Abstract
Background
Lifestyle physical activity (i.e. moderate physical activity during routine daily activities most days of the week) may benefit HIV-positive adults who are at high risk for cardiovascular disease.
Objective
To describe lifestyle physical activity patterns in HIV-positive adults and to examine the influence of lifestyle physical activity on markers of cardiovascular health. Our secondary objective was to compare these relationships between HIV-positive adults and well-matched HIV-uninfected adults.
Methods
One-hundred and nine HIV-positive adults and 20 control participants wore an ActiGraph accelerometer, completed a maximal graded cardiopulmonary exercise test, a coronary CT, anthropomorphic measures, and had lipids and measures of insulin resistance measured from peripheral blood.
Results
Participants (n=129) had a mean age of 52 (±7.3) years, 64% were male (n=82), and 88% African American (n=112 of 129). On average, HIV-positive participants engaged in 33 minutes of moderate-to-vigorous physical activity per day (IQR: 17, 55) compared to 48 minutes in controls (IQR 30,62; p=0.05). HIV-POSITIVE adults had poor fitness (VO2 peak: 16.8 (±5.2) ml/min/kg and VE/VCO2: 33.1 (4.6). A marker of HIV disease (current CD4+ T cell) was associated with reduced VO2peak (r=−0.20; p<0.05) and increased insulin resistance (r=0.25, p<0.01), but not with physical activity or other markers of cardiovascular health (p’s≥0.05). After controlling for age, gender, BMI, and HIV status, physical activity was not significantly associated with VO2 peak or VE/VCO2.
Conclusions
HIV-positive adults have poor physical activity patterns and diminished cardiovascular health. Future longitudinal studies should examine whether HIV infection blunts the beneficial effects of physical activity on cardiovascular health.
Keywords: HIV, Exercise, Cardiovascular Diseases, Exercise Test
Approximately 1.2 million people are living with HIV (PLWH) in the United States, and there are over 30 million PLWH worldwide. HIV antiretroviral therapy (ART) has significantly increased the life expectancy among PLWH.1,2 Long term HIV infection, HIV treatment, and lifestyle factors have led to PLWH experiencing age-related comorbid conditions earlier and more frequently than HIV-uninfected individuals.3 Specifically, PLWH experience chronic inflammation, long-term use of ART, and higher rates of lifestyle risk factors which increase their risk for cardiovascular disease (CVD)3 and create an urgent need for interventions that reduce this risk.
Physical activity includes activities performed as part of daily life (e.g. walking), as well as planned, more vigorous physical activity.4 Seminal research has shown that physical activity can improve cardiovascular and metabolic health.5 Conversely, physical inactivity is associated with poor glycemic control and reduced triglyceride clearance, resulting in an increased risk of mortality and cardiometabolic complications.6 Further, PLWH also have higher rates of insulin resistance, fatigue, pain, depression, and smoking and alcohol use, compounding their risk for CVD.7–13 PLWH can benefit tremendously from physical activity, but their objectively-measured physical activity patterns, and the influence of those patterns on cardiovascular health, are not well understood. The aims of this study were to describe physical activity patterns in PLWH who did not meet AHA recommended guidelines for physical activity, and to examine the relationship of these patterns to markers of cardiovascular health. Furthermore, we aimed to compare these relationships between PLWH and a well-matched HIV-uninfected control group.
METHODS
Design
These data derive from a cross-sectional analysis of baseline data from a clinical trial (parent study) testing the effect of a self-management intervention on exercise and cardiovascular outcomes in a group of PLWH, compared with well-matched HIV-uninfected control participants (NCT02553291).
Sample and Recruitment
One-hundred and nine PLWH and 20 well-matched control participants were recruited via IRB-approved letters to an HIV research registry and flyers posted in HIV care organizations in Cleveland, Ohio. HIV uninfected participants were recruited using ResearchMatch and flyers posted in primary care clinics in Cleveland, Ohio. Those interested in participating telephoned a Research Assistant who screened callers for eligibility. HIV-uninfected participants were matched to PLWH on race, sex, age (± 3years). All participants had to be >18 years of age and at high risk for developing CVD (Framingham 30-year CVD risk score>20% for females and >30% for males). If prescribed a statin medication, participants had to be taking it for at least 6 months. Additionally, PLWH had to be on antiretroviral therapy with suppressed HIV-1 viremia (<400 copies/mL) for at least one year prior to enrollment. Potential participants were excluded if they: 1) had a medical contraindication for exercise,14 2) met weekly physical activity recommendations of 150 minutes of moderate-to-vigorous physical activity15 (assessed using the 7-day physical activity recall16), 3) were unable to understand spoken English, 4) expected to move out of the immediate area, have surgery or were pregnant or planned on becoming pregnant in the next 6 months, 5) were diabetic (hgA1c>7%), or 6) were enrolled in a weight loss program.
Eligible participants were invited to an initial visit where study staff reviewed study purpose, procedures, risk and potential benefits with them. After confirming understanding, those wishing to proceed signed an informed consent document, completed a blood draw and, if a woman of childbearing age, a urine pregnancy test. The IRB at University Hospitals, Cleveland Medical Center approved this study.
Procedures and Measures
Demographics and HIV Characteristics
All participants completed a self-reported demographic survey assessing gender, race, education and monthly income.17 A research assistant helped those who were unable to complete the self-administered computer survey. Participants also consented to medical chart abstraction from which study staff abstracted medical data including years living with HIV, current CD4+ T cell count and CD4+ T cell nadir.
Physical Activity
Participants were given an ActiGraph GT3X/+ accelerometer (ActiGraph, LLC, Fort Walton Beach, FL).18–20 Participants were instructed to wear the accelerometer during all waking hours for 7 consecutive days, except for when showering and swimming. A research assistant affixed the monitor to adjustable elastic belts and placed it over the participant’s non-dominant hip, and counseled the participant on the importance of wearing it every day. A research assistant called each participant two days after they received the devices to check if they were wearing them correctly, address concerns, and remind them return it in one week. When participants returned the accelerometer, we checked to ensure that data met the minimum quality standards (at least 3 days and at least 10 hours per day).21–23 Those not meeting standards were asked to re-wear it for 7 days. Accelerometer data were processed according to recommendations for adults and were sampled at 30Hz, using 60 second epochs and the normal filter.24 Consistent with Caspersen’s (1985) definition, activity ≥ 2 metabolic equivalents (METS) and ≥ 10 minutes was defined as exercise.25 We used the ActiLife software to calculate the amount of time spent in light, moderate, vigorous and moderate-to-vigorous physical activity per valid day using the Freedson (1998) adult calculation.26,27
Blood Pressure and Body Mass Index (BMI)
Participants were escorted to a clinical research unit where trained research nurses measured their height, weight, and vital signs. Each participant’s height was measured to the nearest 0.1cm by asking him or her to stand straight up against a stadiometer platform with shoes off. After removing everything but a light layer of clothing, the participant stepped on a scale and weight was measured to the nearest kilogram. BMI was calculated by dividing weight in kilograms by height in meters squared.
Cardiovascular Health
Our measures of cardiovascular health included cardiorespiratory fitness, cardiometabolic health indicators (see serum laboratory measures), and cardiac computed tomography (CT) scans. Cardiopulmonary exercise tests were performed using a computer-controlled Lodi bicycle ergometer (Groninger, Netherlands) with a MGC Diagnostics Cardiopulmonary Express system (MGC Diagnostics, St. Paul, MN). A trained investigator performed all of the tests using a 20 watt/minute ramp protocol. We measured cardiorespiratory fitness using a peak oxygen uptake (peak VO2) measure. Peak VO2 was defined as the maximal value of VO2 during the final 30 seconds of exercise. The Wasserman-Hansen equation28 was used to determine the percent of predicted peak VO2. Ventilatory efficiency (VE/VCO2 slope) was determined by the linear regression slope of the minute ventilation (VE) and VCO2.29 Anaerobic threshold was manually calculated using the Beaver-Wasserman V-slope method.30
All participants underwent a non-contrast CT scan of the chest for coronary artery calcium scoring. A single reader (blinded to treatment assignment and participant characteristics) quantified total coronary calcium score using the Agatston method.31 All scans were performed on a 64-slice multidetector CT scanner (Somatom Sensation 64, Siemens Medical Solutions USA) with 30×0.6mm collimation, 330ms rotation time, and 120kV tube voltage. Three-millimeter slices were obtained from the carina to the diaphragm with prospective ECG gating at 60% of the R-R interval. Calcified coronary lesions were defined as areas of ≥6 pixels with density >130 Hounsfield units (HU).
Serum Laboratory Measures
Serum studies were used to evaluate cardiometabolic health indicators and inflammation. All participants underwent a 12-hour fasting blood draw at the clinical research unit where a trained phlebotomist drew approximately 20 mL of blood. Serum measures of HgA1c, glucose, insulin, and hsCRP were analyzed fresh samples using standard clinical procedures and commercially available assays at the hospitals lab. We used participants’ fasting glucose and insulin measures to calculate the homeostatic model assessment of insulin resistance (HOMA-IR) for each individual.32 IL-6 levels were measured in batch from plasma stored at −80 degrees C using the IL-6 Quantikine HS ELISA Kit from R&D Systems, INC (Minneapolis, MN). All assays were conducted according to the manufactures’ instructions.
DATA ANALYSIS
All statistical analyses were performed using Stata version 14.0 (College Station, Texas). Data were cleaned and met assumptions for inferential statistics. We analyzed demographic, HIV, physical activity and cardiovascular health characteristics by decade of age and HIV status. Categorical variables were summarized using frequencies and percentages. Continuous variables, depending on their distribution, were summarized with either means and standard deviations or medians and interquartile ranges. We used Pearson’s correlation coefficient to analyze the relationships between physical activity and cardiovascular health, as well as HIV biomarkers in the PLWH. We used adjusted linear regression to identify independent associations between physical activity, cardiovascular health indicators, and HIV status. We adjusted for clinically relevant covariates known to influence these relationships, including age, gender, BMI, and IL-6.33
RESULTS
Demographics and HIV Characteristics
A total of 109 PLWH and 20 well-matched HIV uninfected participants were enrolled in the study. Among PLWH, 70 (64%) were male, 94 (86%) were African American, 56 (51% had a high school degree or less education, and 9 (8%) were employed. PLWH were less likely to be employed (8% vs. 35%), engage in less moderate daily physical activity (33.3 vs 47.8 minutes), had less ventilatory efficiency (33.1 vs. 30.2), and had a lower systolic blood pressure (124 mmHg vs132 mmHG), compared to the controls. Otherwise, there were no statistically significant differences between the two groups. Demographic, HIV and inflammatory characteristics are summarized in Table 1.
Table 1.
Demographic, HIV, and Physical Activity Characteristics of the Sample
| HIV-Infected Subjects (n=109) |
HIV uninfected subjects (n=20) |
p-value1 | ||
|---|---|---|---|---|
| Age in years (Range 31–71) | 52.8 (7.27) | 49.6 (6.86) | 0.06 | |
| Male (%)2 | 70 (64) | 12 (60) | 0.703 | |
| Race (%) | ||||
| African American | 94 (86) | 19 (95) | 0.273 | |
| Caucasian/White/Other | 15 (14) | 1 (5) | ||
| Education (%) | ||||
| High School or less | 56(51) | 9 (45) | 0.443 | |
| Two years or less of college/advanced training | 37 (34) | 5 (25) | ||
| College degree or higher | 16 (15) | 6 (30) | ||
| Employed (%) | 9(8) | 7 (35) | <0.013,4 | |
| Monthly Income | ||||
| >$600 | 20 (18) | 5 (25) | 0.833 | |
| $600–999 | 54 (49) | 9 (45) | ||
| ≥ $1000 | 34 (31) | 6 (30) | ||
| HIV and Inflammation Characteristics | ||||
| Years since HIV diagnosis | 15.6 (7.74) | n/a | ||
| Years taking HIV antiretroviral medication | 12.5 (6.12) | n/a | ||
| Current CD4+T cell count (cells/µL) | 703.8 (404.4) | n/a | ||
| CD4+T cell count nadir (cells/µL) | 191.9 (170.0) | n/a | ||
| Il-6 | 3.17 (2.46) | 2.89 (1.81) | 0.64 | |
| Median hsCRP (IQR) | 1.75 (0.7, 4.3) | 1.95 (0.9, 6.6) | 0.373 | |
| Physical Activity Characteristics | ||||
| Engaged in any moderate-to-vigorous physical activity during the past week | 90 (83%) | 19(95%) | 0.203 | |
| Steps per day | 6,537 (3920) | 7,040 (2265) | 0.53 | |
| Median minutes of light physical activity (IQR) per day | 753.6 (706,845) | 752 (684,830) | 0.733 | |
| Median minutes of moderate physical activity (IQR) per day | 33.3 (17, 55) | 47.8 (30,62) | 0.053,4 | |
| Median minutes of vigorous physical activity (IQR) per day | 0 (0,0) | 0 (0,0) | 0.823 | |
| Median minutes of moderate-to-vigorous physical activity (IQR) per day | 35.1 (18, 58) | 55.2 (31,65) | 0.063 | |
| Markers of Cardiometabolic Health | ||||
| VO2 peak achieved (ml/min/kg) | 16.77 (5.2) | 16.85 (5.85) | 0.94 | |
| Predicted VO2 peak (ml/min/kg) | 29.68 (15.58) | 25.61 (7.46) | 0.26 | |
| VO2 peak at anaerobic threshold (ml/min/kg) | 10.39 (4.04) | 10.55 (2.94) | 0.87 | |
| VE/VCO2 | 33.10 (4.64) | 30.2 (2.50) | 0.034 | |
| Percent Peak VO2 at anaerobic threshold (VO2 at AT/VO2Max) | 62% | 63% | ||
| Peak Work Achieved (watts) | 93.6 (43.4) | 111.35 (42.50) | 0.09 | |
| Peak RER | 1.07 (0.11) | 1.08 (0.13) | 0.70 | |
| Number of subjects achieving RER ≥ 1.0 (%) | 84 (76) | 17 (85) | 0.565 | |
| Median Coronary Calcium Score (IQR) | 0 (0,66) | 0 (0,170) | 0.535 | |
| Coronary Calcium Score >0 (%) | 40 (36) | 8 (40) | 0.805 | |
| Blood Pressure (mm Hg) | 124/80 | 132/83 | 0.044 | |
| Body Mass Index (kg/m2) | 29.3 (8.16) | 32.5 (7.65) | 0.11 | |
| Hip-Waist Circumference | 0.94 (0.08) | 0.94 (0.09) | 0.72 | |
| Median HOMA-IR (IQR) | 3.08 (1.7, 4.6) | 3.81 (1.0, 5.6) | 0.89 | |
Analyzed using T-tests, unless otherwise noted;
There were 4 individuals who identified as transgender in the PLWH group and 1 in the control group;
Due to the distribution of the data, differences between PLWH and controls were analyzed using Wilcoxon rank-sum tests;
Difference between PLWH and control groups was ≤ 0.05;
Frequency data were analyzed using Fisher’s 2-sided exact statistic
Physical Activity
A total of 90 PLWH (83%) and 19 (95%) control participants engaged in any moderate-to-vigorous physical activity (MVPA) in the past week. The median engagement in MVPA per day was 35.1 (IQR: 18, 58) minutes in PLWH and 55.2 (IQR 31,65; p=0.06) in controls. Nearly all physical activity was done at moderate intensity.
Cardiovascular Health
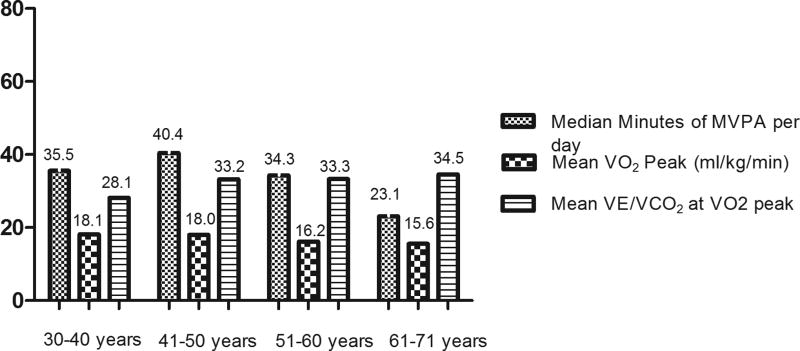
The average peak VO2 achieved (mL/kg/min) for PLWH was 16.8 (±5.2) vs 16.9 (±5.9) for the control group.VE/VC02 for PLWH was 33.1 (4.6) compared to 30.2 (2.5) for the control group. In contrast to exercise, peak VO2 achieved didn’t change with age (Figure 1). Forty (36%) PLWH had a coronary calcium score greater than 1 compared to 8 (40%) in the control group (Table 1). Among PLWH, step counts were associated with decreased IL-6 (r=−0.266, p<0.05), improved VO2peak (r=0.342, p<0.05), and reduced insulin resistance (r=−0.215, p<0.05). There were no other associations between physical activity and markers of cardiovascular health.
Figure 1.
Exercise and Fitness Characteristics of PLHIV
Current CD4+ T cell count and CD4+ T cell nadir are important health indicators for PLWH, but were not associated with physical activity. However, current CD4+T cell count was associated with reduced VO2peak (r=−0.199, p<0.05) and elevated insulin resistance (r=0.248, p<0.05) (Table 2).
Table 2.
Correlations between exercise, Immune characteristics, and markers of Cardiovascular health in people living with HIV1
| Step Counts | Moderate-to- vigorous physical activity |
Il-6 | CD4 + T cell Count(cells/µL) |
CD4 + T cell Nadir(cells/µL) |
VO2 peak achieved (mL/kg/min) |
VO2 peak VE/VCO2 |
|
|---|---|---|---|---|---|---|---|
| Step Counts per Day | 1.0 | 0.81* | |||||
| Minutes of moderate-vigorous physical activity | 0.812 | 1.0 | |||||
| Il-6 | −0.2662 | −0.111 | 1.0 | ||||
| CD4 + T cell Count(cells/µL) | −0.074 | −0.137 | 0.190 | 1.0 | |||
| CD4 + T cell Nadir(cells/µL) | 0.070 | −0.112 | 0.044 | 0.4042 | 1.0 | ||
| VO2 peak achieved (ml/min/kg) | 0.3422 | −0.2042 | −0.2572 | −0.1993 | 0.010 | 1.0 | |
| VO2 peak VE/VCO2 | 0.060 | −0.128 | 0.056 | 0.059 | −0.014 | −0.129 | 1.0 |
| CAC score | 0.172 | 0.121 | 0.202 | −0.029 | −0.115 | −0.159 | 0.188 |
| Systolic Blood Pressure | 0.107 | 0.071 | 0.167 | 0.029 | −0.010 | 0.026 | −0.004 |
| Diastolic Blood Pressure | 0.100 | 0.146 | 0.120 | 0.00 | 0.016 | 0.096 | −0.113 |
| Total Cholesterol | −0.102 | −0.121 | −0.108 | 0.018 | 0.103 | −0.185 | 0.140 |
| HOMA-IR | −0.2153 | −0.176 | 0.161 | 0.2482 | 0.033 | −0.327* | −0.072 |
All associations were analyzed using Pearson Correlation Coefficient;
p≤ 0.05;
p≤ 0.01
The regression analyses indicated that (controlling for age, gender, BMI, and IL-6), physical activity was not significantly associated VO2 peak (Overall Model Adjusted R2=0.42, p<0.01), peak VE/VC02 (Table 3) or coronary calcium score (not shown). However, PLWH exhibited less ventilatory efficiency than controls and our interaction coefficient (β=−1.944, p=0.317) suggests that physical activity may help to mitigate this decreased ventilator efficiency (Table 3).
Table 3.
Quintile regression of physical activity and select covariates on peak VE/VCO2
| Covariates | β | SE(β) | p-value | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Physical Activity (30 minutes/day) | 1.845 | 1.86 | 0.321 | −1.83 | 5.523 |
| HIV Infection | 4.314 | 3.3299 | 0.198 | −2.300 | 10.928 |
| Age per decade | 0.713 | 0.748 | 0.343 | −0.772 | 2.198 |
| Female | 0.558 | 1.348 | 0.680 | −2.119 | 3.236 |
| Transgender | 2.625 | 2.631 | 0.321 | −2.601 | 7.851 |
| Body-Mass-Index (kg/m2) | −0.139 | 0.080 | 0.084 | −0.298 | −0.019 |
| IL-6 | 0.139 | 0.263 | 0.597 | −0.383 | 0.661 |
| HIV × Physical Activity (30 minutes/day) | −1.944 | 1.932 | 0. 317 | −5.781 | 1.893 |
| Constant | 27.309 | 5.721 | <0.000 | 15.944 | 38.674 |
DISCUSSION
Our study is among the first to prospectively examine the relationships between objectively-measured lifestyle physical activity and cardiovascular health in PLWH compared HIV-uninfected controls. We produced several important findings in this study: (1) PLWH engaged in low amounts of physical activity, (2) PLWH achieved low levels of peak VO2 and elevated levels of VE/VCO2, suggesting that HIV may attenuate the effects of physical activity on cardiovascular fitness, and (3) HIV biomarkers (CD4+ T cells and CD4+ T cell nadir) were not associated with physical activity but were associated with peak VO2 achieved. Furthermore, as our sample was largely African American, our work provides evidence that should be considered when developing targeted interventions to this high-risk and often underserved group.
While PLWH engaged in a median of 35 minutes of moderate activity per day, exceeding daily AHA recommendations, almost no one who engaged in vigorous activity suggesting that this is not exercise but rather activity conducted in the course of their activities of daily living. Our results are similar to those in a recent meta-analysis conducted by Vancampfort et al.34 The authors examined self-reported physical activity levels using MET minutes per week in 3,780 PLWH.34 They found that PLWH engaged in mostly light activity (72.8 min/week), followed by moderate (61 min/week), and vigorous activity (12.4 min/week). Approximately 50% of PLWH met recommended physical activity levels (≥150 min/week of MVPA) and no comparisons were made to controls.34 Conversely, in the Multicenter AIDS Cohort Study (MACS),35 physical activity levels of PLWH and non-infected controls (n=1,281) were measured using the International Physical Activity Questionnaire (IPAQ).36 Compared to Vancampfort’s et al. and our results, they found that PLWH engaged in higher levels of MVPA and physical activity behaviors were similar to controls.35 Among MACS participants, HIV infection and low levels of physical activity were associated with higher insulin resistance, further demonstrating the importance of physical activity to cardiometabolic health in PLWH.35,37
Our results build on previous research by providing both objectively-measured physical activity and a well-matched comparison group. The total amount of MVPA our participants engaged in was approximately 30% less that that reported by controls or by MVPA totals in Vancamfort et al’s (2017) review. There are several possible explanations for our comparatively low level of physical activity. First, by using an ActiGraphs, we minimized the risk of over-reporting and behavior change bias associated with visual feedback seen in other wearable devices.38 Second, we enrolled participants who did not meet physical activity recommendations to target those most likely to benefit from an intervention. This may have blunted the overall amount of observed physical activity. However, of the 204 participants screened for this study, only 10 were excluded because they exceeded weekly physical activity recommendations. It is possible that while low, the physical activity patterns observed in our study may be reflective of the PLWH ages 40 and older. Finally, our sample is older than those previously analyzed, and physical activity levels decrease as PLWH age, highlighting the need for increased physical activity interventions in older PLWH.
Given these low levels of physical activity, it was unsurprising to find that some cardiovascular parameters of health were lower in PLWH compared with healthy controls. The peak VO2 achieved for all PLWH was 16.8 ml/min/kg (±5.2) and the VE/VCO2 was 33.1, indicating impaired CO2 removal and a higher risk for heart failure-related mortality.39 Peak VO2 declined and VE/VCO2 increased with age. Similarly, Vancamfort et al. (2016) found that the average peak VO2 among PLWH was 26.4 ml/min/kg, among the lowest levels of those living with chronic diseases.40 Further, the average VO2 peak among healthy adults was 34 (ages 40–59) ml/min/kg ,41 significantly higher that our observed values. These data suggest that PLWH have poor cardiovascular fitness, which may underlie much of the increased CVD burden experienced by this population. These data also illustrate the critical importance of assessing cardiovascular fitness in this population both clinically and as a research variable.42
Cardiovascular health can be improved though regular, moderate-to-vigorous lifestyle physical activity. Recently, O’Brien et al.10 found that combined aerobic and resistance interventions at least three times per week for at least five weeks can significantly improve cardiorespiratory fitness (Peak VO2 Δ ≥ 2 mL/min/kg). However, studies testing home-based interventions among PLWH have largely been unsuccessful, failing to produce meaningful change in physical activity in this population.43–45 This suggests that these interventions were not well targeted to PLWH. Additional, rigorous, developmental research is needed to better understand how to improve physical activity in this population.
Finally, we observed a relationship between CD4+T cell count and VO2peak (r=−0.199, p<0.05). This finding is in contrast to recent studies that have investigated cardiovascular fitness variables and markers of HIV. In O’Brien’s et al. (2017) meta-analysis, for example, five experimental studies examined peak VO2 and markers of HIV, including CD4 T-cell count, nadir CD4 count, and HIV viral load. Consistently, studies showing improved peak VO2 in PLWH had relative stability in markers of HIV. No direct relationship between these variables was observed. However, indirect relationships between these variables have been documented, particularly those related to chronic inflammation experienced by PLWH.34 Our results showed that PLWH had higher levels of the inflammatory cytokine IL-6 compared with controls, and that elevated IL-6 levels, were associated with fewer step per day and diminished peak VO2 in PLWH. While inflammation is not unique to PLWH, HIV infection results in a state of elevated inflammation, particularly when HIV is poorly controlled. As such, higher levels of inflammation experienced by PLWH may play a role in their inability to achieve a similar peak VO2 to controls. This may occur because elevated IL-6 is associated with increased fatigue, limiting how much PLWH can engage in physical activity and exercise. However, recent evidence suggests that physical activity may lead to decreased levels of inflammation,46,47 which may, in part, account for improved peak VO2 among PLWH who exercise. This evidence suggests that there is a complex interplay between factors that influence HIV disease progression, and its ultimate impact on cardiovascular health.
Limitations
Our study has several strengths including prospective data collection using objective measures of physical activity and cardiovascular fitness. However, we have several noteworthy limitations. First, as a cross-sectional study, we were unable to infer causality. Second, this is a single site study, and though we found novel, statistically significant relationships, our findings should be examined in a representative multi-site sample before implementing clinical changes based on our findings. Finally, although consistent with other studies, our results may reflect low levels of physical activity related to aging, as many of our participants were older adults living with HIV.
CONCLUSION
Increasingly, PLWH are aging and developing CVD. Given the complexities of HIV disease, non-pharmacological strategies to mitigate this CVD risk are needed. Physical activity is an evidence-based strategy known to prevent CVD. Our data demonstrate that aging PLWH have poor physical activity patterns and diminished cardiovascular health. Increasing the amount and intensity of lifestyle physical activity is likely to improve aspects of cardiovascular health in this population, especially cardiovascular fitness. However, the relationship between physical activity and cardiovascular health is complex and warrants further study.
Figure 2.

Acknowledgments
CTL has received honoraria from Gilead Sciences and has received research grants from Bristol-Myers Squibb and Medtronic Global Health Foundation.
This project was funded by grants from the American Heart Association (14CRP20380259) and a developmental grant from the University Hospitals/Case Western Reserve University Center for AIDS Research (National Institutes of Health Grant # P30 AI036219)
Footnotes
Conflicts of Interest:For the remaining authors none were declared.
Contributor Information
Allison R. Webel, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA.
Joseph Perazzo, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA.
Christopher T. Longenecker, Harrington Heart and Vascular Institute, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Trevor Jenkins, Harrington Heart and Vascular Institute, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Abdus Sattar, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA.
Margaret Rodriguez, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA.
Nate Schreiner, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA.
Richard A. Josephson, Harrington Heart and Vascular Institute, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
References
- 1.Trickey A, May MT, Vehreschild J-J, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. The Lancet HIV. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HIV Among People Aged 50 and Over. 2016 2015, at https://www.cdc.gov/hiv/group/age/olderamericans/index.html.)
- 3.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clinical Infectious Diseases. 2011:cir627. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 4. [Accessed February 25, 2017];Measuring Physical Activity. 2017 2017, at https://www.hsph.harvard.edu/nutritionsource/mets-activity-table/.)
- 5.Lin X, Zhang X, Guo J, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. Journal of the American Heart Association. 2015;4:e002014. doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Physical Activity. 2017 2017, at https://www.healthypeople.gov/2020/topics-objectives/topic/physical-activity.)
- 7.Webel A, Barkley J, Longenecker C, Mittelsteadt A, Gripshover B, Salata R. A cross-sectional description of age and gender differences in exercise patterns in adults living with HIV. Journal of the Association of Nurses in AIDS Care. 2015;26:176–86. doi: 10.1016/j.jana.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clinical infectious diseases. 2010;51:937–46. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hileman CO, Eckard AR, McComsey GA. Bone loss in HIV—a contemporary review. Current opinion in endocrinology, diabetes, and obesity. 2015;22:446. doi: 10.1097/MED.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien KK, Tynan A-M, Nixon SA, Glazier RH. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC infectious diseases. 2016;16:182. doi: 10.1186/s12879-016-1478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy KP, Parker RA, Losina E, et al. Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: A US-based modeling study. Journal of Infectious Diseases. 2016:jiw430. doi: 10.1093/infdis/jiw430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking Prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Annals of internal medicine. 2015;162:335–44. doi: 10.7326/M14-0954. [DOI] [PubMed] [Google Scholar]
- 13.Perazzo JD, Webel AR, Voss JG, Prince-Paul M. Fatigue Symptom Management in People Living With Human Immunodeficiency Virus. Journal of Hospice & Palliative Nursing. 2017;19:122–7. doi: 10.1097/NJH.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA Guidelines for Exercise Testing: Executive Summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) 1997;96:345–54. doi: 10.1161/01.cir.96.1.345. [DOI] [PubMed] [Google Scholar]
- 15.Webel AR, Perazzo J, Decker M, Horvat-Davey C, Sattar A, Voss J. Physical activity is associated with reduced fatigue in adults living with HIV/AIDS. Journal of Advanced Nursing. 2016;72:3104–12. doi: 10.1111/jan.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden-Wade HA, Coleman KJ, Sallis JF, Armstrong C. Validation of the telephone and in-person interview versions of the 7-day PAR. Medicine and Science in Sports and Exercise. 2003;35:801–9. doi: 10.1249/01.MSS.0000064941.43869.4E. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the american heart association. Circulation. 2013;128:2259–79. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 19.Berntsen S, Hageberg R, Aandstad A, et al. Validity of physical activity monitors in adults participating in free-living activities. Br J Sports Med. 2010;44:657–64. doi: 10.1136/bjsm.2008.048868. [DOI] [PubMed] [Google Scholar]
- 20.Anastasopoulou P, Tubic M, Schmidt S, Neumann R, Woll A, Hartel S. Validation and comparison of two methods to assess human energy expenditure during free-living activities. PLoS One. 2014;9:e90606. doi: 10.1371/journal.pone.0090606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitz MM, Conway PG, Palcher JA, McCarty CA. Using PhenX toolkit measures and other tools to assess urban/rural differences in health behaviors: recruitment methods and outcomes. BMC Res Notes. 2014;7:847. doi: 10.1186/1756-0500-7-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haskell WL, Troiano RP, Hammond JA, et al. Physical activity and physical fitness: standardizing assessment with the PhenX Toolkit. Am J Prev Med. 2012;42:486–92. doi: 10.1016/j.amepre.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton CM, Strader LC, Pratt JG, et al. The PhenX Toolkit: get the most from your measures. Am J Epidemiol. 2011;174:253–60. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Medicine. 2017:1–25. doi: 10.1007/s40279-017-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Freedson PS, Lyden K, Kozey-Keadle S, Staudenmayer J. Evaluation of artificial neural network algorithms for predicting METs and activity type from accelerometer data: validation on an independent sample. J Appl Physiol (1985) 2011;111:1804–12. doi: 10.1152/japplphysiol.00309.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing 1–3. American Review of Respiratory Disease. 1984;129:S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 29.Sun X-G, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy participants. American journal of respiratory and critical care medicine. 2002;166:1443–8. doi: 10.1164/rccm.2202033. [DOI] [PubMed] [Google Scholar]
- 30.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. Journal of applied physiology. 1986;60:2020–7. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 31.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vancampfort D, Mugisha J, De Hert M, et al. Global physical activity levels among people living with HIV: a systematic review and meta-analysis. Disability and Rehabilitation. 2016:1–10. doi: 10.1080/09638288.2016.1260645. [DOI] [PubMed] [Google Scholar]
- 35.Monroe AK, Brown TT, Cox C, et al. Physical activity and its association with insulin resistance in multicenter AIDS cohort study Men. AIDS research and human retroviruses. 2015;31:1250–6. doi: 10.1089/aid.2015.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 37.Willig AL, Overton ET. Metabolic consequences of HIV: pathogenic insights. Current HIV/AIDS Reports. 2014;11:35–44. doi: 10.1007/s11904-013-0191-7. [DOI] [PubMed] [Google Scholar]
- 38.Hills AP, Mokhtar N, Byrne NM. Assessment of physical activity and energy expenditure: an overview of objective measures. Frontiers in nutrition. 2014;1:5. doi: 10.3389/fnut.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Influence of Participant Effort on the Prognostic Value of Peak VO2 and the VE/VCO2 Slope in Patients With Heart Failure. Journal of Cardiopulmonary Rehabilitation and Prevention. 2004;24:317–20. doi: 10.1097/00008483-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Vancampfort D, Mugisha J, Rosenbaum S, et al. Cardiorespiratory fitness levels and moderators in people with HIV: A systematic review and meta-analysis. Preventive Medicine. 2016;93:106–14. doi: 10.1016/j.ypmed.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Wolsk E, Bakkestrøm R, Thomsen JH, et al. The Influence of Age on Hemodynamic Parameters During Rest and Exercise in Healthy Individuals. JACC: Heart Failure. 2016 doi: 10.1016/j.jchf.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Ross R, Blair SN, Arena R, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016 doi: 10.1161/CIR.0000000000000461. CIR. 0000000000000461. [DOI] [PubMed] [Google Scholar]
- 43.Cutrono SE, Lewis JE, Perry A, Signorile J, Tiozzo E, Jacobs KA. The Effect of a Community-Based Exercise Program on Inflammation, Metabolic Risk, and Fitness Levels Among Persons Living with HIV/AIDS. AIDS and Behavior. 2016;20:1123–31. doi: 10.1007/s10461-015-1245-1. [DOI] [PubMed] [Google Scholar]
- 44.Jaggers JR, Sneed JM, Lobelo RLF, et al. Results of a nine month home-based physical activity intervention for people living with HIV. 2016. 2016;3:14. [Google Scholar]
- 45.Webel AR. A Clinical Trial of SystemCHANGE to Improve Exercise, Diet and Health in HIV-Infected Adults. clinicaltrials.gov. 2016
- 46.Dirajlal-Fargo S, Webel AR, Longenecker CT, et al. The effect of physical activity on cardiometabolic health and inflammation in treated HIV infection. Antiviral therapy. 2016;21:237–45. doi: 10.3851/IMP2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonato M, Galli L, Passeri L, et al. A pilot study of brisk walking in sedentary combination antiretroviral treatement (cART)-treated patients: benefit on soluble and cell inflammatory markers. BMC Infectious Diseases. 2017;17:61. doi: 10.1186/s12879-016-2095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]