Abstract
Sixteen disulfides derived from disulfiram (Antabuse™) were evaluated as antibacterial agents. Derivatives with hydrocarbon chains of seven and eight carbons in length exhibited antibacterial activity against Gram-positive Staphylococcus, Streptococcus, Enterococcus, Bacillus, and Listeria spp. A comparison of the cytotoxicity and microsomal stability with disulfiram further revealed that the eight carbon chain analog was of lower toxicity to human hepatocytes and has a longer metabolic half-life. In the final analysis, this investigation concluded that the S-octylthio derivative is a more effective growth inhibitor of Gram-positive bacteria than disulfiram and exhibits more favorable cytotoxic and metabolic parameters over disulfiram.
Keywords: disulfiram, disulfides, antibiotic, Staphylococcus, MRSA, VISA, VRSA
Graphical Abstract
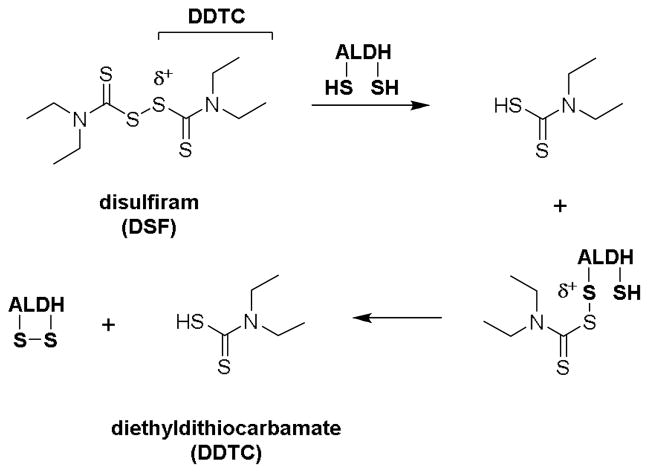
Disulfiram (Antabuse™) is an oral prescription drug for the treatment of alcohol abuse disorder [1]. Upon absorption, disulfiram (DSF) [2] and/or its metabolites [3] inhibit aldehyde dehydrogenase (ALDH) enzymes that oxidize acetaldehyde from ethanol metabolism into acetic acid. The inactivation of hepatic ALDH leads to buildup of toxic acetaldehyde in the body, which manifests ‘hangover’ symptoms (e.g., headache, nausea) to deter alcohol consumption [4].
By chemical nature, electrophilic (δ+) DSF is readily cleaved by thiol-bearing substances such as cysteine enzymes. The thiol-disulfide exchange reactions result in the simultaneous addition and release of diethyldithiocarbamate (DDTC). In the case of ALDH, in vitro studies have shown that a second cysteine residue near the addition site may cleave the labile DDTC adduct with concomitant intramolecular disulfide bond formation (Figure 1) [2]. As a versatile inhibitor of cysteine enzymes, DSF has been evaluated as a treatment for other clinical conditions. Recent U.S. clinical trials using repurposed DSF in treatment include: methamphetamine dependence (NCT00731133); cocaine addiction (NCT00395850); melanoma (NCT00256230); muscle atrophy in pancreatic cancer (NCT02671890); and HIV infection (NCT01286259) [5]. In the area of infectious disease, we recently established that DSF inhibits the in vitro growth of methicillin-resistant Staphylococcus aureus (MRSA) at a minimum inhibitory concentration (MIC) range of 4 – 32 μg/mL and exhibits synergism with vancomycin (VAN) against VAN-resistant S. aureus (VRSA) [6]. The mechanism of MRSA inhibition was also attributed to the transfer of DDTC from DSF to thiophilic substances involved in the regulation of bacterial cell growth. Due to its labile chemical nature, we hypothesized that replacement of the DDTC component in DSF (Figure 1) with S-alkylthio groups would increase antibacterial activity and metabolic stability.
Figure 1.
Proposed route of in vitro aldehyde dehydrogenase (ALDH) inactivation by disulfiram [2].
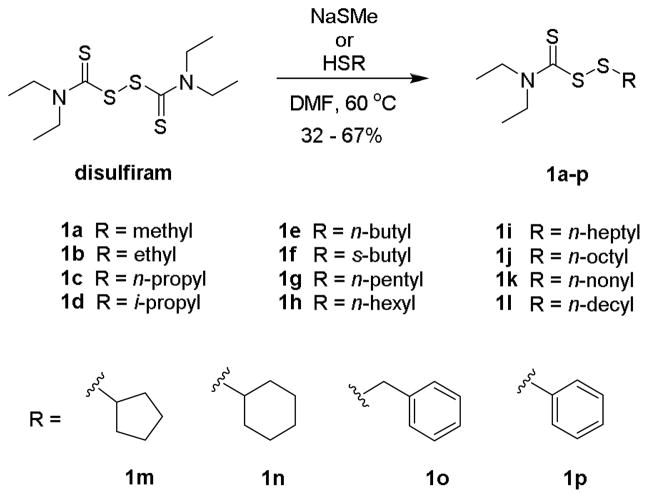
To test this hypothesis, we first synthesized sixteen DSF-derived asymmetric disulfides (1a-p) to deduce the relationship of structure on antibacterial activity (Scheme 1). The compounds were readily prepared by a thiol-disulfide exchange reaction between DSF and respective thiol in DMF [7]. Purification by silica gel chromatography afforded the products as nonaromatic oils in a yield range of 32 – 67% and median yield of 53%. Spectroscopic data and physical characteristics of the compounds were in agreement with previous findings [8].
Scheme 1.
Synthesis of disulfides 1 from disulfiram via a thiol-disulfide exchange reaction.
Antibacterial testing was performed by the broth microdilution assay in 96-well plate format [9,10]. The test agents were initially evaluated against Staphylococcus, Streptococcus, and Enterococcus spp. as our previous research on DSF indicated that Gram-positive cocci would be susceptible [6]. Table 1 shows the MICs of analogs 1a-p in comparison with DSF and VAN. MRSA and Staphylococcus epidermidis exhibited the greatest overall susceptibility to the DSF analogs followed by group A Streptococcus pyogenes (GAS), VAN-resistant Enterococcus faecium (VRE), Streptococcus pneumoniae (SP), and group B Streptococcus agalactiae (GBS). For VISA and VRSA variants of MRSA, the S-heptyl (1i) and S-octyl (1j) derivatives displayed equal or greater antibacterial activity than DSF and VAN.
Table 1.
Susceptibility of Gram-positive cocci to disulfides 1.
| test agent | speciesa | MIC (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| MRSA | VISA | VRSA | VISE | GAS | GBS | SP | VRE | |
| 1a | 32 | 8 | 16 | 32 | 32 | >32 | >32 | >32 |
| 1b | 16 | 8 | 16 | 32 | 32 | >32 | >32 | >32 |
| 1c | 16 | 8 | 16 | 32 | 32 | >32 | >32 | >32 |
| 1d | >32 | 32 | >32 | >32 | 32 | >32 | >32 | >32 |
| 1e | 16 | 16 | 16 | 32 | 32 | >32 | >32 | >32 |
| 1f | >32 | >32 | >32 | >32 | 32 | >32 | >32 | >32 |
| 1g | 16 | 8 | 16 | 8 | >32 | >32 | >32 | >32 |
| 1h | 16 | 4 | 8 | 8 | >32 | >32 | >32 | >32 |
| 1i | 4 | 2 | 4 | 4 | 16 | >32 | 32 | 8 |
| 1j | 4 | 4 | 4 | 4 | 16 | 32 | 16 | 8 |
| 1k | 8 | 8 | 16 | 4 | 8 | 32 | 8 | 8 |
| 1l | 16 | 16 | 32 | 16 | 4 | 16 | 4 | 16 |
| 1m | 32 | 16 | 32 | 32 | 32 | >32 | >32 | >32 |
| 1n | 32 | 16 | 32 | 32 | 16 | >32 | >32 | >32 |
| 1o | 8 | 2 | 8 | 8 | >32 | >32 | >32 | 32 |
| 1p | 32 | 16 | 16 | 16 | >32 | >32 | >32 | >32 |
| disulfiram | 32 | 8 | 32 | 32 | 16 | >32 | >32 | >32 |
| vancomycin | 1 | 4 | >32 | 8 | ≤0.5 | ≤0.5 | ≤0.5 | >32 |
methicillin-resistant Staphylococcus aureus COL (MRSA); vancomycin-intermediate resistant S. aureus ADR-217 (VISA); vancomycin-resistant S. aureus HIP14300 (VRSA); vancomycin-intermediate Staphylococcus epidermidis NRS6 (VISE); group A Streptococcus pyogenes H293 (GAS); group B Streptococcus agalactiae SGBS005 (GBS); Streptococcus pneumoniae TCH8431 (SP); vancomycin-resistant Enterococcus faecium ATCC 700221 (VRE).
The data in Table 1 further reveals a distinct correlation between the length of the S-alkylthio chain and antibacterial activity against Gram-positive cocci. Alkyl chains of seven (1i) and eight (1j) carbons were optimal lengths for antistaphylococcal activity with a MIC of 2 – 4 μM (0.6 – 1.2 μg/mL). By comparison, the MIC ranges of DSF and VAN were 8 – 64 μM (2.4 – 19 μg/mL) and 1 – >32 μM (1.5 – >48 μg/mL), respectively. Short straight chain analogs of one to five carbons were less active than their longer chain counterparts, but were more effective growth inhibitors of MRSA than DSF. Branch and cyclic carbon chain disulfides 1d, 1f, 1m, and 1n similarly had lower activity compared to the straight chain analogs and their respective unbranched equivalents 1c, 1e, 1g, and 1h.
Additional antibacterial testing of the compounds included the select agents Bacillus anthracis (anthrax), Francisella tularensis (tularemia) and Yersinia pestis (plague). Gram-positive B. anthracis exhibited the highest sensitivity to the DSF analogs followed by Gram-negative F. tularensis and Y. pestis (Table 2). In B. anthraces, it was noteworthy that a definitive structure-activity relationship could not be established for analogs with alkyl chains of one to eight carbons in length as seen in S. aureus. Moreover, DSF exhibited greater overall activity for all B. anthraces strains, but not to comparator ciprofloxacin (CIP), which was also the superior test agent against Y. pestis and F. tularensis.
Table 2.
Antibacterial activity of disulfides 1, disulfiram (DSF), and ciprofloxacin (CIP) against select agents.
| species | strain | MIC (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1a | 1c | 1g | 1h | 1i | 1j | 1o | DSF | CIP | ||
| Bacillus anthracis | Ames35 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 1 | 0.5 |
| Sterne 34F2 | 4 | 4 | 4 | 4 | 2 | 2 | 4 | 2 | ≤0.5 | |
| UM23-1 | 2 | 4 | 4 | 2 | 1 | 1 | 2 | 1 | ≤0.5 | |
| Weybridge | 4 | 4 | 8 | 8 | 2 | 2 | 4 | 2 | 0.5 | |
| Francisella tularensis | Utah 112 | 32 | 32 | 32 | 32 | 16 | 16 | 32 | 32 | ≤0.5 |
| Yersinia pestis | Kim (D2) | 16 | 16 | 32 | 32 | 32 | 32 | 16 | 32 | ≤0.5 |
| Kuma (D7) | 16 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | ≤0.5 | |
To further delineate the antibacterial activity spectrum, the compounds were tested on nineteen additional Gram-positive (n = 5) and Gram-negative (n = 14) species. Table 3 shows that the inhibitory activity was confined to Gram-positive bacteria with Bacillus cereus exhibiting the greatest susceptibility followed by another rod-shaped species, Listeria monocytogenes. Similar to B. anthraces, activity was not predicated on chain length in B. cereus; however, chain lengths of seven (1i) and eight (1j) carbons were the most effective inhibitors of L. monocytogenes as observed with S. aureus. Micrococcus luteus and Rhodococcus erythropolis were also moderately susceptible at a MIC range of 16 – 32 μM. Conversely, the Gram-negative species panel as a whole displayed negligible susceptibility to the analogs. Based on the overall test results, it was concluded that DSF and analogs 1 possess similar narrow-spectrum profiles and straight chain derivatives of seven and eight carbons in length are the most potent growth inhibitors of Gram-positive cocci.
Table 3.
Antibacterial activity of disulfides 1 against Gram-positive and Gram-negative species.
| Gram-positive | -negative | strain | MIC (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1a | 1c | 1g | 1h | 1i | 1j | 1o | DSF | CIP | ||
| Bacillus cereus | Gibson 971 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 4 | 0.5 |
| Corynebacterium striatum | FS-1 | >32 | >32 | 32 | 16 | 16 | 16 | 32 | >32 | >32 |
| Listeria monocytogenes | Gibson | 32 | 16 | 8 | 8 | 4 | 4 | 8 | >32 | 4 |
| Micrococcus luteus | SK58 | 32 | 16 | 16 | 16 | 16 | >32 | 16 | 32 | 4 |
| Rhodococcus erythropolis | SK121 | 16 | 16 | 16 | 8 | 16 | 16 | 16 | 16 | ≤0.5 |
|
| ||||||||||
| Acinetobacter baumannii | AB5075 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | ≤0.5 |
| Brucella neotomae | 5K33 | >32 | >32 | 32 | >32 | >32 | >32 | 32 | >32 | 1 |
| Burkholderia cepacia | UCB 717 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | 4 |
| Burkholderia multivorans | CF2 | 32 | 32 | >32 | >32 | >32 | >32 | 32 | 32 | 8 |
| Citrobacter freundii | 4_7_47CFAA | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | ≤0.5 |
| Escherichia coli | DC10B | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | ≤0.5 |
| Klebsiella pneumoniae | 700603 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | 2 |
| Proteus mirabilis | HM-752 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | ≤0.5 |
| Pseudomonas aeruginosa | 15442 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | 1 |
| Salmonella typhi | Ty2 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | ≤0.5 |
| Shigella dysenteriae | Newcastle 1934 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | 4 |
| Vibrio cholera | TS (D4) | 32 | 32 | >32 | >32 | >32 | >32 | >32 | >32 | ≤0.5 |
| Yersinia enterocolitica | WA-314 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | 2 |
| Yersinia pseudotuberculosis | P61 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | ≤0.5 |
With a potential application as a treatment for resistant staphylococcal infections, analog 1j was further evaluated for synergistic potential in comparison with DSF. The MICs of different VAN-1j and VAN-DSF concentration combinations were determined using the checkerboard microdilution assay in 96-well plate format [11,12]. Isobologram analysis revealed that analog 1j and DSF lowered the MIC of VAN in MRSA, VISA, and VISE by comparable additive effects (Table 3) [13]. In VRSA, a synergistic effect was observed for both analog 1j and DSF. From these studies it was concluded that disulfide 1j and DSF can similarly lower the MIC of VAN and, therefore, both may have therapeutic utility as antibiotic adjuvants for staphylococcal infections with reduced VAN susceptibility.
Table 3.
Results of in vitro synergy studies.
| strain | MIC (μg/mL)a | ΣFICd | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| VAN | DSF | 1j | VANb/DSFc | VANb/1j | VANb/DSFc | VANb/1jc | |
| MRSA COL | 2 | 8 | 2 | 1/1 | 1/0.25 | 0.63 (+) | 0.75 (+) |
| VISA AR-217 | 4 | 4 | 0.5 | 2/1 | 0.5/0.25 | 0.75 (+) | 0.56 (+) |
| VRSA HIP14300 | >128 | 16 | 2 | 8/2 | 1/1 | <0.16 (++) | 0.5 (++) |
| VISE NRS53 | 8 | 4 | 2 | 4/2 | 2/1 | 1 (+) | 0.75 (+) |
vancomycin: VAN; disulfiram: DSF
lowest MIC of VAN in combination with DSF or 1j
lowest MIC of DSF or 1j in combination with VAN
lowest ΣFIC measurement; synergy (++) ≤ 0.5; additive (+) 0.5 < to ≤ 1; indifferent (±) 1< to ≤ 4; antagonism (−) > 4 [12]
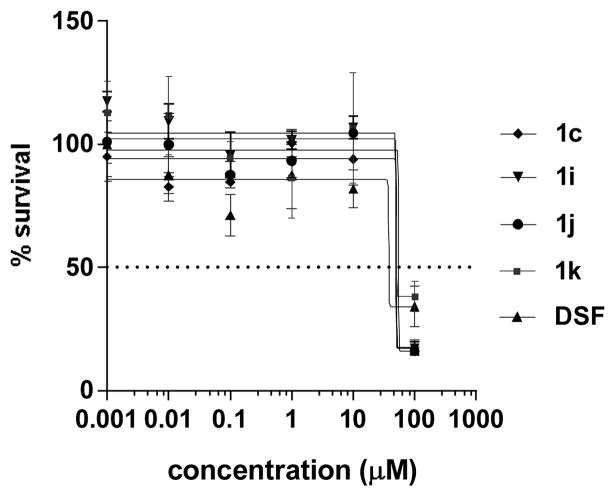
The investigation also compared the cytotoxicity of the analogs with DSF in human liver HepG2 carcinoma cells using the MTT assay [14]. The dose-response curve in Figure 2 revealed that the half-maximal inhibitory concentrations (IC50) of analogs 1c (51 μM), 1h (51 μM), 1j (50 μM), and 1k (55 μM) were above DSF (38 μM), thereby indicating lower cytotoxicity. The >13:1 ratio of S. aureus MIC (4 μM) to IC50 further suggests that compound 1j is selectively toxic to the bacterium. The apparent selectivity was partially attributed to the higher glutathione content in mammalian cells [15]. The thiophilic tripeptide, which is found in low abundance in Gram-positive bacteria, has been shown to inactivate disulfide-based antibacterials through a thiol-disulfide exchange reaction [6,14,16].
Figure 2.
Dose-response curve for liver HepG2 cells.
Additional studies of analog 1j included comparisons of microsomal stability and physiochemical properties to DSF (Table 4). Measurement of the in vitro metabolic stability using pooled rat liver microsomes indicated that the elimination half-life (t½) and intrinsic clearance (CLint) was longer for the DSF analog [17,18]. A comparison of the physiochemical chemical properties also revealed that both DSF and derivative 1j are hydrophobic compounds. The higher clogP and lower molecular polar surface area (PSA) for disulfide 1j suggest that replacement of the DDTC component in DSF with the more lipophilic S-octylthio group may confer better tissue and membranes penetration [19]. This marked difference with DSF could partially account for the increased susceptibility of S. aureus to analog 1j if cell entry is required of both agents to inhibit growth.
Table 4.
Microsomal stability and calculated physiochemical properties.
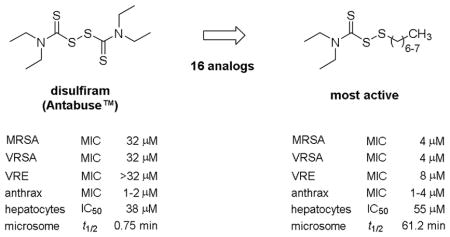
In the final analysis, S-alkylthio analogs of DSF exhibited up to eight times greater antibacterial activity compared to DSF. The activity spectrum of the analogs was similar to DSF with Gram-positive Staphylococcus and Bacillus spp. exhibiting the highest level of susceptibility. Analogs with S-heptylthio (1i) and S-octylthio (1j) groups were found to be the most effective inhibitors of MRSA growth and retained their potency against VISA and VRSA. The select antibacterial activity was partly attributed to the low abundance of redox-buffering glutathione in the cytoplasm of Gram-positive bacteria [14]. Glutathione has been shown to inactivate disulfide-based antibacterial agents [6,14,16] and the higher abundance in Gram-negative bacteria may account for the Gram-type selectivity. This investigation also considered that the outer membrane barrier in Gram-negative bacteria could be a factor; however, the data from Table 3 indicated that increasing the lipophilic property of the compounds, which would facilitate cell membrane permeation, did not effect antibacterial activity in Gram-negative bacteria. Other factors that would account for the Gram-type selectivity, but were not investigated during the study, are the existence of potential pharmacological targets and cellular pathways required for antibacterial action.
This research further resolved that disulfide 1j can lower the MIC of VAN in VISA and VISE, suggesting possible therapeutic utility as an antibiotic adjuvant in VAN-intermediate infections. Preliminary assessment of cytotoxicity and microsomal stability in comparison with DSF also confirmed that the S-octylthio analog was of lower toxicity to human hepatocytes and had a longer half-life, a parameter with implications on dosing frequency. Future research will focus on defining the mechanisms of action and resistance development for disulfide-based antibacterials. In addition, pharmacokinetic-pharmacodynamic (PK-PD) studies will be performed in vivo to determine their viability as antibiotic adjuvants in VAN therapy. The PK studies will be used to guide dosing with a regimen that accounts for the influence of tissue glutathione on the disulfide concentration and gives a preferred trough VAN concentration of 4 to 5 times the MIC [22] with the disulfide for the test pathogen (e.g., VISA).
Acknowledgments
This research was supported by the Marshall University School of Pharmacy FRS Grant Program. The authors also thank the Marshall University Department of Chemistry for use of the NMR facility. Bacteria were acquired from the American Type Culture Collection, FDA-CDC Antimicrobial Resistance Isolate Bank, and the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) for distribution by BEI Resources, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ellis PM, Dronsfield AT. Antabuse’s diamond anniversary: still sparkling on? Drug Alcohol Rev. 2013;32:342–344. doi: 10.1111/dar.12018. [DOI] [PubMed] [Google Scholar]
- 2.Shen ML, Lipsky JJ, Naylor S. Role of disulfiram in the in vitro inhibition of rat liver mitochondrial aldehyde dehydrogenase. Biochem Pharmacol. 2000;60:947–953. doi: 10.1016/s0006-2952(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 3.Shen ML, Johnson KL, Mays DC, Lipsky JJ, Naylor S. Determination of in vivo adducts of disulfiram with mitochondrial aldehyde dehydrogenase. Biochem Pharmacol. 2001;61(5):537–45. doi: 10.1016/s0006-2952(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 4.Wright C, Moore RD. Disulfiram treatment of alcoholism. Am J Med. 1990;88:647–655. doi: 10.1016/0002-9343(90)90534-k. [DOI] [PubMed] [Google Scholar]
- 5.ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2018. Mar 2, [Google Scholar]
- 6.Long TE. Repurposing Thiram and Disulfiram as Antibacterial Agents for Multidrug-Resistant Staphylococcus aureus Infections. Antimicrob Agents Chemother. 2017;61(9) doi: 10.1128/AAC.00898-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preparation of thiuram disulfide 1; general procedure: Disulfiram (200 mg, 0.68 mmol) and thiol (0.74 mmol) were combined in 1 mL of dry DMF and stirred for 10–18 h at 60°C. The solution was then cooled to room temperature, diluted with water and extracted twice with hexanes. The organic layers were combined, washed twice with water, dried over MgSO4, filtered, and concentrated in vacuo Silica gel flash chromatography with 0–10% EtOAc in hexanes gave disulfide 1 as an oil. Spectra data of representative compound 1-[(diethylcarbamothioyl)disulfanyl]octane (1j): Yield 53%; pale oil; TLC (SiO2) Rf 0.52 (9:1 hexanes:EtOAc); 1H NMR (400 MHz, CDCl3) δ 4.07–4.02 (m, 2H), 3.84–3.78 (m, 2H), 2.84 (t, J = 7.2 Hz, 2H), 1.68–1.62 (m, 2H), 1.38–1.26 (m, 16H), 0.86 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 196.4, 51.8, 47.1, 38.8, 32, 29.3, 28.7, 22.9, 14.3, 13.3, 11.6; ESI–MS: m/z 294.2 [M+H]+.
- 8.Cilibrasi V, Tsang K, Morelli M, Solfa F, Wiggins HL, Jones AT, Westwell AD. Synthesis of substituted carbamo(dithioperoxo)thioates as potential BCA2-inhibitory anticancer agents. Tetrahedron Lett. 2015;56:2583–2585. [Google Scholar]
- 9.Jorgensen JH, Turnidge JD. Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. ASM Press; Washington, DC: 2007. pp. 1152–1172. [Google Scholar]
- 10.Susceptibility testing. Bacteria were initially grown on Mueller-Hinton agar, Brucella agar, or tryptic soy agar with 5% sheep’s blood from frozen stocks stored at −80°C. Test cultures were obtained from uniform colonies and enumerated overnight at 26 – 37°C ± 5% CO2 in standard media: Mueller Hinton broth for Bacillus spp., M. luteus and Staphylococcus spp.; Todd Hewitt broth for Streptococcus spp.; tryptic soy broth for Enterobacteriaceae spp., Burkholderia spp. and P. aeruginosa; Brucella broth for B. neotomae and C. freundii; and brain heart infusion (BHI) broth for E. faecium, F. tularensis, L. monocytogenes, P. multocida, and R. erythropolis. All bacteria except, R. erythropolis (30°C) and Yersinia spp. (28 °C) were cultured at 37°C. C. freundii, E. faecium, F. tularensis and Streptococcus spp. were incubated under 5% CO2 atmosphere. Briefly, overnight cultures adjusted to a 0.5 McFarland standard and diluted to 1:100 in the appropriate growth medium were treated with serial dilutions of disulfide 1 and disulfiram prepared as 1 mM or 1 mg/mL stocks in DMSO. Controls vancomycin and ciprofloxacin were prepared in ultrapure pure water. The plates were then incubated for 20 hours and the MICs were recorded as the lowest drug concentration that gave complete inhibition of visual growth.
- 11.Moody JA. Synergism testing: Broth microdilution checkboard and broth macrodilution methods. In: Isenberg HD, editor. Clinical Procedures Handbook. ASM Press; Washington, DC: 1992. pp. 5.18.1–5.18.28. [Google Scholar]
- 12.Synergy testing. Evaluation of synergistic activity was performed by isobologram analysis using the checkerboard microdilution assay. A 1:100 dilution of 0.5 McFarland bacterial suspension was used to inoculate a 96-well plate containing 2-fold serial dilutions of disulfide 1j and VAN in 50 μL CAMHB. The plates were sealed with adhesive film and incubated for 20 h at 37°C. The fractional inhibitory concentration (FIC) index was calculated by dividing the MIC of the VAN-1j combination by the MIC of VAN or 1j alone. The summative ΣFIC was calculated from the FIC values and interpreted according to the standard metrics: synergy ≤ 0.5; additive 0.5 < to ≤ 1; indifferent 1< to ≤ 4; antagonism > 4.
- 13.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 14.Sheppard JG, Mcaleer JP, Saralkar P, Geldenhuys WJ, Long TE. Allicin-inspired pyridyl disulfides as antimicrobial agents for multidrug-resistant Staphylococcus aureus. Eur J Med Chem. 2018;143:1185–1195. doi: 10.1016/j.ejmech.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loi VV, Rossius M, Antelmann H. Redox regulation by reversible protein S-thiolation in bacteria. Front Microbiol. 2015;6:187. doi: 10.3389/fmicb.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramaraju P, Gergeres D, Turos E, Dickey S, Lim DV, Thomas J, Anderson B. Synthesis and antimicrobial activities of structurally novel S,S′-bis(heterosubstituted) disulfides. Bioorg Med Chem Lett. 2012;22(11):3623–31. doi: 10.1016/j.bmcl.2012.04.056. [DOI] [PubMed] [Google Scholar]
- 17.Shave D, Alden P. Application note. Waters Corporation; Milford, MA U.S.A: 2008. Determination of microsomal stability by UPLC/MS/MS. [Google Scholar]
- 18.Microsome study. Experimental solutions for the metabolic stability were prepared as per the method described in the Waters Corporation application note [15]. The metabolic reactions were initiated by adding the enzyme to the pre-incubated mixture of test compound and NADPH solutions in a 2 mL Eppendorf tube. The mixtures were incubated at 37°C for 0, 5, 10, 15, 30, and 60 mins with mild shaking. The reaction was terminated after each specified time point by placing it in an ice bath and adding 500 μl of cold MeCN. The incubation mixtures were then centrifuged for 10 mins at 3000 rpm and an aliquot of each supernatant transferred for analysis. Test compounds are quantified using LC/MS/MS, and its metabolic half-life in the in vitro test system is derived from percent remaining vs. time data. The LC-MS/MS setup consisting of Agilent 6490 triple quadrupole mass spectrometer equipped with an Agilent 1260 Infinity HPLC unit. Positive ESI LC-MS/MS was done using MRM transitions 297.06 > 214.8 and 294.14>148.1 for DSF and disulfide analog 1j, respectively. Separation was performed on Agilent Eclipse plus C18 column eluted with MeOH-water gradient acidified with 0.1% formic acid.
- 19.Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45(12):2615–23. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 20.Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02.
- 21.Calculated from ChemDraw Ultra Software 8.0.
- 22.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–44. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]