Abstract
This integrative multistage study was aimed to identify circulating microRNAs (miRNAs) as prognostic biomarkers and investigate the treatment target for early-stage non-small cell lung cancer (NSCLC) patients. In stage I–II NSCLC patients, we screened and validated the miRNA ratio signatures predictive of prognosis in serum. In tumor, we found that the expression of miR-150 in identified miRNA signatures was also associated with survival. Increased miR-150 expression promoted NSCLC cell proliferation and migration and vice versa. Specific mRNA cleavage sites targeted by endogenous miR-150 in 3′ untranslated region (UTR) of SRCIN1 was identified by utilizing our recently developed novel Stem-Loop-Array reverse-transcription polymerase chain reaction (SLA-RT-PCR) assay. The blocking action of miR-150 resulted in repressed NSCLC cell growth in vitro and knockdown of miR-150 caused substantial tumor volume reduction in vivo. Our findings suggest that miR-150 binding on specific recognition sites in 30 UTR of tumor suppressor gene SRCIN1 present a potential therapeutic target for NSCLC.
Early-stage non-small cell lung cancer (NSCLC) can be curatively treated with surgery alone or with adjuvant chemotherapy; however, about half of patients experience disease recurrence and eventually die of NSCLC.1–3 MicroRNAs (miRNAs) are a group of small noncoding RNAs that have been known to regulate the expression of a wide spectrum of genes, resulting in altered cellular processes such as cell proliferation, differentiation, and apoptosis.4 Researchers have found that miRNAs can act as either oncogenes or tumor suppressor genes and their dysregulated expression has been found in most cancer types.5,6 As a result, miRNAs are promising biomarkers and therapeutic targets for cancer.4,7–11 In lung cancer patients, definitive preoperative diagnosis of pulmonary nodules using computed tomography is challenging. Therefore, the identification of miRNA and target genes that can be used to predict clinical outcomes and select therapeutic strategies for early-stage NSCLC is clearly needed. The potential diagnostic and prognostic utility of circulating miRNAs in patients with lung cancer has attracted extensive interest.12 Moreover, lung cancer phenotypes can be modified by altered miRNA expression.12 While previous studies have addressed the association of circulating miRNAs in lung cancer patients and clinical outcome, most of those investigations focused on patients with late-stage disease.13,14 Only a few studies have assessed the prognosis in early-stage NSCLC patients, mostly using tumor tissues,15–17 and the results were inconsistent. In addition, few studies included functional investigation to examine biological relevance.
In this multistage, integrative study, we determined circulating miRNA ratio signatures that could predict clinical outcomes in a large series of patients with early-stage (stages I and II) NSCLC. We further carried out functional validation in vitro and in vivo, identifying specific mRNA cleavage sites in the 3′ untranslated region (UTR) of tumor suppressor gene SRC kinase signaling inhibitor 1 gene (SRCIN1) targeted by endogenous miR-150 by utilizing our recently developed novel stem-loop-array reverse-transcription polymerase chain reaction (SLA-RT-PCR) assay. These results suggest for the first time that miR-150 is a potential therapeutic target for NSCLC.
Results
Patient characteristics and serum miRNA profiling
A schematic of the study design is shown in Supplemental Figure S1. We studied circulating miRNAs in blood samples taken from 171 Caucasian patients with early-stage NSCLC. The clinical and sociodemographic characteristics of the patients are listed in Supplemental Table S1. We split the patients into discovery and validation cohorts. The median follow-up durations for the discovery, validation, and overall cohorts were 47.3, 49.3, and 47.9 months, respectively. We found no significant differences (P > 0.05) between the discovery and validation cohorts with respect to age, sex, smoking history, disease recurrence, treatment modality, clinical stage, or follow-up time. We selected 89 miR-NAs for large-scale miRNA profiling from two sources: 1) previously published studies of stably detected circulating miRNAs in lung cancer,18–21 and 2) our pilot miRNA profiling of 20 individuals using a TaqMan Array Human MicroRNA Card Set containing 754 human miRNAs.13 Among the 89 examined miRNAs, 56 passed quality control of the Fluidigm platform; we included them in a subsequent analysis (Supplemental Table S2).
Association of serum miRNA expression with NSCLC 5-year recurrence and survival
We calculated the ratio between any two of the 56 candidate miRNAs, as reported previously.18 A total of 43 miRNA ratios were consistently significantly associated with 5-year recurrence rates (P < 0.05) in both the discovery and validation cohorts. After excluding highly correlated miRNA ratios (rho > 0.7), 22 miRNAs were included in final analysis (Table 1). The miRNA ratio most strongly associated with disease recurrence was miR-18a/328 (hazard ratio (HR), 0.18; 95% confidence interval (CI), 0.08–0.39; P = 1.04 × 10−5). Thirteen miRNA ratios were consistently associated with 5-year survival rates (P < 0.05) in both the discovery and validation sets (Table 2). Of these, miR-27a/150 was strongly associated with risk of death from NSCLC (HR, 3.92; 95% CI, 1.94–7.94; P = 1.46 × 10−4).
Table 1. Association of selected microRNA ratios and 5-year cancer recurrence rates in patients with early-stage non-small cell lung cancer.
| Expression levle | Discovery | Validation | Combined | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recurrence (N, %) |
No recurrence (N, %) |
HRa (95% CI) |
P | Recurrence (N,%) |
No recurrence (N,%) |
HRa (95% CI) |
P | Recurrence (N,%) |
No recurrence (N,%) |
HRa (95%CI) |
P | ||
| miR-18a/25 | Low | 10 (45.45) | 12 (54.55) | 1 | 8 (33.33) | 16 (66.67) | 1 | 18 (39.13) | 28 (60.87) | 1 | |||
| High | 9 (20.00) | 36 (80.00) | 0.27 (0.09-0.76) | 1.33E-02 | 11 (24.44) | 34 (75.56) | 0.28 (0.09-0.87) | 2.69E-02 | 20 (22.22) | 70 (77.78) | 0.3 (0.14-0.63) | 1.55E-03 | |
| miR-18a/93 | Low | 9 (42.86) | 12 (57.14) | 1 | 11 (42.31) | 15 (57.69) | 1 | 20 (42.55) | 27 (57.45) | 1 | |||
| High | 9 (18.75) | 39 (81.25) | 0.20 (0.06-0.66) | 8.03E-03 | 10 (21.74) | 36 (78.26) | 0.27 (0.1-0.76) | 1.33E-02 | 19 (20.21) | 75 (79.79) | 0.3 (0.15-0.61) | 9.05E-04 | |
| miR-18a/328 | Low | 10 (50.00) | 10 (50.00) | 1 | 11 (44.00) | 14 (56.00) | 1 | 21 (46.67) | 24 (53.33) | 1 | |||
| High | 9 (20.45) | 35 (79.55) | 0.17 (0.06-0.52) | 1.95E-03 | 8 (17.78) | 37 (82.22) | 0.17 (0.05-0.52) | 2.16E-03 | 17 (19.10) | 72 (80.90) | 0.18 (0.08-0.39) | 1.04E-05 | |
| miR-19b/339-3p | Low | 5 (14.29) | 30 (85.71) | 1 | 6 (17.14) | 29 (82.86) | 1 | 11 (15.71) | 59 (84.29) | 1 | |||
| High | 12 (35.29) | 22 (64.71) | 3.42 (1.10-10.65) | 3.36E-02 | 15 (40.54) | 22 (59.46) | 3.82 (1.32-11.07) | 1.35E-02 | 27 (38.03) | 44 (61.97) | 3.36 (1.58-7.14) | 1.65E-03 | |
| miR-17/486-5p | Low | 3 (13.04) | 20 (86.96) | 1 | 4 (16.00) | 21 (84.00) | 1 | 7 (14.58) | 41 (85.42) | 1 | |||
| High | 17 (33.33) | 34 (66.67) | 4.52 (1.02-20.16) | 4.77E-02 | 16 (34.78) | 30 (65.22) | 5.26 (1.38-20.07) | 1.51E-02 | 33 (34.02) | 64 (65.98) | 3.91 (1.5-10.18) | 5.25E-03 | |
| miR-20a/24 | Low | 10 (21.74) | 36 (78.26) | 1 | 14 (26.92) | 38 (73.08) | 1 | 24 (24.49) | 74 (75.51) | 1 | |||
| High | 8 (33.33) | 16 (66.67) | 3.03 (1.00-9.18) | 4.99E-02 | 8 (32.00) | 17 (68.00) | 3.19 (1.07-9.52) | 3.76E-02 | 16 (32.65) | 33 (67.35) | 2.37 (1.12-5) | 2.40E-02 | |
| miR-27a/146a | Low | 7 (16.67) | 35 (83.33) | 1 | 5 (15.15) | 28 (84.85) | 1 | 12 (16.00) | 63 (84.00) | 1 | |||
| High | 12 (40.00) | 18 (60.00) | 3.80 (1.23-11.72) | 2.02E-02 | 16 (37.21) | 27 (62.79) | 4.87 (1.07-22.2) | 4.10E-02 | 28 (38.36) | 45 (61.64) | 4.49 (1.87-10.75) | 7.60E-04 | |
| miR-27a/324-3p | Low | 3 (15.00) | 17 (85.00) | 1 | 4 (15.38) | 22 (84.62) | 1 | 7 (15.22) | 39 (84.78) | 1 | |||
| High | 15 (31.91) | 32 (68.09) | 6.14 (1.13-33.41) | 3.59E-02 | 16 (36.36) | 28 (63.64) | 4.48 (1.24-16.23) | 2.22E-02 | 31 (34.07) | 60 (65.93) | 3.09 (1.25-7.59) | 1.41E-02 | |
| miR-27a/331-3p | Low | 3 (12.50) | 21 (87.50) | 1 | 3 (12.50) | 21 (87.50) | 1 | 6 (12.50) | 42 (87.50) | 1 | |||
| High | 15 (32.61) | 31 (67.39) | 5.71 (1.23-26.43) | 2.58E-02 | 17 (35.42) | 31 (64.58) | 12.89 (2.35-70.74) | 3.25E-03 | 32 (34.04) | 62 (65.96) | 3.03 (1.22-7.52) | 1.67E-02 | |
| miR-27a/339-3p | Low | 6 (13.33) | 39 (86.67) | 1 | 9 (21.43) | 33 (78.57) | 1 | 15 (17.24) | 72 (82.76) | 1 | |||
| High | 9 (45.00) | 11 (55.00) | 6.17 (1.86-20.46) | 2.95E-03 | 10 (41.67) | 14 (58.33) | 7.51 (2.12-26.62) | 1.79E-03 | 19 (43.18) | 25 (56.82) | 4.07 (1.87-8.87) | 4.18E-04 | |
| miR-27a/484 | Low | 10 (19.61) | 41 (80.39) | 1 | 12 (24.00) | 38 (76.00) | 1 | 22 (21.78) | 79 (78.22) | 1 | |||
| High | 10 (41.67) | 14 (58.33) | 2.72 (1.07-6.93) | 3.63E-02 | 9 (34.62) | 17 (65.38) | 4.63 (1.48-14.51) | 8.57E-03 | 19 (38.00) | 31 (62.00) | 3.12 (1.53-6.35) | 1.69E-03 | |
| miR-25/93* | Low | 6 (20.69) | 23 (79.31) | 1 | 11 (26.19) | 31 (73.81) | 1 | 17 (23.94) | 54 (76.06) | 1 | |||
| High | 13 (32.50) | 27 (67.50) | 3.98 (1.18-13.5) | 2.64E-02 | 10 (29.41) | 24 (70.59) | 3.86 (1.28-11.59) | 1.62E-02 | 23 (31.08) | 51 (68.92) | 2.58 (1.22-5.44) | 1.31E-02 | |
| miR-30b/150 | Low | 6 (13.95) | 37 (86.05) | 1 | 11 (22.92) | 37 (77.08) | 1 | 17 (18.68) | 74 (81.32) | 1 | |||
| High | 9 (40.91) | 13 (59.09) | 8.38 (2.26-31.11) | 1.48E-03 | 8 (34.78) | 15 (65.22) | 3.32 (1.06-10.34) | 3.87E-02 | 17 (37.78) | 28 (62.22) | 4.97 (2.2-11.22) | 1.14E-04 | |
| miR-30c/423-5p | Low | 7 (16.67) | 35 (83.33) | 1 | 8 (18.18) | 36 (81.82) | 1 | 15 (17.44) | 71 (82.56) | 1 | |||
| High | 8 (36.36) | 14 (63.64) | 3.57 (1.16-11.01) | 2.69E-02 | 7 (33.33) | 14 (66.67) | 5.03 (1.28-19.68) | 2.03E-02 | 15 (34.88) | 28 (65.12) | 4.24 (1.84-9.77) | 6.75E-04 | |
| miR-106b/126 | Low | 12 (25.00) | 36 (75.00) | 1 | 9 (20.93) | 34 (79.07) | 1 | 21 (23.08) | 70 (76.92) | 1 | |||
| High | 8 (44.44) | 10 (55.56) | 4.34 (1.4-13.39) | 1.07E-02 | 10 (35.71) | 18 (64.29) | 3.46 (1.26-9.54) | 1.63E-02 | 18 (39.13) | 28 (60.87) | 3.42 (1.67-7) | 7.56E-04 | |
| miR-106b/320 | Low | 10 (23.26) | 33 (76.74) | 1 | 12 (23.53) | 39 (76.47) | 1 | 22 (23.40) | 72 (76.60) | 1 | |||
| High | 8 (34.78) | 15 (65.22) | 3.44 (1.13-10.44) | 2.95E-02 | 8 (33.33) | 16 (66.67) | 3.45 (1.21-9.82) | 2.04E-02 | 16 (34.04) | 31 (65.96) | 2.54 (1.25-5.16) | 1.01E-02 | |
| miR-142-3p/195 | Low | 11 (26.19) | 31 (73.81) | 1 | 12 (21.82) | 43 (78.18) | 1 | 23 (23.71) | 74 (76.29) | 1 | |||
| High | 9 (29.03) | 22 (70.97) | 3.98 (1.26-12.6) | 1.87E-02 | 7 (38.89) | 11 (61.11) | 4.74 (1.32-17.01) | 1.69E-02 | 16 (32.65) | 33 (67.35) | 2.10 (1.03-4.27) | 4.09E-02 | |
| miR-142-3p/342-3p | Low | 9 (18.75) | 39 (81.25) | 1 | 10 (19.61) | 41 (80.39) | 1 | 19 (19.19) | 80 (80.81) | 1 | |||
| High | 11 (37.93) | 18 (62.07) | 3.67 (1.15-11.7) | 2.80E-02 | 9 (42.86) | 12 (57.14) | 4.98 (1.52-16.27) | 7.91E-03 | 20 (40.00) | 30 (60.00) | 3.81 (1.84-7.9) | 3.26E-04 | |
| miR-186/342-3p | Low | 7 (14.58) | 41 (85.42) | 1 | 15 (27.27) | 40 (72.73) | 1 | 22 (21.36) | 81 (78.64) | 1 | |||
| High | 11 (39.29) | 17 (60.71) | 7.75 (2.38-25.22) | 6.78E-04 | 9 (36.00) | 16 (64.00) | 3.02 (1.06-8.67) | 3.93E-02 | 20 (37.74) | 33 (62.26) | 3.61 (1.77-7.37) | 4.32E-04 | |
| miR-191/331-3p | Low | 3 (15.79) | 16 (84.21) | 1 | 5 (20.00) | 20 (80.00) | 1 | 8 (18.18) | 36 (81.82) | 1 | |||
| High | 13 (29.55) | 31 (70.45) | 12.95 (1.63-103.05) | 1.55E-02 | 15 (32.61) | 31 (67.39) | 7.59 (1.48-38.88) | 1.50E-02 | 28 (31.11) | 62 (68.89) | 2.62 (1.07-6.42) | 3.44E-02 | |
| miR-151-3p/423-5p | Low | 12 (28.57) | 30 (71.43) | 1 | 12 (25.00) | 36 (75.00) | 1 | 24 (26.67) | 66 (73.33) | 1 | |||
| High | 3 (14.29) | 18 (85.71) | 0.07 (0.01-0.57) | 1.23E-02 | 5 (21.74) | 18 (78.26) | 0.16 (0.04-0.74) | 1.85E-02 | 8 (18.18) | 36 (81.82) | 0.40 (0.17-0.96) | 4.06E-02 | |
| miR-223*/342-3p | Low | 7 (19.44) | 29 (80.56) | 1 | 9 (24.32) | 28 (75.68) | 1 | 16 (21.92) | 57 (78.08) | 1 | |||
| High | 13 (37.14) | 22 (62.86) | 4.71 (1.47-15.09) | 8.98E-03 | 13 (32.50) | 27 (67.50) | 4.20 (1.47-11.98) | 7.29E-03 | 26 (34.67) | 49 (65.33) | 2.39 (1.23-4.65) | l.00E-02 | |
HR, hazard ratio; CI, confidence interval.
Adjusted for age, sex, smoking history, clinical stage, and treatment regimen.
Table 2. Association of selected microRNA ratios and 5-year survival rates in patients with early-stage non-small cell lung cancer.
| Expression level | Discovery | Validation | Combined | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dead (N, %) |
Alive (N, %) |
HRa (95% CI) |
P | Dead (N, %) |
Alive (N, %) |
HRa (95% CI) |
P | Dead (N, %) |
Alive (N, %) |
HRa (95% CI) |
P | ||
| miR-19b/484 | Low | 9 (20.00) | 36 (80.00) | 1 | 14 (35.90) | 25 (64.10) | 1 | 23 (27.38) | 61(72.62) | 1 | |||
| High | 18 (46.15) | 21 (53.85) | 2.70 (1.06-6.89) | 3.74E-02 | 27 (61.36) | 17 (38.64) | 2.34 (1.13-4.85) | 2.15E-02 | 45 (54.22) | 38 (45.78) | 2.51 (1.45-4.34) | 9.68E-04 | |
| miR-24/142-3p | Low | 20 (35.71) | 36 (64.29) | 1 | 26 (59.09) | 18 (40.91) | 1 | 46 (46.00) | 54 (54.00) | 1 | |||
| High | 2 (9.52) | 19 (90.48) | 0.19 (0.04-0.89) | 3.48E-02 | 9 (31.03) | 20 (68.97) | 0.43 (0.19-0.98) | 4.51E-02 | 11 (22.00) | 39 (78.00) | 0.35 (0.18-0.70) | 3.07E-03 | |
| miR-27a/142-3p | Low | 19 (37.25) | 32 (62.75) | 1 | 26 (60.47) | 17 (39.53) | 1 | 45 (47.87) | 49 (52.13) | 1 | |||
| High | 3 (14.29) | 18 (85.71) | 0.25 (0.07-0.89) | 3.19E-02 | 7 (26.92) | 19 (73.08) | 0.41 (0.17-0.98) | 4.44E-02 | 10 (21.28) | 37 (78.72) | 0.38 (0.19-0.75) | 5.75E-03 | |
| miR-27a/146a | Low | 14 (26.42) | 39 (73.58) | 1 | 18 (38.30) | 29 (61.70) | 1 | 32 (32.00) | 68 (68.00) | 1 | |||
| High | 8 (42.11) | 11 (57.89) | 2.58 (1.04-6.42) | 4.14E-02 | 20 (68.97) | 9 (31.03) | 2.06 (1.06-4.03) | 3.35E-02 | 28 (58.33) | 20 (41.67) | 2.04 (1.20-3.47) | 8.31E-03 | |
| miR-27a/150 | Low | 4 (14.81) | 23 (85.19) | 1 | 6 (27.27) | 16 (72.73) | 1 | 10 (20.41) | 39 (79.59) | 1 | |||
| High | 18 (40.91) | 26 (59.09) | 3.29 (1.06-10.26) | 3.97E-02 | 31 (59.62) | 21 (40.38) | 4.13 (1.61-10.58) | 3.11E-03 | 49 (51.04) | 47 (48.96) | 3.92 (1.94-7.94) | 1.46E-04 | |
| miR-27a/339-3p | Low | 11 (24.44) | 34 (75.56) | 1 | 16 (38.10) | 26 (61.90) | 1 | 27 (31.03) | 60 (68.97) | 1 | |||
| High | 10 (50.00) | 10 (50.00) | 4.33 (1.52-12.38) | 6.17E-03 | 17 (70.83) | 7 (29.17) | 2.97 (1.32-6.66) | 8.26E-03 | 27 (61.36) | 17 (38.64) | 2.56 (1.41-4.63) | 1.93E-03 | |
| miR-27a/532-3p | Low | 11 (22.92) | 37 (77.08) | 1 (reference) | 19 (39.58) | 29 (60.42) | 1 | 30 (31.25) | 66 (68.75) | 1 | |||
| High | 10 (43.48) | 13 (56.52) | 2.92 (1.06-8.03) | 3.85E-02 | 16 (64.00) | 9 (36.00) | 2.68 (1.29-5.58) | 8.44E-03 | 26 (54.17) | 22 (45.83) | 1.94 (1.14-3.31) | 1.51E-02 | |
| miR-29a/142-3p | Low | 18 (36.73) | 31 (63.27) | 1 | 23 (57.50) | 17 (42.50) | 1 | 41 (46.07) | 48 (53.93) | 1 | |||
| High | 4 (19.05) | 17 (80.95) | 0.28 (0.09-0.92) | 3.52E-02 | 6 (25.00) | 18 (75.00) | 0.36 (0.14-0.91) | 3.12E-02 | 10 (22.22) | 35 (77.78) | 0.36 (0.18-0.74) | 5.14E-03 | |
| miR-30c/150 | Low | 9 (20.00) | 36 (80.00) | 1 | 19 (41.30) | 27 (58.70) | 1 | 28 (30.77) | 63 (69.23) | 1 | |||
| High | 10 (43.48) | 13 (56.52) | 2.74 (1.03-7.33) | 4.44E-02 | 14 (70.00) | 6 (30.00) | 2.37 (1.12-5.01) | 2.42E-02 | 24 (55.81) | 19 (44.19) | 2.55 (1.43-4.55) | 1.51E-03 | |
| miR-30c/532-3p | Low | 8 (17.78) | 37 (82.22) | 1 | 17 (39.53) | 26 (60.47) | 1 | 25 (28.41) | 63 (71.59) | 1 | |||
| High | 9 (42.86) | 12 (57.14) | 2.71 (1.03-7.17) | 4.42E-02 | 14 (63.64) | 8 (36.36) | 2.62 (1.17-5.89) | 1.94E-02 | 23 (53.49) | 20 (46.51) | 2.38 (1.28-4.44) | 6.19E-03 | |
| miR-106b/92a | Low | 10 (22.22) | 35 (77.78) | 1 | 21 (47.73) | 23 (52.27) | 1 | 31 (34.83) | 58 (65.17) | 1 | |||
| High | 9 (52.94) | 8 (47.06) | 3.23 (1.09-9.55) | 3.40E-02 | 16 (64.00) | 9 (36.00) | 2.26 (1.05-4.87) | 3.71E-02 | 25 (59.52) | 17 (40.48) | 2.91 (1.62-5.24) | 3.70E-04 | |
| miR-106b/221 | Low | 7 (20.00) | 28 (80.00) | 1 | 12 (42.86) | 16 (57.14) | 1 | 19 (30.16) | 44 (69.84) | 1 | |||
| High | 11 (45.83) | 13 (54.17) | 4.24 (1.29-13.95) | 1.74E-02 | 22 (61.11) | 14 (38.89) | 3.08 (1.28-7.42) | 1.22E-02 | 33 (55.00) | 27 (45.00) | 3.89 (1.94-7.79) | 1.32E-04 | |
| miR-106b/532-3p | Low | 9 (21.95) | 32 (78.05) | 1 | 20 (41.67) | 28 (58.33) | 1 | 29 (32.58) | 60 (67.42) | 1 | |||
| High | 10 (43.48) | 13 (56.52) | 3.21 (1.12-9.19) | 2.94E-02 | 13 (59.09) | 9 (40.91) | 2.27 (1.05-4.92) | 3.75E-02 | 23 (51.11) | 22 (48.89) | 1.86 (1.06-3.26) | 3.01E-02 | |
HR, hazard ratio; CI, confidence interval.
Adjusted for age, sex, smoking history, clinical stage, and treatment regimen.
We then assessed the discriminatory accuracy of each miRNA ratio in predicting recurrence and survival rates by constructing receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC). Among the different combinations of miRNA ratios, risk scores for recurrence and survival based on a 7-miRNA ratio signature (miR-18a/328, miR-142-3p/342-3p, miR-186/342-3p, miR-18a/93, miR-19b/339-3p, miR-17/486-5p, and miR-151-3p/423-5p) and a 5-miRNA ratio signature (miR-24/142-3p, miR-27a/150, miR-27a/339-3p, miR-27a/532-3p, and miR-106b/532-3p) exhibited the most improvement in predictive accuracy compared with clinical factors. The high-risk group identified using the 7-miRNA ratio signature had a high disease recurrence rate of 41%, with a more than 24-fold (HR, 24.13; 95% CI, 3.05–190.79; P= 0.003) higher risk of developing recurrence than the low-risk patients, who had a low recurrence rate of 4.8% in the combined set (Figure 1a). The high-risk group identified using the 5-miRNA ratio signature had a high 5-year death rate of 63%, with an approximately 4-fold (HR, 3.88; 95% CI, 1.94–7.76, P= 0.0001) higher risk of death than the low-risk patients, who had a 23% death rate in the combined set (Figure 1b).
Figure 1.
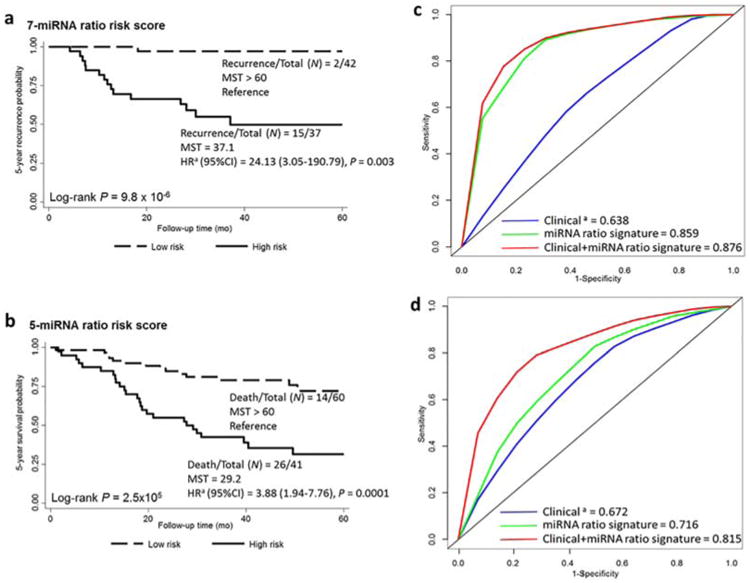
Kaplan–Meier curves and discriminatory accuracy of the models. Kaplan–Meier curves for the 5-year recurrence (a) and survival (b) rates in 171 patients with early-stage NSCLC in high-risk (solid line) and low-risk (dashed line) groups. The HR for association of the miRNA-ratio risk score with recurrence or death within 5 years was calculated; adjusted for age, sex, smoking history, clinical stage, and treatment modality. N, number of patients with the event (recurrence or death) at 5 years/total number of patients in the dataset. MST, median survival time. (c) Receiver operating characteristic curve showing the discriminatory accuracy of clinical factors, the 7-miRNA ratio signature, and a combination of the 7-miRNA ratio signature and clinical factors for predicting 5-year NSCLC recurrence. (d) Receiver operating characteristic curve showing the discriminatory accuracy of clinical factors, the 5-miRNA ratio signature, and a combination of the 5-miRNA ratio signature clinical factors for predicting death from NSCLC within 5 years. The key shows the areas under the curve for all groups. aIncluding age, sex, smoking history, clinical stage, and treatment modality. [Color figure can be viewed at wileyonlinelibrary.com]
A Kaplan–Meier curve comparing 5-year recurrence rates demonstrated that patients with high risk scores had a significantly shorter median recurrence-free survival time (37.1 months) and median overall survival time (29.2 months) than did the low-risk group in the combined set (>60 months, log-rank P= 9.8 × 10−6 and 2.5 × 10−5) (Figure 1a,b). We compared the AUCs of three models: the first model only considered clinical variables, including age, sex, smoking history, clinical stage, and treatment modality; the second model only included the miRNA ratio signatures; and the third one combined the 7-miRNA or 5-miRNA ratio signatures with the clinical variables (Figure 1c,d). In the combined dataset, the AUCs of the three models for predicting 5-year recurrence were 0.638, 0.859, and 0.876, respectively. For predicting 5-year survival, the AUCs were 0.672, 0.716, and 0.815, respectively. With the addition of the 7-miRNA or 5-miRNA ratio signature, the AUCs for recurrence and survival improved ∼37% and 21% compared with clinical factors, respectively.
Expression of miR-150 in tumor is associated with 5-year survival
Next, we built univariate and multivariate Cox proportional hazards models to verify the association of expression of the identified miRNAs and survival in an independent dataset consisting of 246 primary NSCLC tumors collected at the University of Texas MD Anderson Cancer Center (Supplemental Table S3). A total of eight miRNAs were available in the dataset. The expression of miR-150 in the tumor tissues was significantly associated with 5-year survival rates in both the univariate (HR, 0.83, 95% CI = 0.73–0.94, P= 0.001) and multivariate (HR, 0.87; 95% CI, 0.76–0.99; P = 0.04) Cox models. In a stratified analysis by disease stage, the expression of miR-150 was significantly associated with 5-year survival rates in the univariate (HR, 0.81; 95% CI, 0.68–0.95; P = 0.01) and multivariate (HR, 0.79; 95% CI, 0.66–0.95; P = 0.01; Supplemental Table S4) models for patients with early-stage disease, but not those with late-stage disease.
MiR-150 promotes NSCLC proliferation and colony formation in vitro
MiR-150 is frequently upregulated in NSCLC cells compared to normal bronchial epithelia cells (HBEC) in initial functional analysis (Supplemental Figure S2). To elucidate the biological activity of miR-150, we transfected cells with miR-150 precursor (pre-miR-150) or scrambled miRNA precursor control (precontrol). The loss-of-function analyses in vitro by antagonizing miR-150 with an miR-150 inhibitor reduced expression of the miR-150 level (Figure 2a). Inhibition of miR-150 significantly decreased the growth rates for A549 and H1299 (XTT assay; Figure 2b), whereas increasing the miR-150 level by pre-miR-150 treatment significantly increased the expression of miR-150 (Figure 2a). And overexpression of miR-150 significantly enhanced tumor cell proliferation in both A549 and H1299 cells (Figure 2c). Similarly, treatment with the miR-150 inhibitor abrogated the colony-forming ability of H1299 and A549 cells. Conversely, ectopic expression of miR-150 increased colony-formation ability of H1299 and A549 cells (Figure 2d).
Figure 2.
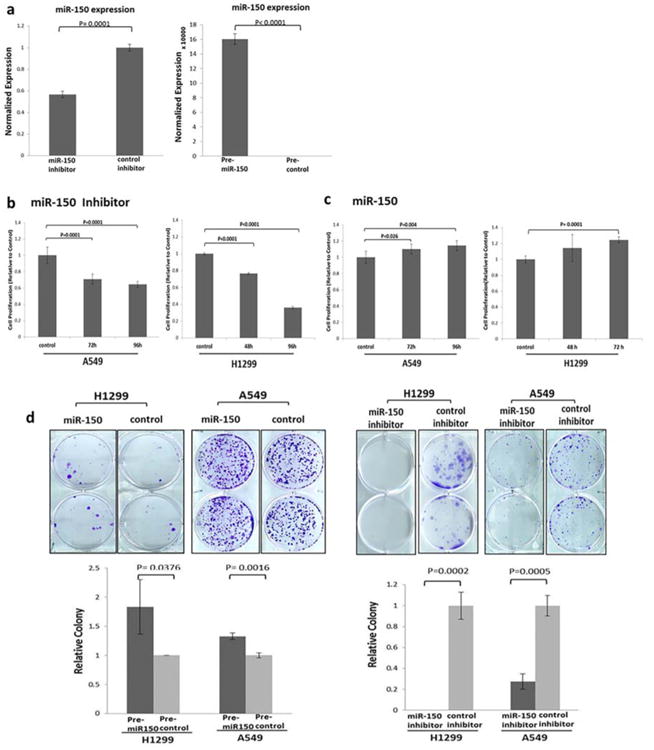
In vitro effects of miR-150 expression on NSCLC cells. (a) Results of quantitative real-time PCR analysis of miR-150 expression in H1299 cells transfected with a miR-150 inhibitor or a pre-miR-150 plasmid. (b) Results of an XTT assay showing the effects of miR-150 inhibition on A549 and H1299 cell proliferation (mean ± SD, n = 6). (c) XTT assay showing the effects of miR-150 overexpression on cell proliferation in A549 and H1299 cells (mean ± SD, n = 6). (d) Representative images (top panels) and quantitative analysis results (bottom panels) showing the effects of miR-150 inhibition and control on tumor cell-induced colony formation in H1299 and A549 cells (mean ± SD, n = 3). Differences between the two groups were analyzed using a two-tailed t-test. [Color figure can be viewed at wileyonlinelibrary.com]
SRCIN1 is a direct target of miR-150
To identify the mechanism underlying the oncogenic role of miR-150 in NSCLC, we investigated putative targets of miR-150 using the TargetScan (www.targetscan.org)22 and miRDB (www.mirdb.org)23 program, and found 19 potential target genes of miR-150. Among them, the expression of SRCIN1, a newly identified inhibitor of Src activity and signaling, was the only gene that was downregulated upon miR-150 overexpression and upregulated upon miR-150 inhibition (Figure 3a). To confirm that F3 SRCIN1 is a direct functional target of miR-150, we compared SRCIN1 mRNA and protein expression in H1299 cells transfected with pre-miR-150 or precontrol using real-time PCR and western blotting. We found markedly decreased SRCIN1 mRNA and protein expression when miR-150 was overexpressed (Figure 3b,c). In contrast, suppression of miR-150 expression by transfection with an miR-150 inhibitor increased SRCIN1 mRNA and protein expression (Figure 3b,c). The interactive mechanism of miRNA-150 and SRCIN1 was further investigated. The predicted interactions between miR-150 and the target sites in the SRCIN1 3′ UTR based on the sequence of seed region are illustrated in Figure 3d, and we identified a total of six potential hybrids. To validate and identify specific miR-150 binding and cleaved sites, we developed and previously demonstrated24 a high-sensitive novel SLA-RT-PCR assay to detect the potential miR-150 mediated target SRCIN1 mRNA cleavage. We designed SLA-RT primers with 6-random nucleotide probes at 5′ termini to match the miR-150 binding sites on SRCIN1 mRNA. We reverse-transcribed a cleaved 5′-mRNA fragment and determined its relative abundance using PCR. Accumulation of the cleaved 5′ -mRNA fragment was represented by the relative intensity of each specific SLA-RT-PCR amplicon and resolved on an agarose gel. The SLA-RT-PCR assay clearly demonstrated miR-150 cleaved 5′-fragments of SRCIN1 mRNA (Figure 3e). The result of the SLA-RT-PCR assay showed elevated amplicon intensities for PCR 3#, which suggested that miR-150 mediated SRCIN1 mRNA cleavage specifically at corresponding recognition site in the 3′ UTR of SRCIN1: 36923318 to 36923339 (Homo sapiens chromosome 17, alternate assembly CHM1_1.1) (Figure 3d, site 4). These results confirmed that SRCIN1 is an endogenous target of miR-150 in NSCLC cells under physiological conditions.
Figure 3.
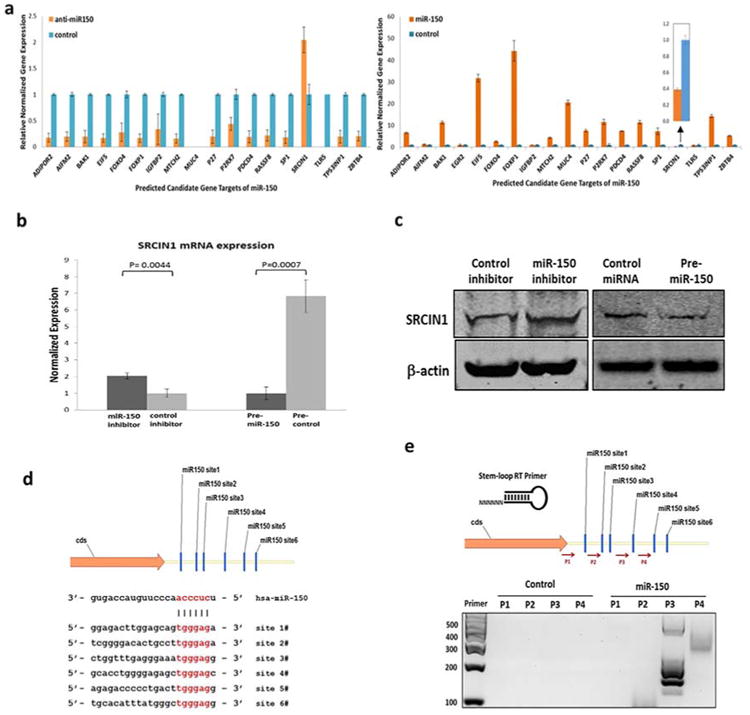
SRCIN1 is the direct target of miR-150. (a) Quantitative real-time PCR analysis of expression levels of potential miR-150 target mRNAs in H1299 cells transfected with a pre-miR-150 plasmid (bottom) or a miR-150 inhibitor (top). (b) The mRNA expression levels of SCRIN1 in H1299 cells transfected with a pre-miR-150 plasmid or a miR-150 inhibitor detected using Quantitative real-time PCR (mean ± SD, n = 3). GAPDH was used as a control. Predicted miR-150 target sequences in the 3′ untranslated regions (UTRs) of SRCIN1 (c) Representative western blots showing SRCIN1 protein expression levels in H1299 cells transfected with either a pre-miR-150 plasmid or inhibitor. (d) A schematic of six conserved miR-150 binding sites in the 3′ UTR region of SRCIN1 mRNA is shown. The seed regions of miR-150 and its corresponding recognition sites in the SRCIN1 3′ UTR are indicated in red. (e) SLA-RT-PCR and agarose gel electrophoresis results showing miR-150-mediated cleavage activities on endogenous SRCIN1 mRNA transcripts in H1299 cells transfected with pre-miR-150 constructs. [Color figure can be viewed at wileyonlinelibrary.com]
Inhibition of miR-150 represses NSCLC growth in vivo
To investigate the function of miR-150 in vivo, we stably infected H1299 cells with a green fluorescent protein (GFP) lentivirus construct containing either an miRzip-150 inhibitor or miRzip-control. We confirmed downregulation of miR-150 expression after infection with the miRzip-150 inhibitor using a real-time PCR assay. As expected, inhibition of miR-150 expression by miRzip-150 also increased SRCIN1 mRNA expression (Figure 4a), suppressed tumor cell migration (transwell assay, Figure 4b), and colony formation (Figure 4c).
Figure 4.
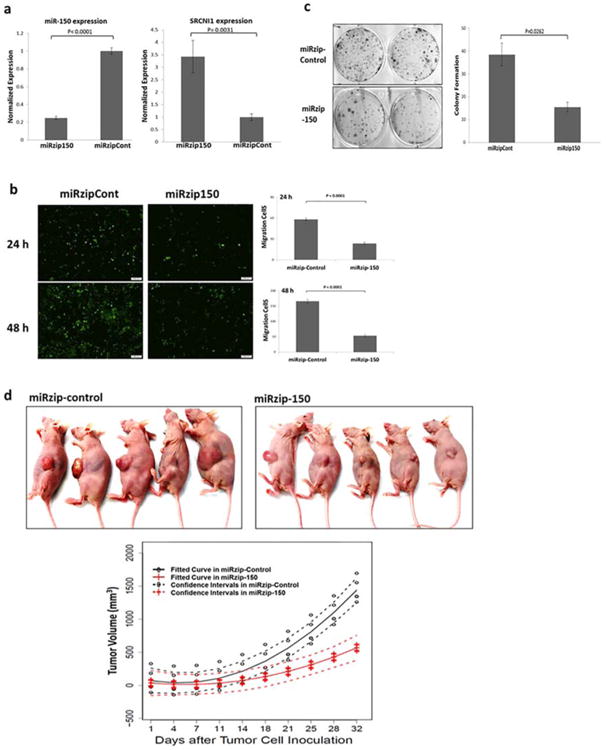
Inhibition of miR-150 expression inhibits NSCLC growth in vivo. (a) H1299 cells were infected with a green fluorescent protein lentivirus construct containing either an miRzip-150 inhibitor (miRzip150) or miRzip-control (miRzipCont). Quantitative real-time PCR assays detected downregulation of miR-150 expression and increased SRCIN1 mRNA expression upon infection with miRzip-150. (b) Transwell assay results showing suppression of H1299 cell migration upon inhibition of miR-150 expression with miRzip-150. (c) Colony-formation assay results showing suppression of H1299 cell colony formation by inhibition of miR-150 expression with miRzip-150. (d) Inhibition of miR-150 expression by infection with miRzip-150 resulted in a marked decrease in NSCLC growth in vivo. Nude mice were subcutaneously injected with 6 × 106 H1299 cells that stably expressed either miRzip-150 or miRzip-control lentiviral vectors. The resulting tumors were measured every 3 days beginning at 14 days after tumor-cell injection. [Color figure can be viewed at wileyonlinelibrary.com]
The H1299-miRzip-150 and H1299-miRzip-control cells were injected into the flank of nude mice (n = 5) per group and tumor growth was measured every 3 days, beginning 2 weeks after injection. Knockdown of miR-150 in H1299 cells caused a substantial reduction in tumor volume in vivo. The mean tumor size in the miRzip-150 treatment group was significantly smaller than that in the miRzip-control group at 21 days (P < 0.005, Figure 4d). These results clearly demonstrated the NSCLC oncogenesis role of miR-150 functions in vivo.
Discussion
The clinical outcomes, including treatment response, disease recurrence, and survival times, of patients with early-stage NSCLC with similar clinical and tumor characteristics vary considerably.25,26 Noninvasive biomarkers that can be used to predict clinical outcomes in patients with early-stage NSCLC have yet to be developed. In the present study, we developed serum miRNA signatures that could predict disease recurrence and survival in a large cohort of patients with NSCLC. Of the identified miRNAs in the signatures, the expression of miR-150 in tumor tissues also appeared significantly associated with survival in patients with NSCLC. The function of miR-150 in the signatures was then determined and validated both in vitro and in vivo. Our results suggest that the miRNA signature serves as a prognostic bio-marker for outcomes of early-stage NSCLC and that miR-150 is an oncogenic miRNA that promotes NSCLC cell proliferation, invasion, and metastasis by directly targeting the SRCIN1 both in vitro and in vivo.
Circulating miRNAs have attracted tremendous interest in cancer diagnosis and prognosis because they are small and remarkably stable in the circulation and can be detected using high-throughput techniques. Only a few studies have investigated the association of miRNA expression with recurrence and survival in early-stage NSCLC patients.15,27 In a study focusing on stage I lung adenocarcinomas, Edmonds and Eischen27 found that aberrant miRNA expression in tumors played important roles in lung adenocarcinoma development and recurrence, but they did not measure circulating miRNAs in their study. Kaduthanam et al.15 identified miR-142-3p as a serum biomarker that predicted tumor recurrence in patients with early-stage pulmonary adenocarcinoma. However, they only examined pulmonary adenocarcinoma and found an overall predictive AUC of 0.78. Sanfiorenzo et al.28 recently found that three plasma miRNA signatures (high miR-155-5p, high miR-223-3p, and low miR-126-3p expression) were markedly associated with high risk for disease progression, but they examined only 52 plasma samples. These inconsistencies may be attributable to the studies' heterogeneous patient populations and relatively small sample sizes. To our knowledge, the current study is one of the largest to identify serum miRNA signatures in patients with stages I and II NSCLC. One strength of this study is that our initial global miRNA screening focused only on miRNAs that could be reliably detected in serum. We adopted a commonly used miRNA ratio analysis29–31 to avoid potential bias when analyzing serum miRNA expression data because the ideal internal normalization control for measurements of circulating miRNA has not been established.18
A single miRNA can regulate a wide spectrum of gene-expression and cellular processes in intracellular signaling path-ways.4 Most of the miRNAs identified in our ratio signatures have previously been implicated in cancer development. Wang et al.32 first demonstrated that miR-27a functions as an oncogene in human tumorigenesis, and Krutilina et al.33 demonstrated that miR-18a represses distant metastasis via a hypoxia-inducible factor 1-alpha-dependent pathway. Garofalo et al.19 showed that gefitinib-based treatment can trigger programmed death of HCC827 and PC9 lung cancer cells via downregulation of miR-30b, miR-30c, miR-221, and miR-222 expression and the consequent upregulation of apoptosis-regulating protease activating factor 1 (APAF1) and Bcl-2-like protein 11 (BCL2L11, BIM) expression in the proapoptotic pathway. In our identified miRNA ratios from recurrence and survival analysis, miR-18a, miR-142-3p, miR-19b, and miR-150 are immune-associated miRNAs and related to adoptive T-cell immunotherapy.34 This suggested that immune-related miRNAs identified in this study can affect the clinical outcome of NSCLC patients. Taken together, these results, which confirmed that miRNA expression is closely associated with cancer cell survival, may contribute to the development of new miRNA-based strategies to prognosticate and treat NSCLC.
In the independent group of NSCLC tumor samples, expression of miR-150 was also significantly associated with survival in tumors. However, the mechanism by which miR-150 acts in NSCLC development remains unknown. We selected this miRNA for comprehensive functional analysis in vitro and in vivo and found increased miR-150 expression promoted NSCLC cell proliferation and migration. In contrast, blocking the action of miR-150 inhibited NSCLC cell proliferation and migration, and knockdown of miR-150 resulted in substantial tumor volume reduction in vivo. These results suggested that miR-150 plays a crucial role in NSCLC progression. Aberrant expression of miR-150 was reported to be involved in malignant hematopoiesis and associated with leukemia development.35,36 Higher expression of miR-150 was associated with short postoperative survival times in patients with gastric cancer.37 In addition, researchers have shown that miR-150 was a potential biomarker for prognosis and therapeutic outcomes in patients with mixed-lineage leukemia gene-associated leukemia.38 In lung cancer, a previous study investigated the relationship between miR-150 and p53, and demonstrated that miR-150 promotes NSCLC cell growth by suppressing p53 expression, suggesting the latter as an miR-150 target. However, in vivo study is needed to confirm the relationship.39
The Cap family protein p140CAP, encoded by SRCIN1, is mainly expressed in the brain, testes, colon, kidney, and lung tissues, and the level of p140CAP expression was reduced in tumors.40,41 The Cap protein p140CAP functions as a tumor suppressor protein, the silencing of which promotes anchorage-independent growth of cancer cells and tumor development and growth.42,43 The mechanism of molecular signaling of SRCIN1 in lung cancer is unclear, and especially the role and mechanism of miRNA in regulation of SRCIN1 expression and tumor progression is lacking. Previous studies suggested that chromosome rearrangements may account for the altered expression of SRCIN1 and result in tumor initiation and progression.41,44 The CAP protein p140CAP inhibits Src signaling pathways and downstream focal adhesion kinase, epidermal growth factor receptor, and Ras/extracellular signal-related kinase activity and functions as a potent tumor suppressor.42 Src is a cellular tyrosine kinase that is frequently overexpressed or aberrantly activated in cancer cells. In particular, researchers have reported high levels of Src activity in lung cancer cells.45 Src is normally maintained in an inactive state via the phosphorylation of Tyr527 in the carboxy-terminal of the protein. P140CAP activates the Csk kinase, which phosphorylates the negative-regulatory Tyr527 and induces a conformational change in the structure of Src.43 MiR-150, therefore, may exert its oncogenic function by targeting SRCIN1, a concept which has been evaluated by Cao et al.,46 and confirmed by the current study. The repression of SRCIN1 by miR-150 may trigger activation of the Src signaling pathways, which promote the proliferation and migration of lung cancer cells.
The success of precision medicine depends on discovering and validating clinically applicable biomarkers that can be used to better classify cancer patients into high-risk populations with poor prognosis and determine optimal targets and strategies for treatment. While many molecular biomarkers have been reported in the past decades with the availability of new technology, including miRNA and all “omics”-based markers, few were translated into clinical utilization to improve patients' outcomes and/or quality of life. This challenge exists because most of the molecular biomarker studies lacked comprehensive validation, reproducibility of results, and/or low statistical power.47 Similar limitations of biomarker discovery also occur in miRNA studies,15,28,46 as just discussed. This study carefully followed the methodology of circulating biomarker discovery, from global screening and validation to functional analysis. The identified miRNAs could combine with other biomarkers and clinical information to facilitate clinical outcome prediction and personalized treatment for the management of early-stage NSCLC patients. Independent validation, assay development, analytical validation, and clinical prospective validation are needed to translate initial discoveries to clinical implementation that have the potential to improve patients' outcomes and quality of life.48–50
This study had several strengths. The patients were all of the same race and received similar treatment regimens at a single institution. We collected comprehensive clinical and epidemiologic information and included relatively long follow-up periods (median = 4 years). The large sample size allowed us to split patients into discovery and validation sets. Utilizing our recently developed novel SLA-RT-PCR assay enabled us to identify authentic miRNA targets by determining mRNA cleavage sites targeted by endogenous miRNAs. Our comprehensive investigation of the function of identified miR-150 both in vitro and in vivo consolidates its role for NSCLC as a prognostic biomarker and therapeutic target. Future independent patient cohorts are needed to confirm our results.
In conclusion, we identified two distinct miRNA ratio signatures that predicted disease recurrence and survival rates in a large cohort of patients with early-stage NSCLC. Moreover, functional characterization of miR-150 in vitro and in vivo, and identified specific mRNA cleavage sites targeted by endogenous miR-150 in 3′ UTR of tumor suppressor gene SRCIN1, suggest a promising novel bio-marker and therapeutic target for NSCLC. The discovery may facilitate personalized treatment of early-stage NSCLC patients.
Methods
A detailed description of the methods can be found in the Supplemental Methods.
Patient population and clinical data collection
The analysis of circulating microRNAs (miRNAs) included 171 white patients with stage I or II NSCLC who were enrolled at the University of Texas MD Anderson Cancer Center since 1991 (Table S1). The expression of tumor miRNAs was analyzed in an independent 246-patient population from MD Anderson and patients' characteristics are listed in Table S3. All study participants gave written informed consent and the study was approved by MD Anderson's Institutional Review Board.
Total RNA isolation and miRNA expression analysis
The detailed methods used for serum RNA isolation and miRNA profiling were described previously.13 The initial global miRNA expression screening was performed with a testing set of 20 white individuals consisting of 10 healthy people and 10 with early-stage NSCLC. Expression of selected miRNAs were measured in serum samples using a high-throughput BioMark HD real-time PCR system, as previously described.13
Cell culture and plasmid construction
Cells were plated and cultured overnight. For siRNA transfection, miRNA-150 knockdown was transfected with miRIDIAN miR-150 inhibitors by DharmaFECT 1 transfection reagent. MiR-150 precursor (pre-miR-150) expression and scramble miRNA precursor control plasmids were constructed. All oligonucleotides were synthesized by Sigma-Aldrich (St. Louis, MO) and annealed as inserts for pre-miR-150 expression plasmid and scramble miRNA precursor control plasmid.
Cell proliferation and colony formation assay
The effect of selected miRNAs on NSCLC proliferation was determined by using an improved XTT assay. Cell viability was assessed by a scanning multiwell spectrophotometer. Single-cell suspension of H1299 and A549 cells were plated in 6-well plates at a density of 200 cells/well. On the second day after plating, cells were transfected with pre-miRNA plasmids or miRNA inhibitors. Every 3 days, the medium was replaced with fresh medium. After 12 days of treatment, the medium was removed, and the cell colonies were stained with crystal violet and counted.
Lentivirus production and transduction
An miR-150 inhibitor (miRzip-150) and a negative control miRNA inhibitor (miRzip-control) were cloned into the pLV3 vector to generate miRzip-150 and miRzip-control, respectively. The recombinant lentiviral plasmids were then cotransfected into HEK293T human embryonic kidney cells with the packaging vector pGag/Pol, pRev, and the envelope vector pVSV-G. The H1299 cells were then infected with the viral supernatant containing polybrene. The transduced cells were then selected with puromycin for 14 days to obtain cells that stably expressed the constructs.
Real-time PCR and western blot assay
Total RNA was isolated by using TRIzol reagent and additional phenol. Mature miR-150 expression was detected using a quantitative real-time PCR assay with miR-150-specific primers and a TaqMan miR-150 probe. The relative amount of SRCIN1 mRNA was normalized to that of GAPDH.
For western blot assay, cell lysates were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to a nitrocellulose membrane. The membranes were probed with corresponding antibodies. The protein levels were normalized with β-actin.
SLA-RT-PCR assay
The details of SLA-RT-PCR assay have been previously demonstrated.24 RNA was reverse transcribed in combination with stem-loop RT-primer. PCR was performed with the TaqMan Gene Expression PCR Master Mix with universal primer and transcript-specific primers. PCR products were analyzed by agarose gel electrophoresis and visualized using a UV transilluminator.
Cell migration assay
The migratory ability of H1299 cells infected with a green fluorescent protein (GFP) lentivirus construct containing either the miRzip-150 inhibitor or miRzip-control was tested in a Transwell Boyden Chamber. The cells were harvested 3 days after miRzip-150 lentivirus infection and suspended in fetal bovine serum (FBS)-free culture medium. Cells were added to the upper chamber and the transwell-containing plates were then incubated for 24 h. The cells remaining on the upper surface of the filter membrane were scraped off gently with a cotton swab. The lower surfaces exhibiting GFP fluorescence were captured using a photomicroscope.
In vivo animal study
Female 6-week-old specific pathogen-free nu/nu nude mice were purchased from Charles River Laboratories (Wilmington, MA), and housed at MD Anderson Cancer Center under conventional conditions. Animal experiments were carried out in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals and the MD Anderson institutional guidelines. H1299 cells transfected with a lentivirus expressing miRzip-150 or miRzip-control were harvested and resuspended in RPMI1640 medium. Next, 6 × 106 cells were injected subcutaneously into the flanks of 10 mice. Five mice were inoculated with H1299-miRzip-150 cells and five other mice were inoculated with H1299-miR-zip-control. Beginning 2 weeks after the tumor cell inoculation, when the tumors had grown to 3 to 5 mm in diameter, the tumors were measured with calipers every 3 days.
Statistical analysis
Time to recurrence and of survival were calculated from the date of diagnosis to the date of recurrence or death or last follow-up. The median time from diagnosis of NSCLC to study enrollment (date of blood draw) was 24 days. Due to the challenge of normalizing of miRNA expression data with an internal control, the ratio between each miRNA was calculated as reported previously.18 To assess the association of serum miRNA ratios with 5-year recurrence and survival rates, serum miRNA ratios were dichotomized using either median cutoff or tertile cutoff. Estimated HRs and 95% CIs were calculated using the Cox proportional hazard model and adjusted for age, sex, smoking history, clinical stage, and treatment regimen. Risk scores for disease recurrence and 5-year survival were calculated for all the possible combinations of the 22- and 13-significant miRNA ratios. The risk score was defined as the weighted sum of the dichotomized miRNA ratios, where the weight was determined by the adjusted log of the miRNA ratio to recurrence and survival. The discriminatory accuracy of each miRNA signature for predicting recurrence and survival was assessed by constructing time-dependent receiver operating characteristic curves and calculating the AUC. All patients were dichotomized according to the median risk score, and individuals with a risk score higher or lower than the median were classified into high-risk and low-risk groups, respectively.
Supplementary Material
Study Highlights.
What is the Current Knowledge on the Topic?
☑ Early-stage non-small cell lung cancer (NSCLC) patients with similar clinical characteristics of tumor vary considerably in clinical outcome. For early-stage NSCLC patients, surgery, supplemented with adjuvant platinum-based chemotherapy, offers the best chance for cure. While early-stage NSCLC patients can be curatively treated by surgery alone or with adjuvant chemotherapy, ∼50–60% of surgically resected early-stage NSCLC patients develop recurrence and eventually die from this disease.
What Question did this Study Address?
☑ This integrative multistage study aimed to identify circulating miRNAs as prognostic biomarkers and investigate treatment target for early-stage NSCLC patients.
What This Study Adds To Our Knowledge
☑ Our findings indicate that the miRNA ratio signatures could serve as prognostic biomarkers for early-stage NSCLC and miR-150 binding on specific recognition sites in 3′ UTR of tumor suppressor gene SRCIN1 present a potential therapeutic target for NSCLC.
How This Might Change Clinical Pharmacology Or Translational Science
☑ This comprehensive investigation of the function of identified miR-150 both in vitro and in vivo consolidates its role for NSCLC as a prognostic biomarker and therapeutic target that may facilitate personalized treatment of early-stage NSCLC patients.
Acknowledgments
This work was supported in part by grants from the Cancer Prevention and Research Institute of Texas (RP1300502); and National Cancer Institute (P50 CA070907 and R01 CA176568). Additional funding was provided by MD Anderson institutional support for the Center for Translational and Public Health Genomics and Duncan Family Institute for Cancer Prevention and Risk Assessment.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of Interest: The authors have no potential conflicts of interest to declare.
Author Contributions: L.Z. and J.L. wrote the article; X.W., L.Z., J.L., Y.Y., J.G., I.W., J.A.R., and L.J. designed the research; L.Z., J.L., T.O., E.G., and J.L. performed the research; L.Z., J.L., Y.Y., J.W., and Y.Z. analyzed the data.
References
- 1.Pignon JP, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 2.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 3.Scagliotti GV, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell lung cancer. J Natl Cancer Inst. 2003;95:1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Qiu C, Zhang H, Wang J, Cui Q, Yin Y. Human microRNA oncogenes and tumor suppressors show significantly different biological patterns: from functions to targets. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esquela-Kerscher A, Slack FJ. Oncomirs — microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CJ, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–110. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 9.Calin GA, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 10.Volinia S, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, et al. MicroRNA-related genetic variants in iron regulatory genes, dietary iron intake, microRNAs and lung cancer risk. Ann Oncol. 2017;28:1124–1129. doi: 10.1093/annonc/mdx046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol. 2014;11:145–156. doi: 10.1038/nrclinonc.2014.5. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, et al. Pathway-based serum microRNA profiling and survival in patients with advanced stage non-small cell lung cancer. Cancer Res. 2013;73:4801–4809. doi: 10.1158/0008-5472.CAN-12-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, et al. Cell-free microRNA expression profiles in malignant effusion associated with patient survival in non-small cell lung cancer. PLoS One. 2012;7:e43268. doi: 10.1371/journal.pone.0043268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaduthanam S, et al. Serum miR-142-3p is associated with early relapse in operable lung adenocarcinoma patients. Lung Cancer. 2013;80:223–227. doi: 10.1016/j.lungcan.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Saito M, et al. The association of microRNA expression with prognosis and progression in early-stage, non-small cell lung adenocarcinoma: a retrospective analysis of three cohorts. Clin Cancer Res. 2011;17:1875–1882. doi: 10.1158/1078-0432.CCR-10-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vosa U, et al. Identification of miR-374a as a prognostic marker for survival in patients with early-stage nonsmall cell lung cancer. Genes Chromosomes Cancer. 2011;50:812–822. doi: 10.1002/gcc.20902. [DOI] [PubMed] [Google Scholar]
- 18.Boeri M, et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A. 2011;108:3713–3718. doi: 10.1073/pnas.1100048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garofalo M, et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med. 2012;18:74–82. doi: 10.1038/nm.2577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Lei Z, et al. MiR-142-3p represses TGF-beta-induced growth inhibition through repression of TGFbetaR1 in non-small cell lung cancer. FASEB J. 2014;28:2696–2704. doi: 10.1096/fj.13-247288. [DOI] [PubMed] [Google Scholar]
- 21.Li J, et al. microRNA-146 up-regulation predicts the prognosis of non-small cell lung cancer by miRNA in situ hybridization. Exp Mol Pathol. 2014;96:195–199. doi: 10.1016/j.yexmp.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 23.Wong N, Wang X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucl Acids Res. 2015;43:D146–152. doi: 10.1093/nar/gku1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Xu K, Roth JA, Ji L. Detection of siRNA-mediated target mRNA cleavage activities in human cells by a novel stem-loop array RT-PCR analysis. Biochem Biophys Rep. 2016;6:16–23. doi: 10.1016/j.bbrep.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–819. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmonds MD, Eischen CM. Differences in miRNA expression in early stage lung adenocarcinomas that did and did not relapse. PLoS One. 2014;9:e101802. doi: 10.1371/journal.pone.0101802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanfiorenzo C, et al. Two panels of plasma microRNAs as non-invasive biomarkers for prediction of recurrence in resectable NSCLC. PLoS One. 2013;8:e54596. doi: 10.1371/journal.pone.0054596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neely LA, et al. A microRNA expression ratio defining the invasive phenotype in bladder tumors. Urol Oncol. 2010;28:39–48. doi: 10.1016/j.urolonc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Fritz HK, Lindgren D, Ljungberg B, Axelson H, Dahlback B. The miR(21/10b) ratio as a prognostic marker in clear cell renal cell carcinoma. Eur J Cancer. 2014;50:1758–1765. doi: 10.1016/j.ejca.2014.03.281. [DOI] [PubMed] [Google Scholar]
- 31.Avissar M, Christensen BC, Kelsey KT, Marsit CJ. MicroRNA expression ratio is predictive of head and neck squamous cell carcinoma. Clin Cancer Res. 2009;15:2850–2855. doi: 10.1158/1078-0432.CCR-08-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, et al. Upregulation of miR-27a contributes to the malignant transformation of human bronchial epithelial cells induced by SV40 small T antigen. Oncogene. 2011;30:3875–3886. doi: 10.1038/onc.2011.103. [DOI] [PubMed] [Google Scholar]
- 33.Krutilina R, et al. MicroRNA-18a inhibits hypoxia-inducible factor 1-alpha activity and lung metastasis in basal breast cancers. Breast Cancer Res. 2014;16:R78. doi: 10.1186/bcr3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji Y, Hocker JD, Gattinoni L. Enhancing adoptive T cell immunotherapy with microRNA therapeutics. Semin Immunol. 2016;28:45–53. doi: 10.1016/j.smim.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Y, Jiang X, Chen J. The role of miR-150 in normal and malignant hematopoiesis. Oncogene. 2014;33:3887–3893. doi: 10.1038/onc.2013.346. [DOI] [PubMed] [Google Scholar]
- 36.Fayyad-Kazan H, et al. Circulating miR-150 and miR-342 in plasma are novel potential biomarkers for acute myeloid leukemia. J Transl Med. 2013;11:31. doi: 10.1186/1479-5876-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katada T, et al. microRNA expression profile in undifferentiated gastric cancer. Int J Oncol. 2009;34:537–542. [PubMed] [Google Scholar]
- 38.Jiang X, et al. Blockade of miR-150 maturation by MLL-fusion/MYC/LIN-28 is required for MLL-associated leukemia. Cancer Cell. 2012;22:524–535. doi: 10.1016/j.ccr.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang DT, et al. miR-150, p53 protein and relevant miRNAs consist of a regulatory network in NSCLC tumorigenesis. Oncol Rep. 2013;30:492–498. doi: 10.3892/or.2013.2453. [DOI] [PubMed] [Google Scholar]
- 40.Di Stefano P, et al. P130Cas-associated protein (p140Cap) as a new tyrosine-phosphorylated protein involved in cell spreading. Mol Biol Cell. 2004;15:787–800. doi: 10.1091/mbc.E03-09-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer. 2010;10:858–870. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- 42.Damiano L, et al. p140Cap dual regulation of E-cadherin/EGFR cross-talk and Ras signalling in tumour cell scatter and proliferation. Oncogene. 2010;29:3677–3690. doi: 10.1038/onc.2010.128. [DOI] [PubMed] [Google Scholar]
- 43.Di Stefano P, et al. p140Cap protein suppresses tumour cell properties, regulating Csk and Src kinase activity. EMBO J. 2007;26:2843–2855. doi: 10.1038/sj.emboj.7601724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katoh M, Katoh M. Evolutionary recombination hotspot around GSDML-GSDM locus is closely linked to the oncogenomic recombination hotspot around the PPP1R1B-ERBB2-GRB7 amplicon. Int J Oncol. 2004;24:757–763. [PubMed] [Google Scholar]
- 45.Mazurenko NN, Kogan EA, Zborovskaya IB, Kisseljov FL. Expression of pp60c-src in human small cell and non-small cell lung carcinomas. Eur J Cancer. 1992;28:372–377. doi: 10.1016/s0959-8049(05)80056-5. [DOI] [PubMed] [Google Scholar]
- 46.Cao M, et al. miR-150 promotes the proliferation and migration of lung cancer cells by targeting SRC kinase signalling inhibitor 1. Eur J Cancer. 2014;50:1013–1024. doi: 10.1016/j.ejca.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 47.Vargas AJ, Harris CC. Biomarker development in the precision medicine era: lung cancer as a case study. Nat Rev Cancer. 2016;16:525–537. doi: 10.1038/nrc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goossens N, Nakagawa S, Sun X, Hoshida Y. Cancer biomarker discovery and validation. Transl Cancer Res. 2015;4:256–269. doi: 10.3978/j.issn.2218-676X.2015.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McShane LM, et al. Criteria for the use of omics-based predictors in clinical trials. Nature. 2013;502:317–320. doi: 10.1038/nature12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelloff GJ, Sigman CC. Cancer biomarkers: selecting the right drug for the right patient. Nat Rev Drug Discov. 2012;11:201–214. doi: 10.1038/nrd3651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


