Abstract
When a liver transplantation candidate is declined for listing to receive a deceased organ, sometimes a loved one comes forward and offers to be a living donor. This raises the ethical question of whether a patient who is not eligible for deceased donor liver transplantation should be eligible for living donor liver transplantation. We compare living organ donation in kidney and liver transplantation and explore key ethical concepts of justice, fairness, and societal trust. Ultimately, because there is no alternative life-preserving therapy in end-stage liver disease, and because transplantation with a living donor organ does not involve removing a resource from the common pool of transplant organs, we argue that a “slightly less benefit” than what is required for deceased transplantation listing standard should be used to determine the acceptability of living donor liver transplantation.
Keywords: Ethics, liver transplantation, living donor, organ transplantation, justice, trust, risks, benefits
Introduction
A broad consensus has been achieved on numerous ethical issues in organ transplantation including the use of deceased donor organs, acceptability of the brain death standard, and allocation of organs based on urgency and need [2]. Organ scarcity has encouraged liver transplantation (LT) programs to develop alternative life-saving approaches for addressing the needs of patients with advanced liver disease, including living donor liver transplantation (LDLT). Although the transplant community has come to accept LDLT as a viable option for both pediatric and adult patients in need, numerous ethical issues related to LDLT still need to be resolved.
Donor safety has been the primary focus for transplant programs that perform LDLT [3-5]. Comprehensive informed consent processes and robust medical assessments of both donor and recipient have been developed to ensure appropriate ethical practice and safety [6,7]. Situations do arise, however, in which it is unclear whether LDLT should be offered, and what the ethical standard for making such decisions should be. When a LT candidate is declined for listing to receive a deceased donor liver transplantation (DDLT), sometimes a loved one comes forward and offers to be a living donor. This raises the ethical question of whether a patient who is not eligible for DDLT should be eligible for LDLT. Using two clinical cases encountered at our institution, we describe situations in which deceased organ donation was not pursued for psychosocial or medical reasons, and query whether it is ethically acceptable to proceed with LDLT. These cases were adapted from real-life experiences and are modified for the purposes of this manuscript’s discussion.1
Case 1
BH was a 18-year-old boy with biliary atresia. Over time, he developed decompensated liver disease and ultimately needed a LT to survive. He had hepatic encephalopathy, ascites, and a recent hospitalization for esophageal variceal bleeding. His Model for End Stage Liver Disease (MELD) score was 17 making it unlikely that he would receive a deceased donor liver from the UNOS list in the near future. BH had a history of medication non-adherence, frequently missed medical appointments, and skipped laboratory blood draws at the Hepatology Clinic. He was enrolled in school, but he displayed rebellious behavior and had frequent absences, detentions and prior expulsions. His relationship with his parents had also been trying; he repeatedly violated his curfew and moved out of his parents’ home for a time to live with a girlfriend. After that relationship ended, he moved back home with his parents. He had been arrested for legal infractions, such as petty theft, but he was never incarcerated. His pediatrician referred him back to the LT office for evaluation.
The transplant social worker and psychiatrist evaluated BH and identified no substance abuse issues or psychiatric problems. Despite BH’s interest in LT and his understanding of the severity of his liver disease, it was determined that he lacked insight into the ways in which his behavior compromised his health. In light of those reports, the LT team ultimately decided not to list BH because of their concerns about his history of nonadherence and rebellious, unpredictable behavior. They recommended that BH and his parents seek an opinion from another LT center.
The next day, BH’s parents came forward and both volunteered to be living donors for their son. They strongly believed that their involvement in the LT as organ donors would strengthen their bond with BH and promote his post-transplant adherence.
Case 2
ML was a 49-year-old woman with cirrhosis due to chronic hepatitis C virus (HCV) complicated by hepatocellular carcinoma (HCC). She had a 3-centimeter liver lesion for which she received several courses of loco-regional therapy. Although her natural MELD score was 13, she was eligible to receive additional “exception points” related to her HCC. Those additional points gradually increased her MELD score to 30, placing her towards the top of the waiting list for a deceased donor organ.
Later, a scan showed a residual active tumor and a new 1.4 centimeter HCC. The imaging also showed a right breast mass that ultimately was diagnosed as Stage 2 breast cancer after lumpectomy and sentinel node sampling. ML tolerated the procedures well, but her natural MELD rose to 20 with new onset ascites. She was no longer eligible for extra HCC-related points and she was unable to tolerate further treatment for her HCC or adjuvant therapy for the breast cancer. Despite the potential excellent five-year survival with a LT, the transplant team decided to de-list ML because of her recently diagnosed breast cancer and recommended that she seek a second opinion at another center.
The day after the family was informed of the committee’s decision to remove ML from the deceased donor organ waiting list, ML’s husband volunteered to be a living donor because without LT she would not survive. Both he and ML expressed their strong desire to proceed with LDLT despite the potential risks associated with immunosuppression in the setting of breast cancer and the fact that ML would need to undergo post-transplant cancer therapy.
The fundamental question that these cases raise is whether transplant centers should evaluate living donor recipients using the same criteria for deceased donor transplantation. In other words, should the same standards that are used to decline or accept patients on the national United Network for Organ Sharing (UNOS) waiting list, also be used for determining whether a LDLT should be performed? Should the criteria be more or less rigorous? No clear and compelling recommendation has emerged, and transplant centers are left uncertain as to how to proceed in these difficult situations.
Lessons Learned from Living Donation in Kidney Transplantation
The kidney transplantation (KT) community has been dealing with this issue for a long time because living donor kidney transplantation (LDKT) has been an accepted practice for more than 30 years, much longer than in LDLT. Because hemodialysis is an alternative to LDKT, KT programs often require that LDKT recipients have an equal or better prospect of long-term survival than the recipient of a deceased donor kidney transplantation (DDKT) would. To make this point more explicit, consider that a transplant program might require a KT recipient to have at least a 90% chance for five-year graft and patient survival in order to be eligible for DDKT listing. The program might then require that LDKT recipients have a similarly excellent prospect, or an even better chance of success. To justify imposing the inevitable risks and burdens on a healthy kidney donor, LDKT recipients are expected to do just as well, if not better, than those who undergo DDKT.
The most critical difference between liver and kidney transplantation is that kidney patients who are not listed for an allograft have the alternative life-preserving option of hemodialysis. Unlike the situation with KT, a patient who is turned down for both DDLT and LDLT will die. This difference makes it obvious that there is far more at stake for LT candidates when living donation is declined as compared to KT. Moreover, unlike deceased organ donation, living donor transplantation involves significant risks to the donor. These include physical consequences related to loss of an organ and the risks associated with organ procurement surgery. The procedure also involves psychological and emotional risks related to the recovery and aftermath of surgery, and its effects on the relationship between donor, recipient, and others [2,8-10]. Whereas death associated with kidney donation is extraordinarily rare [11], the risks associated with LDLT are markedly greater than the risks to KT living donors [12,13]. Approximately two in a thousand liver donors actually die [14-16]. In the U.S., approximately one third of living liver donors also experience a surgery-related complication, some of which are serious and enduring [17,18]. Living kidney donation is now typically performed laproscopically, thus recovery from kidney donation is faster and easier than LDLT [19]. Living kidney donors typically leave the hospital 2-4 days post-operatively and can return to their normal activities in 8 weeks. The scars from donation are small. By contrast, liver donation involves a major open surgery. Living liver donors typically stay in the hospital for 6-8 days post-operatively, often with some period in an intensive care unit. They then convalesce at home for about 12-16 weeks and cannot return to their normal activities for about 4-6 months. The scar from liver donation results from a long incision down the mid-line of the abdomen and across the abdomen for a significant length [20,21].
Subjecting a healthy person to such risks and burdens for the sake of another individual is remarkably unusual in medical practice where the focus has traditionally been to “do no harm.” In spite of the risks involved, LDLT has been accepted in our society because of the dire circumstances of organ scarcity and because the transplant community has shown that it can be trusted in carrying out these procedures in a safe and ethical manner. In any living donor situation, the harms and burdens to the donor are justified by the significant benefit to the recipient [3,6,7]. This means that the organ donor needs to have a robust understanding of the risks and burdens involved and the capacity to consider them in the context of the values and priorities that the donor finds most salient. To minimize risks, transplant teams assess living donors’ general health and the compatibility of their anatomy for donation. To assure that the donation decision is voluntary, they also investigate whether donors are well informed of the risks and burdens involved. Although such procedures are part of every living donor evaluation, two questions remain: 1) Do the differences between living donation for KT and LT suggest that a different standard be used when deciding whether to proceed with these two procedures? 2) Do additional factors related to the recipient’s expected survival or outcomes and the donor’s potential benefits have to be considered?
Justice and Fairness in Organ Allocation
Aristotle’s well-accepted formal principle of justice requires that similar circumstances be treated similarly and different circumstances be treated differently [22]. This principle applies to the allocation of resources among those who have standing relative to the distribution. Adherence to this principle promotes the fair distribution of scarce resources amongst the entitled claimants. UNOS and the transplant community demonstrate their commitment to this basic ethical requirement in their adoption of allocation rules such as the Model for End-Stage Liver Disease (MELD) system which provides a transparent metric for comparing the urgency of patients’ need for transplantation—the higher the MELD score, the more severe the liver disease and the more urgent the need for LT. By employing that single standard and allocating organs first to those with the highest MELD scores on the LT waiting list, UNOS and transplant medicine show fairness in their treatment of every patient, regardless of why the patient comes to need a transplant.
When it comes to allocation from the pool of deceased donor organs, every LT candidate should be treated fairly; that is, the same allocation scheme should be applied in the distribution of deceased donor organs for all individuals. Because there are not enough deceased donor organs to meet the needs of every candidate who could benefit from DDLT, the transplant community has come to accept the view that those who are not expected to significantly benefit from DDLT should be denied an organ so that others who are more likely to derive a significant benefit can have the chance to receive the gift of life [23]. This approach is an example of medicine’s longstanding commitment to medical triage in circumstances of extreme resource scarcity. Medical triage reflects the view that a just allocation of the insufficient supply of critical resources should aim at avoiding the worst outcome. A greater number of avoidable deaths is accepted as being a worse result than an alternative allocation that is likely to result in fewer deaths.
Aristotle discerned the difficulty involved in determining which features of a situation should be taken into account in deciding that individuals are similarly situated, and which of the generally important factors should be given priority in a particular allocation of resources. Although justice does require equality in the treatment of equals, many incommensurable factors have to be balanced in order to determine what gives different people equal standing and what counts as equal treatment [24]. Providing justice therefore requires moral discernment to identify which factors are significant and how they should be compared in order to achieve a just distribution. In the allocation of extremely scarce medical resources, it is well accepted that physicians should take a low likelihood of survival into account. In that light, medicine employs the concept of medical triage on the battlefield in the allocation of scarce transplant organs.
We have argued elsewhere that given today’s organ scarcity and the rate of people dying for lack of an organ, a 50-70% chance of five-year survival is a reasonable benchmark for listing patients for deceased organ transplantation (Figure 1) [25]. In other words, if a transplant recipient has a less than 50% risk of five-year survival, it is not reasonable to offer DDLT given the poor outcomes expected post-LT. This standard was intended to serve as a benchmark for withholding transplant organs from those with a significantly worse chance of survival than others who are competing for the same deceased donor organ [26]. When the potential transplant recipient is deemed to be medically or psychosocially higher risk than other transplant recipients should the standard benchmark of a 50-70% chance of recipient five-year survival be used for living donation? Additionally, patients who receive LDLT are not taking an organ from the deceased donor organ pool—a living donor organ would only be donated to a specific recipient because of some special feature of their personal relationship. No one else who needs an organ would be in line for that organ. In that way, LDLT does not involve injustice to other candidates on the transplant list.
Figure 1.
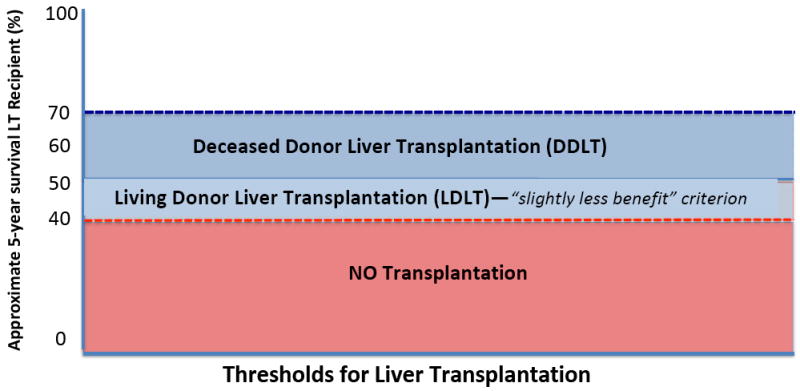
Expected approximate survival standards for LDLT and DDLT
Assuming that 50-70% chance of five-year survival is a reasonable benchmark for listing patients for DDLT, a “slightly less benefit” standard may be acceptable for LDLT.
Trust and Setting Limits in LDLT
Although living donation does not involve taking an organ from the deceased donor pool, there are other important ethical considerations that warrant setting limits in LDLT. These stem from the important fact that LDLT, unlike other medical interventions, involves harm to an otherwise healthy individual (i.e. the donor). Three reasons justify setting limits on LDLT. First, is the medical duty to act in the interest of each patient. Whereas the living donor may see redeeming value in accepting the risks of living liver donation, medical professionals have a fiduciary responsibility to advocate for their donor patient and protect the donor from excessive harm. From the point of view of the medical team they must independently assess the benefits and burdens and conclude that the promised benefits are worth the foreseeable risks involved. Today’s transplant programs are vigilantly attentive in excluding living donors whose pre-transplant workup indicates any medically identifiable additional risk that could make the overall risk to their lives greater than what could otherwise be expected. Numerous medical tests are performed in the evaluation of living donors. A vigilant transplant program will rule out as many as 80-90% of those who come forward as potential liver donors for medical reasons alone [27,28]. Caution is the well-accepted benchmark in donor evaluation. Even when an individual is deemed to be an acceptable donor for LT, strategies to minimize risk including consideration of donor-recipient matching, surgical techniques (e.g. left hepatectomy vs. right hepatectomy) must be utilized to ensure best outcomes.
Second, it is essential that society trusts the transplantation community and that the integrity and reputation of transplantation programs is upheld so as to preserve the future of LDLT. As a society, we grant physicians the authority to make these life-altering decisions, and we hold transplant physicians accountable for the decisions that they make. From the point of view of an eager potential donor, any amount of life-extension for the recipient may be worth the risk. It is important to acknowledge the complex balance of psychological, social, and physical benefits and risks to the donor that may be underappreciated by LT programs. Depending on the intricacies of the relationship between donor and recipient, the donor may stand to gain a lot. For example, the donor may be financially dependent on the recipient or rely on the recipient emotionally in ways that significantly contribute to the donor’s quality of life and wellbeing. From the perspective of society, however, exposing a healthy donor to the risk of death for a brief life extension (e.g., six months), or because the donor risks losing a beloved companion is likely to appear irresponsible. Society trusts the transplant community to make reasonable decisions. For society to continue to regard the risks of living organ donation as acceptable and allow LDLT to proceed, it needs to be confident that decisions involve a careful balance of risks and benefits and that transplant programs refuse to perform LDLT when the benefits are expected to be trifling. Every transplant team is responsible for making its decisions about when to go forward with LDLT. The eagerness of the donor must not factor into that decision. To maintain the trust that allows LDLT to proceed, programs have to be able to justify their decisions in a way that society will regard as trustworthy. This consideration requires program decisions to reflect the kinds of judgment that others will see as appropriate, and not fool-hearty, reckless, or self-serving. As the Ethics Committee of the Transplantation Society noted in the 2004 Amsterdam Forum on the Care of the Live Kidney Donor, living donor transplantation must “be performed in a manner that will minimize the physical, psychological, and social risk to the individual donor and does not jeopardize the public trust of the healthcare community” [29].
The third reason to set limits in LDLT is to preserve the future availability of this practice. The transplant community would like to be able to use LDLT to help patients in the future, including those patients who are declined for deceased donor organ listing. In order to maintain their ability to do so, transplant programs must demonstrate that they behave responsibly in their decisions to undertake LDLT. The continued privileges of the transplant community depend upon the public’s confidence.
What Standard Should Be Used for LDLT?
Because of the unique features of LDLT, we must define the degree of benefit to the potential recipient that can justify the risks incurred by the donor [3]. Yet, it is difficult to balance the benefits and risks because they involve different likely outcomes for different individuals. The primary benefits for the organ recipient are improved health and life extension. The benefits to the donor are social and psychological, whereas the donor’s risks and burdens are a complex assortment of potential physical, social, and psychological outcomes.
Even though it may not be possible to draw a precise line distinguishing permissible from impermissible LDLT, it may be enough to provide a guideline for transplant teams that must make those decisions. Because of the dire consequences for the recipient and the lack of any alternative life-preserving therapy, we suggest allowing LDLT for transplant patients whose likely benefit is slightly less than what is required for deceased donor listing, but not far below that standard. Roughly speaking, the transplant community should accept living donor transplants when the risk-benefit ratio is reasonable, and not when it is unreasonable. For example, LT programs should reject LDLT when it is likely to extend the recipient’s life for only a very short time, as in the case of significant metastatic cancer or advanced HCC. When the life expectancy of the recipient after transplantation is expected to be short, other authors have similarly advised LT programs against pursuing LDLT because they consider the potential donor risks to outweigh the benefits [30,31]. Our proposal goes farther by suggesting the standard for any acceptable use of LDLT as “slightly less benefit than what is required for deceased transplantation listing” (Figure 1).
We understand that our standard of using a “slightly less benefit” than what is required for deceased transplantation listing is somewhat vague. It is hard to say precisely how much less is slightly less benefit. Nevertheless, we usually accept decisions of transplant teams because programs have extensive experience in making these decisions and because, for the most part, their judgments are reasonable. We suggest 40% likelihood of five-year survival as a cut-off for LDLT. We offer it as a possible target and only as an example of how the standard could be implemented (Figure 1). The vagueness of employing a standard based on “slightly less benefit” is also not unique in bioethics. For example, the concept of a slight or minor increase over minimal risk defines the standard for research with children in The Common Rule and other research guidelines. As Aristotle cautioned in Book I, Chapter 3 of his Nicomachean Ethics, “we must not expect more precision than the subject-matter admits” [22]. In other words, ethics is not pharmacology. Vague principles may be as far as we can go in ethics, and guidelines for making difficult decisions should nevertheless be recognized as useful moral tools for navigating difficult decisions. The slightly less benefit standard is aimed to be a launching point for discussions and offer a concrete framework by which LT programs can weigh risks and benefits and ultimately decide an acceptable threshold at which to perform LDLTs.
The Confounding Problem of Graft Failure
Unfortunately, there are instances in which liver transplantation fails immediately. Primary non-function, a rare complication of LT, occurs immediately after the transplant procedure in less than 3-5% of all transplantations [32,33]. Graft failure can also occur as the result of a physiological event such as ischemic cholangiopathy, other biliary complications, or hepatic artery thrombosis. The possibility of such an occurrence complicates decisions about LDLT. When graft failure occurs the organ recipient will die soon unless another organ transplantation is performed. The real possibility of graft failure complicates the approach to LDLT.
Patients who are declined for listing and then accepted for LDLT are required to be placed on the UNOS list until they receive their LDLT. If graft failure should ensue shortly after LDLT, should these patients, who otherwise were ineligible for DDLT be allotted an organ from the deceased donor pool? Participants at a 2010 international consensus conference in Zurich, Switzerland, on liver transplantation for HCC concluded that “based on utility, justice, and equity, they did not support re-transplantation for patients who were beyond these [standard eligibility] criteria, because these patients would not have qualified for DDLT [deceased donor liver transplantation] in the first place” [26]. We agree with that statement, but recognize that the conclusion has broader implications than what may have been contemplated by its authors.
A patient with acute graft failure has an urgent need for re-transplantation. In such a circumstance, it is easy to imagine that the transplant team and surgeons who explanted the patient’s native organ would feel guilty and find it emotionally difficult to allow their patient to die without attempting re-transplantation. They are likely to feel as if they had failed their patient and that withholding re-transplantation could be tantamount to abandonment. The anticipation of that reaction may make transplant teams reluctant to undertake LDLT without having the option of re-transplantation via DDLT. After all, the possibility of re-transplantation has been an important element in the success of solid organ transplant programs. An additional reason that could seem relevant to supporting re-transplantation following organ failure after LDLT is the reality of transplant program viability. Transplant program outcomes are reported and monitored. Graft failures and patient deaths have a negative impact on a program’s record, and could have serious repercussions for the program’s future. Awareness of that possibility may make programs less willing to offer LDLT without the option of relisting patients who suffer graft failure.
Nevertheless, these considerations fly in the face of the justice, fairness, and trust considerations that were previously discussed. Using a deceased organ from the common pool to transplant a patient who was determined to be ineligible for that organ would be unjust to another patient who is entitled to the gift of life because of the great likelihood of deriving a significant benefit from it. Allocating a deceased organ to an otherwise ineligible graft failure patient following LDLT would amount to circumventing a just policy. Making difficult and uncomfortable decisions is an inherent feature of medicine that involves allocation of critically scarce resources. In critical care, physicians must at times withhold or withdraw acutely limited critical treatment resources from patients who are not likely to derive significant benefit from them, when those resources could instead make a drastic difference in the treatment of another patient [34,35]. Transplant surgeons and other members of transplant teams regularly make arduous decisions to deny listing to patients who are unlikely to derive a significant benefit and suspend the listing of patients when their medical condition deteriorates. The same should hold for extending eligibility of LDLT patients when graft failure happens during or shortly after transplantation. Whereas withholding re-transplantation may feel far more difficult than refusing to list a patient who would be ineligible for a deceased donor organ, the moral justification for both decisions is the same.
Despite the emotional burden of withholding the opportunity for re-transplantation following organ failure, transplant teams should not offer a deceased organ to a living donor recipient with acute graft failure given the injustice to others on the transplant list. We envision that the personal involvement and sense of failure when treatment is not successful inclines physicians to do even more, particularly for your patient. When the life of another patient is on the line, however, the preference for generosity must be resisted. Doing so is especially hard when the person who will be saved is distant and not personally known to you. The emotional pain for team members, patients, and families involved in such trying decisions can be mitigated by sharing the policy decision with all stakeholders, including potential donors and recipients, in advance of having to make a difficult call. By including explicit statements about the extended eligibility LDLT patient’s continued ineligibility for organs from the deceased donor pool in the event of graft failure, everyone involved will be aware and prepared for accepting the decision if it has to be made.
Applying the Standard
In light of the above discussion, how do we reconcile the issue of whether to pursue LDLT in the two cases that we presented at the beginning? In case 1, BH’s parents feel that their life-saving gesture of volunteering as organ donors demonstrates their persistent love to their son. They are hopeful that their commitment will help him to comply better with his post-transplant regimen.
The LT team and BH’s parents ultimately agreed that in this unique case BH’s behavior would likely improve after his parents’ demonstrated their commitment to him. With BH’s evolving maturity, the team was sufficiently optimistic that the issues of post-LT adherence and follow-up could be mitigated by his parents’ involvement, so as to make his benefit from LDLT sufficiently likely. If BH could adhere to his post-transplant care, he would be expected to have an excellent chance for long-term survival. So long as BH’s parents complete the standard rigorous LDLT evaluation protocol and are accepted as candidates by the independent donor advocacy team, LDLT is reasonable in this case. This case’s unique family dynamics, however, may not be generalizable to other patients who demonstrate noncompliance. The same expectation for future adherence might not hold for other adolescents or for adults with more ingrained behaviors that would require structured behavioral modifications and robust reliable social supports.
In case 2, ML’s husband would also have to meet the same rigorous acceptance requirements as other living donors. He would have to undergo a comprehensive informed consent process and demonstrate that he clearly understood that his wife’s survival might be worse than other LT patients due to her recent cancer, especially given the worse outcomes associated with breast cancer after transplantation [36]. He also would have to demonstrate understanding of the uncertainties related to additional risks of chemotherapy on allograft function, and the effects of immunosuppression on the natural history of the cancer. The original transplant committee decision to de-list ML reflected the view that it would not be fair to allocate an organ to her when another patient on the waiting list would be likely to derive a significantly greater benefit from it. However, given that her life expectancy was not significantly lower than the standard used for deciding DDLT (i.e. 40% 5-year survival), LDLT was thought to be a reasonable option.
Concluding Thoughts
LDLT is a potentially lifesaving option for individuals who are denied deceased donor listing and who still have a reasonably good chance of long-term survival post-transplantation. Because each case that involves rejecting a patient from listing is likely to be heart-wrenching and involve its own idiosyncrasies, it is important to develop a programmatic policy in advance that can be the basis for guiding these difficult decisions. Criteria for declaring that the use of a living donor is not unreasonable should be articulated as clearly as possible. Ideally, they should be endorsed by a transplant team consensus that is developed when there is no particular case at issue that could color the team’s judgment.
In this paper, we propose using a modified standard for pursuing LDLT. It is acceptable to pursue LDLT so long as it offers only “slightly less benefit” than what is required for deceased transplantation listing. This criterion can be used for deciding when it would be reasonable to perform a LDLT and when that option should be avoided. Further defining the standard is likely to be challenging because this criterion may not translate into a precise survival rate or objective outcome. To ensure that LDLT continues to be performed safely and effectively, the well-accepted donor screening standards must continue to be upheld. The focus on minimizing donor risks by excluding donors for medical and psychosocial reasons, as well as the employment of strict criteria for the acceptability of LDLT, will help to maintain society’s trust in organ transplantation.
Acknowledgments
Dr. Lieber is supported by a grant from the National Institutes of Health (T32DK07634).
Footnotes
The actual cases that inspired this discussion did not receive liver transplantation. In both cases, LRD was considered, but transplantation was ultimately not pursued.
Disclosures: In this paper we draw upon our previous publication, expand our discussion of the salient ethical issues, and revise our stance on one controversial issue [1]. We received permission from Clinical Liver Disease to adapt and re-use content from the prior publication.
Conflicts of Interest: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schiano TD, Rhodes R. The Ethics of living related liver transplantation when deceased donation is not an option. Clin Liver Dis. 2015;6:112–6. doi: 10.1002/cld.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutmann T, Land W. Ethics regarding living-donor organ transplantation. Langenbeck’s Arch Surg / Dtsch Gesellschaft Für Chir. 1999;384:515–22. doi: 10.1007/s004230050237. [DOI] [PubMed] [Google Scholar]
- 3.Panocchia N, Bossola M, Silvestri P, Midolo E, Teleman AA, Tazza L, et al. Ethical Evaluation of Risks Related to Living Donor Transplantation Programs. Transplant Proc. 2013;45:2601–3. doi: 10.1016/j.transproceed.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 4.Gordon EJ. Informed consent for living donation: a review of key empirical studies, ethical challenges and future research. Am J Transplant. 2012;12:2273–80. doi: 10.1111/j.1600-6143.2012.04102.x. [DOI] [PubMed] [Google Scholar]
- 5.Gordon EJ, Rodde J, Skaro A, Baker T. Informed consent for live liver donors: A qualitative, prospective study. J Hepatol. 2015;63:838–47. doi: 10.1016/j.jhep.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Petrini C, Hester M, Schaefer GO, Emanuel EJ, Wertheimer A, Miller FG, et al. Ethical issues with informed consent from potential living kidney donors. Transplant Proc. 2010;42:1040–2. doi: 10.1016/j.transproceed.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 7.Jennings T, Grauer D, Rudow DL. The role of the independent donor advocacy team in the case of a declined living donor candidate. Prog Transplant. 2013;23:132–6. doi: 10.7182/pit2013299. [DOI] [PubMed] [Google Scholar]
- 8.Cronin DC, Millis JM. Living donor liver transplantation: The ethics and the practice. Hepatology. 2008;47:11–3. doi: 10.1002/hep.22150. [DOI] [PubMed] [Google Scholar]
- 9.Knibbe ME, Maeckelberghe ELM, Verkerk MA. Confounders in voluntary consent about living parental liver donation: no choice and emotions. Med Health Care Philos. 2007;10:433–40. doi: 10.1007/s11019-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 10.Strong RW, Lynch SV. Ethical issues in living related donor liver transplantation. Transplant Proc. 1996;28:2366–9. [PubMed] [Google Scholar]
- 11.Bruzzone P, Berloco PB, Ciszek M, Paczek L, Rowinski W, Gracida C, et al. Ethical aspects of renal transplantation from living donors. Transplant Proc. 2003;39:1785–6. doi: 10.1016/j.transproceed.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Hashikura Y, Ichida T, Umeshita K, Kawasaki S, Mizokami M, Mochida S, et al. Donor complications associated with living donor liver transplantation in Japan. Transplantation. 2009;88:110–4. doi: 10.1097/TP.0b013e3181aaccb0. [DOI] [PubMed] [Google Scholar]
- 13.Iida T, Ogura Y, Oike F, Hatano E, Kaido T, Egawa H, et al. Surgery-related morbidity in living donors for liver transplantation. Transplantation. 2010;89:1276–82. doi: 10.1097/TP.0b013e3181d66c55. [DOI] [PubMed] [Google Scholar]
- 14.Middleton PF, Duffield M, Lynch SV, Padbury RTA, House T, Stanton P, et al. Living donor liver transplantation--adult donor outcomes: a systematic review. Liver Transpl. 2006;12:24–30. doi: 10.1002/lt.20663. [DOI] [PubMed] [Google Scholar]
- 15.Lee JG, Lee K-W, Kwon CHD, Chu CW, Kim B-W, Choi DL, et al. Donor safety in living donor liver transplantation: The Korean organ transplantation registry study. Liver Transpl. 2017;23:999–1006. doi: 10.1002/lt.24778. [DOI] [PubMed] [Google Scholar]
- 16.Beavers K, Sandler RS, Shrestha R. Donor morbidity associated with right lobectomy for living donor liver transplantation to adult recipients: A systematic review. Liver Transplant. 2002;8:110–7. doi: 10.1053/jlts.2002.31315. [DOI] [PubMed] [Google Scholar]
- 17.Suh K-S, Suh S-W, Lee J-M, Choi Y, Yi N-J, Lee K-W. Recent advancements in and views on the donor operation in living donor liver transplantation: A single-center study of 886 patients over 13 years. Liver Transplant. 2015;21:329–38. doi: 10.1002/lt.24061. [DOI] [PubMed] [Google Scholar]
- 18.Patel S, Orloff M, Tsoulfas G, Kashyap R, Jain A, Bozorgzadeh A, et al. Living-Donor Liver Transplantation in the United States: Identifying Donors at Risk for Perioperative Complications. Am J Transplant. 2007;7:2344–9. doi: 10.1111/j.1600-6143.2007.01938.x. [DOI] [PubMed] [Google Scholar]
- 19.Weitz J, Koch M, Mehrabi A, Schemmer P, Zeier M, Beimler J, et al. Living-donor kidney transplantation: risks of the donor? benefits of the recipient. Clin Transplant. 2006;20:13–6. doi: 10.1111/j.1399-0012.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 20.Pascher A, Sauer IM, Walter M, Lopez-Haeninnen E, Theruvath T, Spinelli A, et al. Donor evaluation, donor risks, donor outcome, and donor quality of life in adult-to-adult living donor liver transplantation. Liver Transplant. 2002;8:829–37. doi: 10.1053/jlts.2002.34896. [DOI] [PubMed] [Google Scholar]
- 21.Ladner DP, Dew MA, Forney S, Gillespie BW, Brown RS, Merion RM, et al. Long-term quality of life after liver donation in the adult to adult living donor liver transplantation cohort study (A2ALL) J Hepatol. 2015;62:346–53. doi: 10.1016/j.jhep.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aristotle . The Nicomachean Ethics of Aristotle. London: Oxford University Press; 1971. [Google Scholar]
- 23.Keller EJ, Kwo PY, Helft PR. Ethical considerations surrounding survival benefit-based liver allocation. Liver Transpl. 2014;20:140–6. doi: 10.1002/lt.23780. [DOI] [PubMed] [Google Scholar]
- 24.Martin DK, Meslin EM, Kohut N, Singer PA. The incommensurability of research risks and benefits: practical help for research ethics committees. IRB. 1995;17:8–10. [PubMed] [Google Scholar]
- 25.Schiano TD, Bourgoise T, Rhodes R. High-risk liver transplant candidates: An ethical proposal on where to draw the line. Liver Transpl. 2015;21:607–11. doi: 10.1002/lt.24087. [DOI] [PubMed] [Google Scholar]
- 26.Clavien P-A, Lesurtel M, Bossuyt PMM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renz JF, Roberts JP. Long-term complications of living donor liver transplantation. Liver Transpl. 2000;6:S73–6. doi: 10.1053/jlts.2000.18686. [DOI] [PubMed] [Google Scholar]
- 28.Abdullah K, Abdeldayem H, Salama IA-K, Badah K, Al-Somali B, Abdulkareem A. Retrospective analysis of the causes of rejection of potential donors for living related liver transplantation. Hepatol Int. 2007;1:431–6. doi: 10.1007/s12072-007-9013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ethics Committee of the Transplantation Society. The consensus statement of the Amsterdam Forum on the Care of the Live Kidney Donor. Transplantation. 2004;78:491–2. doi: 10.1097/01.tp.0000136654.85459.1e. [DOI] [PubMed] [Google Scholar]
- 30.Pomfret EA, Lodge JPA, Villamil FG, Siegler M. Should we use living donor grafts for patients with hepatocellular carcinoma? Ethical considerations. Liver Transpl. 2011;17(Suppl 2):S128–32. doi: 10.1002/lt.22356. [DOI] [PubMed] [Google Scholar]
- 31.Volk ML, Marrero JA, Lok AS, Ubel PA. Who decides? Living donor liver transplantation for advanced hepatocellular carcinoma. Transplantation. 2006;82:1136–9. doi: 10.1097/01.tp.0000245670.75583.3d. [DOI] [PubMed] [Google Scholar]
- 32.Lock JF, Schwabauer E, Martus P, Videv N, Pratschke J, Malinowski M, et al. Early diagnosis of primary nonfunction and indication for reoperation after liver transplantation. Liver Transplant. 2010;16:172–80. doi: 10.1002/lt.21973. [DOI] [PubMed] [Google Scholar]
- 33.Taner CB, Bathala V, Nguyen JH. Primary Nonfunction in Liver Transplantation: A Single-Center Experience. Transplant Proc. 2008;40:3566–8. doi: 10.1016/j.transproceed.2008.07.137. [DOI] [PubMed] [Google Scholar]
- 34.Fleck L. Just Rationing in the ICU: What Benefits Count? APA Newsl Philos Med. 2016;15:6–10. [Google Scholar]
- 35.Danis M. The Moral Permissibility of Removing Patients from Intensive Care. APA Newsl Philos Med. 2016;15:14–5. [Google Scholar]
- 36.Wong G, Au E, Badve SV, Lim WH. Breast Cancer and Transplantation. Am J Transplant. 2017;17:2243–53. doi: 10.1111/ajt.14368. [DOI] [PubMed] [Google Scholar]


