significantly improved the function of tumor-infiltrating T cells, which is in line with another study that showed co-blockade of TIGIT and PD-1 to improve the effector responses of tumor-antigen specific CD8+ T cells from melanoma patients (10). Together, these observations support synergy between the TIGIT and PD-1 pathways and indicate non-redundant functions for these receptors in controlling the anti-tumor immune response in mice and humans.
As the current therapeutic targets of co-inhibitory receptors expand from CTLA-4 and PD-1 to include novel inhibitory molecules such as TIGIT, it is essential to deepen our understanding of their mechanism of action and both their cell- and tissue-specific functions. The antibodies presented in this study will serve as important tools to the scientific community to address these questions in vivo and the knowledge gained will provide valuable insight for the development of novel therapeutic approaches targeting TIGIT both in autoimmune diseases and cancer.
Supplementary Material
Acknowledgments
We would like to thank members of the Kuchroo Group, the Joller Group, and the Jain Group for helpful discussions.
This work was supported by grants from the NIH (R01 NS30843 and AI093671, AI056299, AI073748 to V.K.K., and R01 CA187975 to A.C.A.); the Arthritis National Research Foundation (to N.J.); the Swiss National Science Foundation (PP00P3_150663, to N.J.); the European Research Council (677200 to N.J.); the Zuercher Universitaetsverein (ZUNIV-FAN to N.J.); the Olga Mayenfisch Stiftung (to N.J.); the NCI (P01-CA080124 and R35-CA197743 to R.K.J.); the National Foundation for Cancer Research (R.K.J.); the Ludwig Center at Harvard (R.K.J.); and by a sponsored research grant from Potenza Therapeutics to A.C.A. Z.A. is supported by a Tosteson fellowship award for medical discovery from Massachusetts General Hospital.
Abbreviations used in this article
- TIGIT
T cell immunoglobin and ITIM domain
- DC
dendritic cell
- Treg
regulatory T cell
- MOG
myelin oligodendrocyte glycoprotein
- dLN
draining lymph node
- EAE
experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
Footnotes
Disclosures
A.C.A. is a member of the SAB for Potenza Therapeutics, Tizona Therapeutics, and Idera Pharmaceuticals, which have interests in cancer immunotherapy. V.K.K. has an ownership interest and is a member of the SAB for Potenza Therapeutics and Tizona Therapeutics. A.C.A.’s and V.K.K.’s interests were reviewed and managed by the Brigham and Women’s Hospital and Partners Healthcare in accordance with their conflict of interest policies. T. Kondo is an employee of Mitsubishi Tanabe Pharma Corporation and was supported by its Scholarship Program. R.K.J. received consultant fees from Merck, Ophthotech, Pfizer, SPARC, SynDevRx, XTuit; owns equity in Enlight, Ophthotech, SynDevRx, XTuit; and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, Tekla World Healthcare Fund. Neither any reagent nor any funding from these organizations was used in this study.
References
- 1.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, Sharpe AH, Quintana FJ, Mathis D, Benoist C, Hafler DA, Kuchroo VK. Treg Cells Expressing the Coinhibitory Molecule TIGIT Selectively Inhibit Proinflammatory Th1 and Th17 Cell Responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 4.Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK, Anderson AC. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125:4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The Immunoreceptor TIGIT Regulates Antitumor and Antiviral CD8(+) T Cell Effector Function. Cancer cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D, Hebb L, Wolf A, Bukowski TR, Rixon MW, Kuijper JL, Ostrander CD, West JW, Bilsborough J, Fox B, Gao Z, Xu W, Ramsdell F, Blazar BR, Lewis KE. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41:902–915. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hafler JP, Maier LM, Cooper JD, Plagnol V, Hinks A, Simmonds MJ, Stevens HE, Walker NM, Healy B, Howson JM, Maisuria M, Duley S, Coleman G, Gough SC, Worthington J, Kuchroo VK, Wicker LS, Todd JA. CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immun. 2009;10:5–10. doi: 10.1038/gene.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiti AK, Kim-Howard X, Viswanathan P, Guillen L, Qian X, Rojas-Villarraga A, Sun C, Canas C, Tobon GJ, Matsuda K, Shen N, Chernavsky AC, Anaya JM, Nath SK. Non-synonymous variant (Gly307Ser) in CD226 is associated with susceptibility to multiple autoimmune diseases. Rheumatology (Oxford) 2010;49:1239–1244. doi: 10.1093/rheumatology/kep470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 13.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, Yu V, Dalvie N, Amelung RL, Datta M, Song JW, Askoxylakis V, Taylor JW, Lu-Emerson C, Batista A, Kirkpatrick ND, Jung K, Snuderl M, Muzikansky A, Stubenrauch KG, Krieter O, Wakimoto H, Xu L, Munn LL, Duda DG, Fukumura D, Batchelor TT, Jain RK. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci U S A. 2016;113:4476–4481. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callahan MK, Postow MA, Wolchok JD. Targeting T Cell Co-receptors for Cancer Therapy. Immunity. 2016;44:1069–1078. doi: 10.1016/j.immuni.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 Axis Regulates Human T Cell Function. J Immunol. 2012 doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.