Abstract
Several meta-analyses have attempted to determine the relations between intake of α-linolenic acid (ALA) and prostate cancer, but results were inconclusive. 47,885 men aged 40–75y without prior cancer in the Health Professionals Follow-up Study were prospectively followed from 1986 to 2010. Intake of ALA was determined from validated food frequency questionnaires every four years. We used multivariate Cox proportional hazards models to estimate hazard ratios (HR) with 95% confidence intervals (CIs) for lethal prostate cancer (distant metastasis or prostate cancer death). 386 lethal prostate cancers were diagnosed in the pre-PSA era (before February,1994) and 403 cancers in the PSA era. Intake of ALA was associated with increased risk of lethal prostate cancer in the pre-PSA era (comparing top to bottom quintile of intake, multivariate-adjusted HR =1.78; 95% CI = 1.22–2.06; p trend = 0.003), but not in the PSA era (HR =0.81; 95% CI = 0.56–1.17; p trend = 0.53), and the difference in associations was statistically significant (p for interaction = 0.02). Mayonnaise, a primary food source of ALA intake in our cohort, was likewise only significantly associated with lethal prostate cancer in the pre-PSA era. Among many other fatty acids that are correlated with ALA due to shared food sources, none was associated with lethal prostate cancer in the pre-PSA era. In conclusion, higher intake of ALA was associated with an increased risk of lethal prostate cancer in the pre-PSA era, but not in the PSA era. Potential reasons for the differential associations warrant further investigation.
Keywords: α-linolenic acid, prostate cancer, prospective cohort study
Introduction
Prostate cancer remains the second leading cause of cancer death in American men.1 About 26,000 men are projected to die of prostate cancer in 2016.1 The large geographic variations in the rates of prostate cancer and changing rates in migrant studies suggest that modifiable environmental factors such as dietary factors play a role.2–4
Total Dietary fat was frequently studied in earlier years,5 but recent interest has focused on specific types of fats. Alpha-linolenic acid (ALA) is an 18-carbon omega-3 (n-3) fatty acid found in some vegetable oils, walnuts, leafy green vegetables, grains and animal fats.6 Although commonly regarded as the precursor for long-chain n-3 fatty acids, increasing evidence suggests that ALA has independent and specific effects on some chronic diseases7. Several meta-analyses8–11 have attempted to determine the relationships between intake of ALA and risk of prostate cancer but the interpretations of results have been complicated by substantial inter-study heterogeneity.
Many factors potentially contribute to the inter-study heterogeneity, including variations in the amount of ALA intake, food sources, dietary assessment methods, frequency of dietary assessments, food composition databases, adjustment for confounding factors, and duration of follow-up. For example, studies in Spain (approximate interquartile range (IQR) for intake of ALA, 0.7–2.1g/d)12 and Uruguay (approximate IQR, 0.7–1.6g)13, where ALA intake was derived primarily from meats, showed positive associations, whereas a study in Italy (approximate IQR, 0.6–2.6g)14 where ALA intake came mainly from olive oil and other vegetable sources, showed an inverse association. The divergent associations of ALA indicate that adequate control for confounding by dietary patterns or other components of the diet is essential in future studies.
Widespread PSA screening may have further added to the heterogeneity. In the pre-PSA era, prostate cancers were generally diagnosed due to urinary symptoms, whereas in the PSA era, many indolent cancers were diagnosed that likely would have remained undiagnosed in the absence of screening. Therefore, it has been argued that lethal prostate cancer (those that develop distant metastases or cause death) is a more specific outcome to evaluate risk factors in the PSA era.15
In our past prospective analyses16–18 in the Health Professionals Follow-up Study (HPFS) with repeated dietary assessments and 4–16 years of follow-up, ALA intake was positively associated with risk of prostate cancer, especially for advanced stage disease. However, we lacked power to examine lethal prostate cancers. With an additional 8 years of follow-up that doubled the number of lethal prostate cancers, we sought to provide further insights into the relationship by focusing on lethal prostate cancer and taking PSA screening into consideration.
Methods
Study Population
The HPFS is an ongoing prospective cohort that includes 51,529 male US health professionals aged 40 to 75 years old at baseline in 1986. Cohort participants are followed by questionnaires every 2 years about lifestyle factors and new medical diagnoses, and by food frequency questionnaires (FFQ's) every 4 years to obtain dietary information. At baseline we excluded those who did not adequately complete the baseline FFQ or had a previous diagnosis of cancer (except nonmelanoma skin cancer). 47,885 eligible participants were prospectively followed for prostate cancer incidence, metastasis and mortality until January 31, 2010. The study protocol was approved by the Institutional Review Board at the Harvard T.H. Chan School of Public Health.
Assessment of Dietary Intake
On FFQs, commonly used units or portion sizes were specified for each food item and participants were asked to report how often, on average over the past year, they had consumed each food item (9 possible responses ranging from “≤1 time per month” to “≥ 6 times/day”). The FFQs specifically inquired about the usual kind of fat used for frying, sautéing and baking. The FFQs also inquired about the usual brand and type of margarine using an open-ended question. Such information was taken into account when calculating ALA intake from fried, sautéed, and baked foods prepared at home. The daily nutrient intake was calculated by multiplying the consumption frequency of each food by its nutrient content and then summing across all foods. The nutrient composition data were primarily based on the US Department of Agriculture Nutrient Database supplemented with information from manufacturers and published reports. We adjusted all the nutrient intakes for total energy using the residual method to reflect the composition of the diet.19
In the recently completed Women's Lifestyle Validation Study,20 an extensive validation study that involved more than 700 women from the Nurses’ Health Studies, two cohorts of women with similar FFQs to those in the HPFS, the Spearman correlation between intake of energy-adjusted ALA from the FFQ and from two 1-week diet records was 0.57 (95% CI = 0.48 – 0.65) after correcting for random within-person error in the diet records. Similarly, the de-attenuated correlation for ALA between the FFQ and four 24-hour dietary recalls was 0.58.
Identification of Prostate Cancer Cases
Diagnoses of prostate cancer were initially self-reported on biennial questionnaires by the participants and then confirmed by review of medical records and pathology reports. Participants with confirmed prostate cancer diagnoses were separately followed by a biennial questionnaire to obtain information on prostate cancer treatment, progression and metastasis. Deaths in the cohorts were ascertained through reports by family members and searches of National Death Index. Underlying causes of death were determined by review of medical records and death certificates by a study physician, and were based on death certificates alone in the rare cases when the primary medical records were not available. The mortality follow-up rate in the cohort was nearly 100%. The primary study outcome was lethal prostate cancer, defined as cancers that caused death or had distant metastases by the end of follow-up.
Statistical Analysis
Each participant contributed person-time to the analysis from the return of baseline questionnaire to the confirmed initial diagnosis of lethal prostate cancer, death, or the end of follow-up, January 31, 2010, whichever occurred first. To best represent long-term intake and minimize measurement error,21 we calculated the cumulative average intake of ALA by averaging all available FFQs up to the start of each two-year risk interval. All cumulative averages were categorized into quintiles based on the distribution in the entire cohort for that two-year risk interval. Likewise, we calculated the cumulative average intakes of foods and categorized them into pre-specified groups.
We used time-varying Cox proportional hazards model to estimate the hazard ratios (HR’s) and 95% confidence intervals (CI’s) for lethal prostate cancer. Multivariable models were stratified by age in months and calendar year and were adjusted for known and suspected risk factors previously identified in our cohort and other studies. We also adjusted for PSA testing, which was first inquired in 1994 and biennially thereafter. We lagged the PSA testing by one period in the analysis to avoid counting diagnostic PSA testing as screening. For example, PSA testing during 1994–1996 was used to adjust for the 1996–1998 follow up period. We tested the linear trend across quintiles of ALA intake by modeling the median intake of each category as a continuous variable.
We stratified our analysis by the time period before and after the clinical introduction of PSA screening. The pre-PSA screening era was defined as February 1, 1986 to January 31, 1994, and the PSA screening era, February 1, 1994 to January 31, 2010. Cumulative average intake of ALA was calculated separately in two periods. To test if the risk estimates differed between the two periods, we created an interaction term by multiplying a continuous time-varying ALA variable derived from the median intake of each quintile by the binary indicator variable for time period and used a Wald test to ascertain the statistical significance of interaction.
Results
During 24 years (941,461 person-years) of follow-up, we confirmed 789 lethal prostate cancer cases among 47,885 participants. 386 cases had an initial cancer diagnosis date in the pre-PSA era and 403 cases in the PSA era. Many demographic and lifestyle factors did not vary appreciably across quintiles of ALA intake in 1990 (mid-point of pre-PSA era) or 2002 (mid-point of post-PSA era), except that participants with higher intake of ALA in 1990 were less likely to be never smokers or to engage in vigorous physical activity (Table 1). Intake of ALA was positively related to intakes of LA, marine n-3 fatty acids (only for 2002 intake), coffee (only for 1990 intake), and tomato sauce, but inversely related to multivitamin use (only for 1990 intake). Intake of ALA was also positively related to the prudent dietary pattern score in both years whereas intake of ALA was positively related to western dietary pattern only in 1990.
Table 1.
Age-standardized characteristics of the study population according to energy-adjusted intake of α-linolenic acid in 1990 and 2002
| 1990, Quintiles | 2002, Quintiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | Q1 | Q2 | Q3 | Q4 | Q5 | |
|
|
|
|||||||||
| No. of participants | 7,009 | 7,194 | 7,147 | 6,814 | 7,241 | 5,499 | 5,479 | 5,592 | 5,479 | 5,508 |
| Age, year | 59 | 58 | 58 | 58 | 58 | 68 | 68 | 68 | 68 | 68 |
| BMI, kg/m2 | 25.0 | 25.5 | 25.6 | 25.7 | 25.8 | 25.9 | 26.3 | 26.4 | 26.4 | 26.3 |
| Height, inches | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 |
| White, % | 96 | 97 | 97 | 96 | 97 | 96 | 97 | 97 | 96 | 96 |
| Never smokers, % | 46 | 47 | 45 | 45 | 43 | 40 | 41 | 42 | 42 | 43 |
| Current smokers, % | 8 | 7 | 8 | 7 | 8 | 4 | 4 | 4 | 3 | 3 |
| Vigorous activity, % top quintile | 21 | 18 | 17 | 16 | 17 | 16 | 15 | 15 | 15 | 16 |
| Has diabetes, % | 3 | 3 | 3 | 4 | 4 | 5 | 6 | 8 | 8 | 10 |
| Family history of prostate cancer, % | 13 | 14 | 14 | 13 | 14 | 12 | 13 | 13 | 13 | 14 |
| ‡PSA testing in the prior two years,% | NA | NA | NA | NA | NA | 84 | 86 | 85 | 85 | 84 |
| Daily dietary intake | ||||||||||
| Multivitamin use, % | 41 | 38 | 38 | 38 | 39 | 67 | 65 | 66 | 66 | 67 |
| Total calories, kcal | 1,873 | 1,916 | 1,959 | 1,976 | 1,869 | 2,027 | 2,028 | 2,034 | 2,022 | 2,006 |
| α-linolenic acid, mg | 749 | 922 | 1,048 | 1,193 | 1,527 | 809 | 1,011 | 1,163 | 1,347 | 1,966 |
| Linoleic acid, g | 9.4 | 10.3 | 11.0 | 11.9 | 14.0 | 9.1 | 10.3 | 11.2 | 12.3 | 15.4 |
| Marine omega3 fatty acids, mg | 340 | 331 | 337 | 348 | 443 | 306 | 339 | 360 | 382 | 427 |
| trans 18:1, g | 2.4 | 2.8 | 2.9 | 2.9 | 2.9 | 1.8 | 2.0 | 2.1 | 2.1 | 2.0 |
| Supplemental vitamin E, mg | 36 | 32 | 33 | 33 | 41 | 118 | 115 | 116 | 117 | 126 |
| Calcium, mg | 919 | 919 | 913 | 907 | 901 | 1,206 | 1,140 | 1,135 | 1,125 | 1,146 |
| Total coffee, servings | 1.8 | 1.8 | 1.9 | 2.0 | 2.0 | 1.6 | 1.6 | 1.6 | 1.6 | 1.5 |
| Tomato sauce, servings | 0.11 | 0.12 | 0.13 | 0.13 | 0.13 | 0.14 | 0.17 | 0.18 | 0.19 | 0.19 |
| Dietary pattern score | ||||||||||
| Prudent | −0.16 | −0.08 | 0.001 | 0.06 | 0.14 | 0.01 | 0.11 | 0.18 | 0.23 | 0.39 |
| Western | −0.32 | −0.09 | 0.06 | 0.18 | 0.16 | 0.12 | 0.20 | 0.23 | 0.23 | 0.11 |
Values are means(SD) or percentages and are standardized to the age distribution of the study population (except for age).
The number of participants in1990 and 2002 does not equal the number of participants at 1986 baseline due to missing ALA intake in 1990 or 2002.
PSA testing was not asked until 1994.
During the follow-up, the age-adjusted intake of ALA increased about 30% in the HPFS from 1986 (mean ± SD: 1,110 ± 303 mg) to 2006 (mean ± SD: 1,435 ± 745 mg).
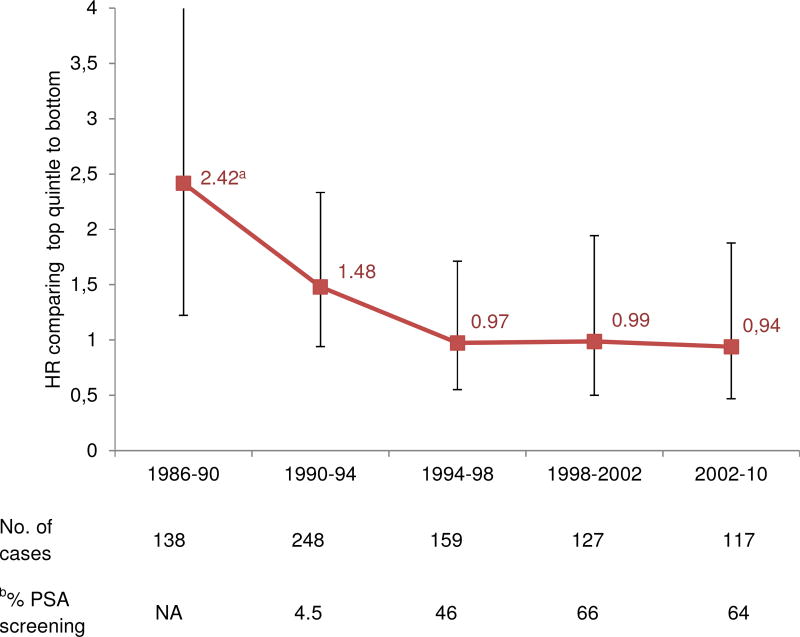
The associations between cumulative averaged intake of ALA and lethal prostate cancer for each 4-year follow-up period decreased over time (Figure). Since 1994, which we used to mark the widespread clinical introduction of PSA screening, the associations were markedly attenuated and no longer significant. In Table 2, we stratified the analysis by the pre-PSA and PSA era. The HR comparing the top to the bottom quintile after extensive adjustment for lifestyle and dietary risk factors (multivariable model 2) was 1.46 (95% CI = 1.04 – 2.04; p trend = 0.04) in the pre-PSA era and 0.77 (95% CI = 0.55 – 1.09; p trend = 0.31) in the PSA era. Further adjusting for LA intake strengthened the association in the pre-PSA era (HR = 1.78; 95% CI = 1.22 – 2.60; p trend = 0.003). The difference in pre and post associations was statistically significance (p=0.02).
Figure. Multivariable-adjusted hazard ratios for the association between intake of α-linolenic acid and lethal prostate cancer at different follow-up period.
ap for trend test across quintiles of ALA intake <0.05.
Multivariable model (MV) included: age, calendar time, race (White, African American, Asian American, or other), current BMI (<21, 21 to 23, 23 to 25, 25 to 27.5, 27.5 to <30, or ≥ 30 kg/m2), height (quartiles), vigorous activity (quintiles, MET-hours/wk), smoking (never, former quit > 10 y ago, former quit ≤ 10 y ago, or current), family history of prostate cancer in father or brother (yes or no), diabetes (Type I or II, yes or no), multivitamin use (yes or no), history of PSA testing (yes or no, lagged by one questionnaire cycle), total calories (quintiles) and linoleic acid (quintiles).
2002–2006 and 2006–2010 intervals were combined into 2002–2010 to increase power due to a small number of cases in each time period
Abbreviations, multivariable model (MV); linoleic acid (LA)
b PSA testing was not asked until 1994.
Table 2.
The associations between intake of α-linolenic acid and lethal prostate cancer in the pre-PSA (1986–1994) and PSA (1994–2010) era
| Quintiles (Q) |
P interaction between two eras |
||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p trend | ||
|
|
|
||||||
| Pre-PSA eraa | |||||||
| Median intake, mg/d | 800 | 950 | 1,070 | 1,205 | 1,445 | ||
| Cases/person years | 63 / 71,601 | 78 / 71,472 | 74 / 71,335 | 88 / 73,909 | 83 / 71,435 | ||
| Age-adjusted model | 1 (ref) | 1.38 (0.98,1.93) | 1.32 (0.94,1.86) | 1.55 (1.11,2.15) | 1.44 (1.03,2.00) | 0.04 | 0.06 |
| Multivariable model 1c | 1 (ref) | 1.34 (0.96,1.88) | 1.27 (0.90,1.80) | 1.48 (1.06,2.05) | 1.41 (1.01,1.96) | 0.06 | 0.04 |
| Multivariable model 2d | 1 (ref) | 1.37 (0.98,1.93) | 1.32 (0.93,1.86) | 1.53 (1.10,2.14) | 1.46 (1.04,2.04) | 0.04 | 0.03 |
| + linoleic acid | 1 (ref) | 1.43 (1.01,2.02) | 1.43 (1.00,2.04) | 1.74 (1.22,2.48) | 1.78 (1.22,2.60) | 0.003 | 0.02 |
| PSA erab | |||||||
| Median intake, mg/d | 813 | 980 | 1,090 | 1,230 | 1,500 | ||
| Cases/person years | 71 / 107,198 | 68 / 106,862 | 95 / 107,187 | 74 / 106,580 | 66 / 106,813 | ||
| Age-adjusted model | 1 (ref) | 0.94 (0.68,1.31) | 1.06 (0.77,1.46) | 1.24 (0.91,1.69) | 0.83 (0.59,1.17) | 0.60 | |
| Multivariable model 1c | 1 (ref) | 0.93 (0.67,1.29) | 1.02 (0.74,1.41) | 1.17 (0.86,1.60) | 0.78 (0.55,1.09) | 0.32 | |
| Multivariable model 2d | 1 (ref) | 0.93 (0.66,1.29) | 1.02 (0.74,1.41) | 1.18 (0.86,1.61) | 0.77 (0.55,1.09) | 0.31 | |
| + linoleic acid | 1 (ref) | 0.94 (0.67,1.31) | 1.04 (0.75,1.45) | 1.21 (0.87,1.68) | 0.81 (0.56,1.17) | 0.53 | |
Analysis in the pre-PSA era was based on cumulatively updated ALA intake calculated from 1986 to 1990 FFQs
Analysis in the PSA era was based on cumulatively updated ALA intake calculated from 1994 to 2006 FFQs
Multivariable model 1 adjusted for age, calendar time, race (White, African American, Asian American, or other), current BMI (<21, 21 to 23, 23 to 25, 25 to 27.5, 27.5 to <30, or ≥ 30 kg/m2), height (quartiles), vigorous activity (quintiles, MET-hours/wk), smoking (never, former quit > 10 y ago, former quit ≤ 10 y ago, or current), family history of prostate cancer in father or brother (yes or no), diabetes (Type I or II, yes or no), multivitamin use (yes or no), history of PSA testing (yes or no, lagged by one questionnaire cycle), total calories (quintiles)
Multivariable model 2 includes all variables in model 1 and intakes of calcium (< 500, 500 to 750, 750 to 1000, 1000 to 1250, 1250 to 1500, 1500 to 2000, > 2000 mg/d), tomato sauce, coffee, supplemental vitamin E and marine omega3 fatty acids (all in quintiles).
Among primary ALA-containing foods, in the pre-PSA era only mayonnaise was significantly associated with higher risk of lethal prostate cancer (adjusted HR comparing 5–6 servings/wk to ≤ 3 months = 1.49; 95% CI = 1.06–2.09; p trend = 0.02) (Table 3). In the PSA era, none of the foods was positively associated with the risk of lethal prostate cancer. However, the highest intake of margarine was inversely associated with the risk of lethal prostate cancer.
Table 3.
Hazard ratios of lethal prostate cancer according major food sources of α-linolenic acid
| Servings | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| % contribution to ALA intakea |
≤ 3/month | 1/week | 2–4/week | 5–6/week | 1/day | ≥ 2/day | P trend |
|
|
|
||||||||
| Pre-PSA erab | ||||||||
| Mayonnaise | 13.5 | 1(ref) | 1.31 (0.98,1.75) | 1.29 (0.97,1.70) | 1.49 (1.06,2.09) | 0.02 | ||
| Oil & vinegar dressing | 11.1 | 1(ref) | 1.25 (0.93,1.69) | 1.08 (0.80,1.44) | 1.15 (0.84,1.56) | 0.46 | ||
| Beef, pork or lamb | 6.3 | 1(ref) | 1.22 (0.92,1.62) | 1.24 (0.94,1.63) | 0.23 | |||
| Margarine | 3.6 | 1(ref) | 0.91 (0.67,1.23) | 1.06 (0.77,1.46) | 0.96 (0.66,1.40) | 0.86 (0.62,1.18) | 0.39 | |
| Cheese | 3.5 | 1(ref) | 0.74 (0.53,1.05) | 0.79 (0.60,1.05) | 1.07 (0.79,1.46) | 0.42 | ||
| PSA erab | ||||||||
| Walnutsc | 18.2 | 1(ref) | 1.16 (0.66,2.03) | 0.83 (0.41,1.66) | 0.72 | |||
| Mayonnaise | 5.7 | 1(ref) | 0.99 (0.72,1.36) | 0.50 (0.30,0.82) | 0.87 (0.54,1.41) | 0.08 | ||
| Light mayonnaise | 1.3 | 1(ref) | 1.04 (0.74,1.46) | 1.23 (0.86,1.77) | 0.59 (0.32,1.06) | 0.44 | ||
| Oil & vinegar dressing | 4.1 | 1(ref) | 1.28 (0.92,1.80) | 0.85 (0.61,1.19) | 0.98 (0.71,1.35) | 0.34 | ||
| Beef, pork or lamb | 0.4 | 1(ref) | 0.95 (0.72,1.25) | 0.95 (0.68,1.31) | 0.76 | |||
| Margarine | 1.9 | 1(ref) | 0.88 (0.66,1.17) | 0.69 (0.48,0.98) | 0.71 (0.44,1.13) | 0.66 (0.46,0.96) | 0.02 | |
| Cheese | 2.5 | 1(ref) | 1.02 (0.74,1.41) | 0.89 (0.65,1.21) | 0.75 (0.53,1.07) | 0.07 | ||
% contribution to ALA intake on the 1986 FFQ in the pre-PSA era and to ALA intake on the 2006 FFQ in the PSA era.
Multivariable models were adjusted for the same variables as multivariable model 1 in the Table 2.
Walnuts were not included in the FFQ until 1998.
Prudent and western dietary patterns are two major dietary patterns identified by factor analysis in the HPFS cohort to reflect overall dietary quality,22 and have been found to be associated with the risk of heart disease23 but not with risk of prostate cancer.24 ALA intake was modestly positively correlated with those two dietary patterns (Supplemental Figure 1). The correlation of ALA with western dietary pattern was higher than that with prudent pattern before 1994 but the pattern reversed thereafter. This finding raised the question if the positive association in the pre-PSA era but the lack of association in the PSA-era is the result of confounding by the "unhealthy" versus "healthy" sources of ALA. We conducted several analyses in the pre-PSA era to address this question. First, we found that neither prudent (comparing the top to the bottom quintile, HR = 1.01; 95% CI = 0.69 – 1.48; p trend=0.90) or western dietary pattern (HR = 1.16; 95% CI = 0.76 – 1.75; p trend=0.44) was associated with the risk of lethal prostate cancer. Second, at baseline, we separated ALA intake into animal and plant sources (such information was not available after 1986). Animal-sourced ALA (mean ± SD: 366 ± 145 mg) and plant-sourced ALA (745 ± 300 mg) were weakly inversely correlated (Pearson r = −0.22). The multivariable-adjusted HR comparing the top to the bottom quintile was 1.31 (95% CI = 0.94 – 1.84; p trend=0.06) for animal-sourced ALA and 1.49 (95% CI = 0.99 – 2.25; p trend=0.04) for plant-sourced ALA. When further mutually adjusting those two sources, the HR was 1.40 (95% CI = 1.00 – 1.97; p trend=0.02) for the animal-sourced ALA and 1.60 (95% CI = 1.05 – 2.43; p trend=0.02) for the plant-sourced ALA. Moreover, we found that none of the many fatty acids that were correlated with ALA, including trans 16:1 (Pearson r=0.26), trans 18:1 (r=0.08), cis 18:1 (r=0.25), trans 18:2 (r=0.17), cis 18:2 (r=0.41), total saturated fatty acids (r=0.30), was associated with lethal prostate cancer (Supplemental Figure 2). However, the association between intake of ALA and lethal prostate cancer persisted when adjusting for each fatty acid (Supplemental Figure 2). Finally, when we restricted to those participants who did not consume walnuts in the PSA era analysis, the HR for intake ALA comparing top to bottom quintile was 0.82 (95% CI = 0.54–1.23; p trend = 0.62; n=307 events) in the fully adjusted model.
Discussion
In the present study with 24 years follow-up, the association between higher intake of ALA and lethal prostate cancer was mainly evident in the pre-PSA era but not in the PSA era. This association in the pre-PSA era could not be attributed to obvious sources of confounding.
Lethal prostate cancer has been increasingly recognized as a more specific outcome to ensure comparability of results in the pre-PSA and PSA era.15,25–27 For example, in the HPFS we had found that dietary lycopene intake was similarly inversely associated with lethal prostate cancer in the pre-PSA and PSA eras, but results for total prostate cancer were heterogeneous by time period.28 Despite of focusing on lethal prostate cancers, we found that ALA intake was only related to the risk of lethal prostate cancer in the pre-PSA era. This difference could reflect methodological reasons or biological reasons. It is possible that the positive association in the pre-PSA era was largely due to confounding, because ALA intake in our cohorts, especially during early follow-up, was mainly derived from foods that often contained partially hydrogenated vegetable oils (e.g. mayonnaise, salad dressing, margarine), processed baked foods and red meat. However, several analyses could not identify a likely confounding factor. First, although the findings from several studies suggested that high meat rather than high ALA intake is responsible for the positive association with prostate cancer,12,13 in the present study, both intakes of animal-sourced and plant-sourced ALA were associated with lethal prostate cancer with an even stronger association for the plant-sourced ALA. Second, we found specificity for the positive association for intake of ALA among many other correlated fatty acids. Lastly, we excluded the possibility that intake of ALA is simply a marker for dietary patterns, because neither prudent nor western dietary pattern was associated with lethal prostate cancer in the pre-PSA era.
Alternatively, the reason for no association in the PSA era could be due to altered prostate cancer epidemiology influenced by PSA screening. Prostate cancers with lethal potential are diagnosed much earlier in their natural history and receive curative treatment. The European trial29 and Göteborg trial30 and one observational study that took advantage of a natural experiment in Sweden31 all showed a significant reduction in prostate cancer mortality by PSA screening. The PLCO trial had a null finding,32 but this trial compared less intensive with more intensive screening; moreover, contamination by intensive PSA screening in the control group may account for the null effect.33–35 Thus, the pre-PSA and PSA eras include a diverse mixture of lethal prostate cancers; in the pre-PSA era there are lethal cancers that would have been potentially curable had they been diagnosed early enough, while in the PSA era, these curable cancers would be removed from the pool of lethal cancer. If ALA only increases risk of a subset of lethal prostate cancers that is curable by treatment, the onset of widespread PSA screening could have largely removed the subset of lethal cancers through curative treatment, leaving only those incurable lethal cancers unrelated to ALA.
Another possibility is that the nature of the exposure “ALA” has changed over time. Trans ALA isomers are formed during partial hydrogenation, deep frying and industrial deodorization.36,37 European scientists found the presence of trans ALA in many foods (e.g. vegetable oils,36,38 low-calorie spreads39 and infant formulas40,41) and in human body composition.42–44 Up to 40% of ALA can be present as trans isomers.37,38. Pre-PSA era coincides with the same time period when the trans fat level were higher and also presumably a higher level of trans ALA compared to the PSA era. A downward trend in intake of total trans fat45 and in plasma levels46 over time was found which is largely due to changes made by food manufacturers in reducing partially hydrogenated oils. Trans ALA can be incorporated into plasma lipids and converted to trans long-chain polyunsaturated fatty acids in humans.44,47 Similarly, long-term feeding rats of a diet high in trans ALA resulted in a significant increase in trans Docosahexaenoic acid (DHA) and a decrease in cis DHA.48,49 Therefore, it is possible that high ALA intake in the pre-PSA era is a marker for trans ALA that promotes prostatic carcinogenesis by interfering with normal DHA function. The possibility related to trans ALA, coupled with bias related to PSA screening, may explain why the positive association was mainly evident in the pre-PSA era. However, this hypothesis related to trans ALA is novel and warrants further study.
Findings from previous prospective cohort studies that examined ALA intake and prostate cancer are mixed. However, stratifying by intensity of PSA screening and characterizing cancers by aggressiveness may offer more clarity. It has been argued that, in the PSA era, even prostate cancer outcomes such as high-grade or advanced stages are not good predictors for the lethal propensity.18,25,26,50 Four prospective studies51–54 that examined non-lethal prostate cancer in the PSA era found no associations, which is not surprising, and consistent with our previous findings in the HPFS.16–18 However, the large NIH-AARP study55 reported a modest (17% higher risk in the highest quintile) but significant positive association with advanced prostate cancer and a nonsignificant positive association with fatal prostate cancer despite widespread PSA screening. It is possible that the use of a single dietary questionnaire collected in the mid 1990’s may partially reflect the effects of pre-PSA diets. One study in Finland56 and anther in the Netherlands57 had no widespread PSA screening. Neither study found a significant association with incident prostate cancer. Possible explanations could be due to a short 6 years of follow-up and high proportion of non-lethal cancers among all incident prostate cancers, or different sources of ALA in those populations.
In addition to the potential for residual confounding, several other limitations in our study are worth noting. Random measurement error in the ALA exposure is inevitable and this would likely lead to an underestimation of the true association. However, we tried to minimize this bias by using cumulative average of multiple assessments of diets over long-period of time. Finally, our cohort consists of primarily white health professionals and results may not be generalizable to other populations. However, such homogeneity of study population minimized confounding by socioeconomical status and differential access to healthcare, and facilitated the high follow-up rate.
In conclusion, higher intake of ALA was associated with an increased risk of lethal prostate cancer in the pre-PSA era; however, ALA as currently consumed does not appear to be a risk factor for lethal prostate cancer. Our findings are important because ALA is considered an essential fatty acid and has important health benefits. Further studies are warranted to determine the causes for the differential associations by PSA era.
Supplementary Material
Novelty and Impact.
ALA is a popular and health-beneficial omega3 fatty acid. However, some prior studies reported a positive link between ALA intake and prostate cancer. We studied 48,000 men over two decades and found that higher intake of ALA was only associated with an increased risk of lethal prostate cancer in the pre-PSA era, but not in the PSA era. This means that current consumption of ALA is not related to developing lethal prostate cancer.
Acknowledgments
We would like to thank the participants and staff of the HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding
The study was funded by the National Institutes of Health (grant No. PO1 CA055075, CA141298, and CA13389, UM1 CA167552). KM Wilson was supported by Prostate Cancer Foundation Young Investigator Awards. The funding bodies had no influence in the design or conduct of the study, analysis and interpretation of the data, or preparation of the article.
Abbreviations
- ALA
α-linolenic acid
- LA
linoleic acid
- DHA
docosahexaenoic acid
- HR
hazard ratios
- CI's
confidence intervals
- n-3
omega-3
- HPFS
Health Professionals Follow-up Study
- FFQ's
food frequency questionnaires
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40:43–68. [PubMed] [Google Scholar]
- 3.Parkin D, Whelan SL, Ferlay J, Teppo L, Thomas D. Cancer incidence in five continents, International agency for research on cancer. Lyon: IARC Scientific Publications. 2003;155 [Google Scholar]
- 4.Yu H, Harris RE, Gao YT, Gao R, Wynder EL. Comparative epidemiology of cancers of the colon, rectum, prostate and breast in Shanghai, China versus the United States. Int J Epidemiol. 1991;20:76–81. doi: 10.1093/ije/20.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Dennis LK, Snetselaar LG, Smith BJ, Stewart RE, Robbins ME. Problems with the assessment of dietary fat in prostate cancer studies. Am J Epidemiol. 2004;160:436–444. doi: 10.1093/aje/kwh243. [DOI] [PubMed] [Google Scholar]
- 6.Nettleton JA. Omega-3 fatty acids: comparison of plant and seafood sources in human nutrition. J Am Diet Assoc. 1991;91:331–337. [PubMed] [Google Scholar]
- 7.Anderson BM, Ma DW. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009;8:33. doi: 10.1186/1476-511X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carleton AJ, Sievenpiper JL, de Souza R, McKeown-Eyssen G, Jenkins DJ. Case-control and prospective studies of dietary alpha-linolenic acid intake and prostate cancer risk: a meta-analysis. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu YQ, Zheng JS, Yang B, Li D. Effect of individual omega-3 fatty acids on the risk of prostate cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. J Epidemiol. 2015;25:261–274. doi: 10.2188/jea.JE20140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carayol M, Grosclaude P, Delpierre C. Prospective studies of dietary alpha-linolenic acid intake and prostate cancer risk: a meta-analysis. Cancer Causes Control. 2010;21:347–355. doi: 10.1007/s10552-009-9465-1. [DOI] [PubMed] [Google Scholar]
- 11.Simon JA, Chen YH, Bent S. The relation of alpha-linolenic acid to the risk of prostate cancer: a systematic review and meta-analysis. Am J Clin Nutr. 2009;89:1558S–1564S. doi: 10.3945/ajcn.2009.26736E. [DOI] [PubMed] [Google Scholar]
- 12.Ramon JM, Bou R, Romea S, Alkiza ME, Jacas M, Ribes J, Oromi J. Dietary fat intake and prostate cancer risk: a case-control study in Spain. Cancer Causes Control. 2000;11:679–685. doi: 10.1023/a:1008924116552. [DOI] [PubMed] [Google Scholar]
- 13.De Stefani E, Deneo-Pellegrini H, Boffetta P, Ronco A, Mendilaharsu M. Alpha-linolenic acid and risk of prostate cancer: a case-control study in Uruguay. Cancer Epidemiol Biomarkers Prev. 2000;9:335–338. [PubMed] [Google Scholar]
- 14.Bidoli E, Talamini R, Bosetti C, Negri E, Maruzzi D, Montella M, Franceschi S, La Vecchia C. Macronutrients, fatty acids, cholesterol and prostate cancer risk. Ann Oncol. 2005;16:152–157. doi: 10.1093/annonc/mdi010. [DOI] [PubMed] [Google Scholar]
- 15.Jahn JL, Giovannucci EL, Stampfer MJ. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era. Int J Cancer. 2015;137:2795–2802. doi: 10.1002/ijc.29408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CG, Willett WC. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 17.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 20.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 2017;185:570–584. doi: 10.1093/aje/kww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 23.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–921. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 24.Wu K, Hu FB, Willett WC, Giovannucci E. Dietary patterns and risk of prostate cancer in U.S. men. Cancer Epidemiol Biomarkers Prev. 2006;15:167–171. doi: 10.1158/1055-9965.EPI-05-0100. [DOI] [PubMed] [Google Scholar]
- 25.McCarty MF, Dinicolantonio JJ, Lavie CJ, O'Keefe JH. RE: Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2014;106:dju014. doi: 10.1093/jnci/dju014. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E. Commentary: Serum lycopene and prostate cancer progression: a re-consideration of findings from the prostate cancer prevention trial. Cancer Causes Control. 2011;22:1055–1059. doi: 10.1007/s10552-011-9776-x. [DOI] [PubMed] [Google Scholar]
- 27.Key TJ, Appleby PN, Travis RC, Albanes D, Alberg AJ, Barricarte A, Black A, Boeing H, Bueno-de-Mesquita HB, Chan JM, Chen C, Cook MB, Donovan JL, Galan P, Gilbert R, Giles GG, Giovannucci E, Goodman GE, Goodman PJ, Gunter MJ, Hamdy FC, Heliovaara M, Helzlsouer KJ, Henderson BE, Hercberg S, Hoffman-Bolton J, Hoover RN, Johansson M, Khaw KT, King IB, Knekt P, Kolonel LN, Le Marchand L, Mannisto S, Martin RM, Meyer HE, Mondul AM, Moy KA, Neal DE, Neuhouser ML, Palli D, Platz EA, Pouchieu C, Rissanen H, Schenk JM, Severi G, Stampfer MJ, Tjonneland A, Touvier M, Trichopoulou A, Weinstein SJ, Ziegler RG, Zhou CK, Allen NE. Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr. 2015;102:1142–1157. doi: 10.3945/ajcn.115.114306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zu K, Mucci L, Rosner BA, Clinton SK, Loda M, Stampfer MJ, Giovannucci E. Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst. 2014;106:djt430. doi: 10.1093/jnci/djt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Paez A, Maattanen L, Bangma CH, Aus G, Carlsson S, Villers A, Rebillard X, van der Kwast T, Kujala PM, Blijenberg BG, Stenman UH, Huber A, Taari K, Hakama M, Moss SM, de Koning HJ, Auvinen A. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, Pihl CG, Stranne J, Holmberg E, Lilja H. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stattin P, Carlsson S, Holmstrom B, Vickers A, Hugosson J, Lilja H, Jonsson H. Prostate cancer mortality in areas with high and low prostate cancer incidence. J Natl Cancer Inst. 2014;106:dju007. doi: 10.1093/jnci/dju007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Isaacs C, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hsing AW, Izmirlian G, Pinsky PF, Kramer BS, Miller AB, Gohagan JK, Prorok PC. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulati R, Tsodikov A, Wever EM, Mariotto AB, Heijnsdijk EA, Katcher J, de Koning HJ, Etzioni R. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer Causes Control. 2012;23:827–835. doi: 10.1007/s10552-012-9951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stampfer MJ, Jahn JL, Gann PH. Further evidence that prostate-specific antigen screening reduces prostate cancer mortality. J Natl Cancer Inst. 2014;106:dju026. doi: 10.1093/jnci/dju026. [DOI] [PubMed] [Google Scholar]
- 35.Shoag JE, Mittal S, Hu JC. Reevaluating PSA Testing Rates in the PLCO Trial. N Engl J Med. 2016;374:1795–1796. doi: 10.1056/NEJMc1515131. [DOI] [PubMed] [Google Scholar]
- 36.Wolff RL. Heat-induced geometrical isomerization of alpha-linolenic acid: effect of temperature and heating time on the appearance of individual isomers. J Am Oil Chem Soc. 1993;70:425–430. [Google Scholar]
- 37.Wolff RL. Further studies on artificial geometrical isomers of alpha-linolenic acid in edible linolenic acid-containing oils. J Am Oil Chem Soc. 1993;70:219–224. [Google Scholar]
- 38.Wolff RL. trans-Polyunsaturated fatty acids in French edible rapeseed and soybean oils. J Am Oil Chem Soc. 1992;69:106–110. [Google Scholar]
- 39.Wolff RL, Sebedio JL. Geometrical isomers of linolenic acid in low-calorie spreads marketed in France. J Am Oil Chem Soc. 1991;68:719–725. [Google Scholar]
- 40.Chardigny JM, Wolff RL, Mager E, Bayard CC, Sebedio JL, Martine L, Ratnayake WMN. Fatty acid composition of French infant formulas with emphasis on the content and detailed profile of trans fatty acids. J Am Oil Chem Soc. 1996;73:1595–1601. [Google Scholar]
- 41.Ratnayake WMN, Chardigny JM, Wolff RL, Bayard CC, Sebedio JL, Martine L. Essential fatty acids and their trans geometrical isomers in powdered and liquid infant formulas sold in Canada. Journal of Pediatric Gastroenterology and Nutrition. 1997;25:400–407. doi: 10.1097/00005176-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Chardigny JM, Wolff RL, Mager E, Sebedio JL, Martine L, Juaneda P. Trans mono- and polyunsaturated fatty acids in human milk. Eur J Clin Nutr. 1995;49:523–531. [PubMed] [Google Scholar]
- 43.Chen ZY, Pelletier G, Hollywood R, Ratnayake WMN. Trans fatty acid isomers in Canadian human milk. Lipids. 1995;30:15–21. doi: 10.1007/BF02537037. [DOI] [PubMed] [Google Scholar]
- 44.Sebedio JL, Vermunt SH, Chardigny JM, Beaufrere B, Mensink RP, Armstrong RA, Christie WW, Niemela J, Henon G, Riemersma RA. The effect of dietary trans alpha-linolenic acid on plasma lipids and platelet fatty acid composition: the TransLinE study. Eur J Clin Nutr. 2000;54:104–113. doi: 10.1038/sj.ejcn.1600903. [DOI] [PubMed] [Google Scholar]
- 45.Honors MA, Harnack LJ, Zhou X, Steffen LM. Trends in fatty acid intake of adults in the Minneapolis-St Paul, MN Metropolitan Area, 1980–1982 through 2007–2009. J Am Heart Assoc. 2014;3:e001023. doi: 10.1161/JAHA.114.001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwenke DC, Foreyt JP, Miller ER, 3rd, Reeves RS, Vitolins MZ. Plasma concentrations of trans fatty acids in persons with type 2 diabetes between September 2002 and April 2004. Am J Clin Nutr. 2013;97:862–871. doi: 10.3945/ajcn.112.046508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermunt SH, Beaufrere B, Riemersma RA, Sebedio JL, Chardigny JM, Mensink RP. Dietary trans alpha-linolenic acid from deodorised rapeseed oil and plasma lipids and lipoproteins in healthy men: the TransLinE Study. Br J Nutr. 2001;85:387–392. doi: 10.1079/bjn2000270. [DOI] [PubMed] [Google Scholar]
- 48.Acar N, Bonhomme B, Joffre C, Bron AM, Creuzot-Garcher C, Bretillon L, Doly M, Chardigny JM. The retina is more susceptible than the brain and the liver to the incorporation of trans isomers of DHA in rats consuming trans isomers of alpha-linolenic acid. Reprod Nutr Dev. 2006;46:515–525. doi: 10.1051/rnd:2006033. [DOI] [PubMed] [Google Scholar]
- 49.Acar N, Chardigny JM, Bonhomme B, Almanza S, Doly M, Sebedio JL. Long-term intake of trans (n-3) polyunsaturated fatty acids reduces the b-wave amplitude of electroretinograms in rats. J Nutr. 2002;132:3151–3154. doi: 10.1093/jn/131.10.3151. [DOI] [PubMed] [Google Scholar]
- 50.Giovannucci E. Does prostate-specific antigen screening influence the results of studies of tomatoes, lycopene, and prostate cancer risk? J Natl Cancer Inst. 2007;99:1060–1062. doi: 10.1093/jnci/djm048. [DOI] [PubMed] [Google Scholar]
- 51.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer. 2007;121:1339–1345. doi: 10.1002/ijc.22805. [DOI] [PubMed] [Google Scholar]
- 52.Koralek DO, Peters U, Andriole G, Reding D, Kirsh V, Subar A, Schatzkin A, Hayes R, Leitzmann MF. A prospective study of dietary alpha-linolenic acid and the risk of prostate cancer (United States) Cancer Causes Control. 2006;17:783–791. doi: 10.1007/s10552-006-0014-x. [DOI] [PubMed] [Google Scholar]
- 53.Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E. A prospective study on dietary fat and incidence of prostate cancer (Malmo, Sweden) Cancer Causes Control. 2007;18:1107–1121. doi: 10.1007/s10552-007-9050-4. [DOI] [PubMed] [Google Scholar]
- 54.Bassett JK, Severi G, Hodge AM, MacInnis RJ, Gibson RA, Hopper JL, English DR, Giles GG. Plasma phospholipid fatty acids, dietary fatty acids and prostate cancer risk. Int J Cancer. 2013;133:1882–1891. doi: 10.1002/ijc.28203. [DOI] [PubMed] [Google Scholar]
- 55.Pelser C, Mondul AM, Hollenbeck AR, Park Y. Dietary fat, fatty acids, and risk of prostate cancer in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2013;22:697–707. doi: 10.1158/1055-9965.EPI-12-1196-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mannisto S, Pietinen P, Virtanen MJ, Salminen I, Albanes D, Giovannucci E, Virtamo J. Fatty acids and risk of prostate cancer in a nested case-control study in male smokers. Cancer Epidemiol Biomarkers Prev. 2003;12:1422–1428. [PubMed] [Google Scholar]
- 57.Schuurman AG, van den Brandt PA, Dorant E, Brants HA, Goldbohm RA. Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study. Cancer. 1999;86:1019–1027. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.