Abstract
The context preexposure facilitation effect (CPFE) is a variant of contextual fear conditioning in which acquisition of the contextual representation and association of the retrieved contextual memory with an immediate foots-hock are separated by 24hrs. During the CPFE, learning- related expression patterns of the early growth response -1 gene (Egr-1) vary based on training phase and brain subregion in adult and adolescent rats (Asok et al., 2013; Shreiber et al., 2014; Chakraborty et al., 2016). The current experiments extended our previous findings by examining Egr-1 expression in infant (PD17) and juvenile (PD24) rats during the CPFE using preexposure protocols involving single-exposure (SE) or multiple-exposure (ME) to context. Following a 5 min preexposure to the training context (i.e. the SE protocol), Egr-1 expression in the medial prefrontal cortex (mPFC), dorsal hippocampus (dHPC) and lateral nucleus of the amygdala (LA) was differentially increased in PD24 rats relative to PD17 rats. In contrast, increased Egr-1 expression following an immediate foot-shock (1s, 1.5mA) did not differ between PD17 and PD24 rats, and was not learning-related. Interestingly, increasing the number of exposures to the training chamber on the preexposure day (i.e. ME protocol) altered training-day expression such that a learning-related increase in expression was observed in the mPFC in PD24 but not PD17 rats. Together, these results illustrate a clear maturation of Egr-1 expression hat is both age- and experience-dependent. In addition, the data suggest that regional activity and plasticity within the mPFC on the preexposure but not the training day may contribute to the ontogenetic profile of the effect. Further studies are necessary to elucidate the causal role of sub-region-specific neuroplasticity in the ontogeny of the CPFE.
Keywords: CPFE, ontogeny, Egr-1, contextual fear conditioning, learning
1. Introduction
Fear conditioning provides a useful tool for assessing the neurobiological substrates of learning and memory. During standard contextual fear conditioning (sCFC), an animal is placed inside of the conditioning chamber (Context A) with sufficient time (usually 2–3 minutes) to acquire a representation of the context and then receives an aversive foot-shock. Conditioned fear (e.g., freezing behavior), measured immediately following foot-shock or after a retention interval, is increased in these animals relative to animals given an immediate foot-shock without adequate exposure time (usually under 10 seconds) to the training context (Fanselow, 1986). In a variant of sCFC, the context preexposure facilitation effect (CPFE; Fanselow, 1986, 1990; Rudy & O’Reilly, 1999), acquisition of the context representation and association of that representation with foot shock are separated by 24hr. Preexposure to the training context allows the immediate foot-shock to be associated with a retrieved representation of the context (for review see Rudy, 2009). The temporal separation of conditioning phases makes the CPFE well suited to separately assess the neurobiological mechanisms of context learning and contextual fear conditioning in the rat.
In addition to serving as a tool to understand the mechanisms of associative learning, contextual fear has been used to explore the ontogeny of hippocampus-dependent learning. The CPFE develops gradually after Postnatal Day (PD) 17 and is robust by PD24 (Jablonski, Schiffino, & Stanton, 2012; Robinson-Drummer & Stanton, 2015; Rudy & Morledge, 1994). In contrast, infant PD17 rats can display contextual fear using sCFC when there is no retention interval between context learning and shock delivery, as reflected in intact post-shock freezing (Pugh & Rudy, 1996; Rudy & Morledge, 1994). These results suggest that infant rats are able to use contextual cues to momentarily acquire a context-shock association but that a context representation is either not consolidated or not retrieved and associated with shock when infant rats experience immediate-shock 24hr later during the CPFE protocol. Studies of the molecular substrates of infantile memory deficits that measure regional gene expression during the CPFE have the potential to inform this issue.
Both the hippocampus and medial prefrontal cortex (mPFC) are crucial for fear conditioning during the CPFE. Importantly, pharmacological antagonism of these regions during preexposure or training disrupts conditioning indicating that they both participate in the acquisition and/or consolidation of the context representation and context-shock association (Chang & Liang, 2012; Heroux, Robinson-Drummer, Sanders, Rosen, & Stanton, 2017; Matus-Amat, Higgins, & Rudy, 2004; Matus-Amat, Higgins, Sprunger, Wright-Hardesty, & Rudy, 2007; Robinson-Drummer, Dokovna, Heroux, & Stanton, 2016; Robinson-Drummer, Heroux, & Stanton, 2017; Schiffino, Murawski, Rosen, & Stanton, 2011). In PD31-33 rats, the CPFE differentially increases mPFC and hippocampal levels of the inducible transcription factor called early growth response-1 gene (Egr-1). Egr-1 is an immediate early gene (IEG) that participates in long-term synaptic plasticity (Alberini, 2009; Veyrac, Besnard, Caboche, Davis, & Laroche, 2014) and regional induction depends on the phase of training and conditioning group (Asok, Schreiber, Jablonski, Rosen, & Stanton, 2013; Chakraborty, Asok, Stanton, & Rosen, 2016; Lee, 2010; Schreiber, Asok, Jablonski, Rosen, & Stanton, 2014).
The importance of Egr-1 activity for contextual fear conditioning has been demonstrated previously in adult rats. Egr-1 is highly expressed in the lateral nucleus (LA) of the amygdala (Malkani & Rosen, 2000) and the hippocampus and reduction of amygdalar or hippocampal Egr-1 levels significantly impairs contextual fear conditioning (Lee, 2010; Malkani & Rosen, 2001; Malkani, Wallace, Donley, & Rosen, 2004). Although both the hippocampus and amygdala are important for CPFE performance, our lab has shown that learning-related changes in Egr-1 expression have only been reliably observed in the mPFC (Asok et al., 2013; Chakraborty et al., 2016; Schreiber et al., 2014 although see Lee, 2010). In both adolescent and adult rodents, following immediate-foot-shock training, mPFC Egr-1 levels in animals that learn the CPFE is significantly elevated above non-associative control animals preexposed to an alternate context. Both the fear conditioned and control groups showed increased Egr-1 expression above homecage controls, revealing a “stair-step” pattern of expression from homecage, to non-associative control to fear conditioned groups. Although the mPFC shows a protracted rate of development, it is currently unknown whether maturation of molecular activity in this brain region or others tracks the ontogenetic profile of the CPFE. The present study sought to examine the ontogenetic profile of Egr-1 expression in the mPFC as well as the amygdala and hippocampus during the CPFE in PD17, 24, and 31 rats.
The CPFE emerges between PD17 and PD24 with freezing levels at test not differing between PD24 and older (adolescent or adult) rats (Jablonski et al., 2012; Robinson-Drummer & Stanton, 2015; Schiffino et al., 2011). We hypothesized that gene expression patterns would change between PD17 and PD24 with the latter displaying adolescent- and adult-like gene expression patterns. In contrast, PD17 rats, which do not exhibit the CPFE, would show different patterns of expression following conditioning reflecting their lack of fear expression. The current experiment presented several novel findings. Preexposure to the training context, using the single-exposure protocol, increased Egr-1 in all regions in PD24 but not PD 17 rats. In contrast, immediate foot-shock training increased Egr-1 expression non-differentially in the mPFC at both ages while PD31 rats showed the previously reported (Asok et al., 2013) learning-related increase in expression in the mPFC. Increasing context exposure on the preexposure day (i.e. multiple-exposure protocol) revealed learning-related expression changes in the mPFC on the training day in the PD24 but not PD17 rats. These results reveal a maturation of Egr-1 expression that is both age- and experience-dependent.
2. General Methods
2.1 Subjects
Animal husbandry was as described in our previous reports (Asok et al., 2013; Schreiber et al., 2014) in accordance with the NIH guidelines and protocols approved by the Institutional Animal Care and Use Committee at the University of Delaware. Subjects were male and female Long-Evans rats born to time-bred dams in the University of Delaware breeding colony, with the date of birth designated as Postnatal day 0 (PD0). Litters were transferred to the in-lab colony room on PD2 and were culled to eight animals and paw-marked on PD3. Litters were provided ad libitum food and water and maintained on a 12:12 h light/dark schedule. Animals beginning conditioning on PD17 were group housed with their dams throughout the procedure. Animals beginning conditioning on PD24 or PD31 were weaned into group cages (45 × 24 × 17 cm3) with 2–4 same-sex littermates on PD21 and then individually housed into individual opaque white cages (45 × 24 × 17 cm3) two days prior to experimental procedures and for the remainder of the study. Four to eight animals per litter were used with no more than one same-sex littermate assigned to a given experimental condition (except on a few occasions where their data were averaged, noted below).
2.2 Apparatus and Stimuli
Full details of the apparatus and stimuli were previously described (Asok et al., 2013; Jablonski et al., 2012; Schreiber et al., 2014). Contextual fear conditioning took place in four clear Plexiglas chambers with a grid floor connected to a shock scrambler which delivered a 2s, 1.5mA foot shock. The chambers were in a 2 × 2 arrangement within a fume hood providing light and ambient noise. Adjoining chamber walls had a white opaque covering to prevent animals from seeing each other during the procedures. Activity was recorded with a camera connected to a computer running FreezeFrame software (Actimetrics, Wilmette, IL) with a bout of freezing defined as a period lasting 0.75s or longer without any change in pixel luminance. The training context (Context A) was as just described. An alternate-preexposure context (Context B) was a modification of Context A that altered the texture, flooring, side walls, and spatial layout of the chamber. Transport cages (11 × 11 × 18 cm3) made of Lexan and surrounded with opaque paper on all four walls were used to move individual rats to and from their colony room home cages. All chambers (Context A and B) were cleaned with a 5% ammonium hydroxide solution, otherwise there was no odor used during conditioning.
2.3 Procedure
The general conditioning procedure has been described in detail previously (Asok et al., 2013; Dokovna, Jablonski, & Stanton, 2013; Jablonski et al., 2012; Murawski & Stanton, 2010, 2011; Schreiber et al., 2014). Preexposure, training and testing occurred a day apart beginning on PD17, PD24 or PD31. Animals were assigned to one of three groups: Pre group (preexposed to Context A), Alt-Pre group (preexposed to context B) or HC (home cage control). Most animals were sacrificed following either the preexposure day or the training day for tissue collection and in situ hybridization of brain regions (see section 2.4 Brain collection). HC controls sacrificed on the training day experienced context preexposure on the previous day to control for the effects of prior behavioral experience on baseline gene expression. Another group of Pre and Alt-Pre littermates were not sacrificed so that they could undergo retention testing of conditioned fear. Brain sections of rats sacrificed after preexposure, training or home-cage controls were assessed for Egr-1 expression (see section 2.5 In situ hybridization and image analysis).
2.3.1 Preexposure
Context preexposure for Experiment 1 consisted of a single 5min exposure (single-exposure protocol) to either Context A or Context B (see Figure 1c for protocol schematic). Animals were removed from their home cages, weighed and transported in groups of four to the waiting area outside of the conditioning room. Each chamber was thoroughly cleaned with a 5% ammonium hydroxide solution immediately prior to each training session. Animals were loaded into the chambers and allowed to freely explore for 5 minutes (i.e. single-exposure protocol), after which they were returned, in their transport boxes, to their home cages. Animals used for gene expression were sacrificed 30 (±3) min following removal from the chambers. HC controls did not receive preexposure and were sacrificed while the Pre and Alt-Pre groups were being preexposed (see section 2.4 Brain collection, below).
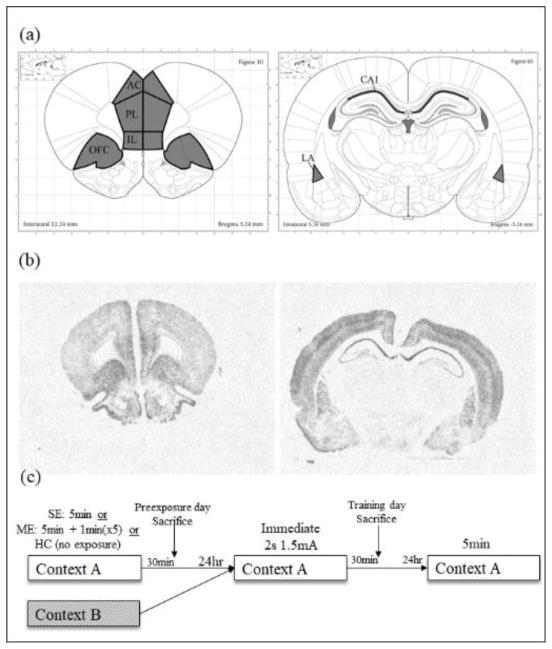
Figure 1.
(a) Illustrations of brain regions analyzed following in situ hybridization. Regions included in Egr-1 analysis are outlined in black and shaded in gray. Guides for CA1 and LA were obtained from plate 60 and guides for PL, IL, AC, and OFC were obtained from plate 10. Images are adapted from The Rat Brain in Stereotaxic Coordinates, 7th Ed (Paxinos & Watson, 2013). (b) Example brain slices with contrast enhancement to illustrate in situ hybridization results for regions in the mPFC (left) and the dHPC and LA (right). (c) Schematic of CPFE and animal sacrifice procedure illustrating the single–exposure and multiple-exposure protocols.
Context preexposure for Experiment 2 and 3 was identical to that for Experimental 1 except that animals (in addition to a single-exposure group in Experiment 2) received additional exposures using the multiple-preexposure protocol (previously described in Dokovna et al., 2013; Matus-Amat et al., 2004; Robinson-Drummer et al., 2016; Robinson-Drummer et al., 2017). Following the initial 5min preexposure period, animals were removed and placed back in their transport boxes for approximately 1min, after which they were returned to the chambers for 1min. This cycle was repeated four additional times yielding a total of five 1min exposures. All other transport, cleaning and brain collection procedures were identical to that of the single-exposure group.
2.3.2 Training
Twenty-four hours after preexposure, animals were again weighed, placed into transport boxes and transported four at a time to the waiting area outside of the conditioning room. Following cleaning with 5% ammonium hydroxide, all animals were individually brought into the conditioning room and received an immediate 2s, 1.5mA foot shock upon placement into Context A. Animals were immediately removed, returned to their transport cages, and returned to their home cage. Animals used for gene expression were sacrificed 30 (±3) min following removal from the conditioning chambers. HC controls did not receive conditioning and were sacrificed while the other animals were being trained (see section 2.4 Brain collection, below).
2.3.3 Retention Testing
Twenty-four hours after immediate-shock training, animals retained for behavioral testing were transported and placed into Context A in an identical manner to training day except no shock was delivered and freezing was recorded over a 5min testing period.
2.4 Brain Collection, in situ hybridization and image analysis
Detailed brain collection and in situ hybridization procedures were the same as previously described (Asok et al., 2013; Schreiber et al., 2014). On the preexposure day, HC animals were completely naïve. However, the training day HC controls were comprised equally of animals exposed to Context A or Context B once (Experiment 1) or after multiple-exposures (Experiment 2 and 3). Two consecutive sixteen micrometer frozen coronal brain sections corresponding to the medial prefrontal cortex [mPFC; anterior cingulate cortex (AC), prelimbic cortex (PL), infralimbic cortex (IL), orbitofrontal cortex (OFC)], dorsal hippocampus (dHPC) and lateral amygdala (LA) were sectioned on a cryostat (Leica Inc., Deerfield, IL) using the Paxinos and Watson stereotaxic rat brain atlas as a guide (Paxinos & Watson, 2013). Regions were targeted during slicing using the following coordinates: +3.24 anterior to bregma for the mPFC and −3.24 posterior to bregma for the hippocampus/amygdala (see Figure 1a).
For in situ hybridization, an antisense RNA probe (riboprobe) was transcribed from a plasmid containing a sense cDNA sequence coding for a 230bp sequence of Egr-1 (gift from J. Milbrandt, Washington University, St. Louis, MO). The transcribed riboprobe incorporated a radioactively labeled 35S UTP (approximately 1×106 dpm) using a T3 RNA polymerase Maxiscript kit according to the manufacturer’s instructions (Life Technologies, Grand Island, NY). After hybridization and washing, the dry slides were exposed to Kodak Biomax MR Film for two days.
Autoradiograms were captured and digitized to 8-bit gray values. Using ImageJ, the background was subtracted (2D-rolling ball radius of 50.0 pixels) and the mean density (mean gray value) for mRNA labeling was measured for each specific brain region per slice using the previously mentioned (Paxinos & Watson, 2013) atlas demarcations as a guide. Scores for each region were averaged across brain sides and then brain slices. A 14C standard with known amounts of radioactivity was exposed and captured with each film. The standard was then used to create a 3rd degree polynomial equation to convert the mean gray values of each slide to nCi/g. The nCi/g values for animals in each experiment were then normalized against the average nCi/g of the home cage animals for that experiment and in the specified region of interest to obtain a proportionate score of the home cage group which equaled 100%. When nCi/g scores fell ±1.96 standard deviations from the proportionate group mean for a region, that score was defined as an outlier and was excluded from further analysis (typically 1 score/group/region was excluded; see Results sections).
2.5 Statistical Analysis
Statistica 13 (Statsoft, Tulsa, Oklahoma) was used for all statistical analyses. Statistical analyses was as described previously (Asok et al., 2013; Murawski & Stanton, 2011; Schiffino et al., 2011; Schreiber et al., 2014). For in situ hybridization data, normalized data in the PD 31 experimental groups (i.e. Pre and Alt-Pre groups) were compared to HC controls using one-way ANOVA followed by Newman-Keul’s post hoc tests. For all other analyses factorial ANOVA followed by Newman-Keul’s post hoc test was used to compare multiple experimental factors. All behavioral data were analyzed using FreezeFrame software (Acimetrics, Wilmette IL) by an observer blind to the experimental condition of the animals. Activity thresholds were adjusted for individual animals to exclude small movements from being recorded as freezing. Behavioral data were analyzed via one-way or factorial ANOVA followed Newman-Keuls post-hoc tests (details of design appear for each experiment below). Behavioral scores ±1.96 standard deviations from the group mean were removed from analyses as outliers. All ANOVAs initially included sex as a factor. However, because main or interaction effects were rarely found (only 3 out of 34 comparisons), data are summarized (Figures) and analyzed (Tables 1–3) collapsed across this variable. Exceptions were not replicated across experiments, were only found in single regions for specific ages and were not reflected in pair-wise comparisons so they are not reported in text. Gene expression data were analyzed separately for each region. To avoid excessive, repetitive statistical reporting in the text, the statistical findings for all gene-expression analyses are summarized in Tables 1–3.
Table 1.
Statistical results (F and p values) and group numbers (n) for Experiment 1. Factorial ANOVA and Newman-Keuls post-hoc tests were used to evaluate changes in gene expression as a function of age at preexposure (using the single-exposure protocol). Gene expression was analyzed in PD17 and PD24 animals following the preexposure (Experiment 1A) and training (Experiment 1B) and in PD31 animals following training (Experiment 1C).
| Experiment 1.A: Single-Exposure CPFE Preexposure day Egr-1 expression PD17 and PD24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| AC | PL | IL | |||||||
| n (HC, Alt-Pre, Pre) | n (HC, Pre, Alt-Pre) | n (HC, Pre, Alt-Pre) | |||||||
|
|
|||||||||
| PD17 | 13, 11, 12 | 13, 11, 11 | 13, 12, 12 | ||||||
| PD24 | 12, 10**, 11 | 12, 10**, 13* | 12, 10***, 12** | ||||||
| F | p | F | p | F | p | ||||
|
|
|||||||||
| Age | 14.36 | <. .001 | PD24>PD17 | 15.63 | <.001 | PD24 >PD17 | 14.63 | <.001 | PD24 > PD17 |
|
|
|||||||||
| Preexposure | 4.15 | 0.02 | Alt-Pre > HC | 4.92 | 0.01 | Pre, Alt-Pre > HC | 16.66 | <.001 | Pre, Alt-Pre > HC |
|
|
|||||||||
| Age x Preexposure | 3.88 | 0.03 | PD 24 Pre, Alt-Pre > PD 17, HC | 4.18 | 0.02 | PD 24 Pre, Alt-Pre > PD 17, HC | 4.04 | 0.02 | PD 24 Pre, Alt-Pre > PD 17, HC |
|
|
|||||||||
| OFC | dHPC | dLA | |||||||
| n (HC, Pre, Alt-Pre) | n (HC, Pre, Alt-Pre) | n (HC, Alt-Pre, Pre) | |||||||
|
|
|||||||||
| PD17 | 13, 12, 11 | 11, 12, 12 | 12, 12, 12 | ||||||
| PD24 | 11, 11***, 12** | 12, 10, 11** | 12, 11***, 10*** | ||||||
| F | p | F | p | F | p | ||||
|
|
|||||||||
| Age | 15.57 | <.001 | PD24>PD17 | 8.04 | <01 | PD24>PD17 | 20.6 | <.001 | PD24>PD17 |
|
|
|||||||||
| Preexposure | 8.74 | .<001 | Pre, Alt-Pre> HC | 4.84 | 0.01 | Pre, Alt-Pre > HC | 8.27 | <.001 | Pre, Alt-Pre > HC |
|
|
|||||||||
| Age x Preexposure | 4.89 | 0.01 | PD24 Pre, Alt-Pre > PD 17, HC | 3.14 | 0.0502 | NS | 6.03 | <.01 | PD 21 Pre, Alt-Pre > PD 17, HC |
|
| |||||||||
| Experiment 1B: Single-Exposure CPFE Training day Egr-1 expression PD17 and PD24 | |||||||||
|
| |||||||||
| AC | PL | IL | |||||||
| n (HC, Alt-Pre, Pre) | n (HC, Pre, Alt-Pre) | n (HC, Pre, Alt-Pre) | |||||||
|
|
|||||||||
| PDI7 | 13, 12**, 11** | 13, 12***, 11*** | 13, 12***, 11** | ||||||
| PD24 | 12, 12**, 10** | 12, 10***, 11** | 12, 10***, 11*** | ||||||
| F | p | F | p | F | p | ||||
|
|
|||||||||
| Age | 0.18 | 0.68 | NS | 0.55 | 0.46 | NS | 9.39 | <.01 | PD24 > PD27 |
|
|
|||||||||
| Preexposure | 6.24 | <.01 | Pre, Alt-Pre > HC | 20.94 | <.001 | Pre. Alt-Pre > HC | 16.12 | <.001 | Pre, Alt-Pre > HC |
|
|
|||||||||
| Age x Preexposure | 0.23 | 0.8 | NS | 1.54 | 0.22 | NS | 2.46 | 0.09 | NS |
|
|
|||||||||
| OFC | dHPC | dLA | |||||||
| n (HC, Pre, Alt-Pre) | n (HC, Pre, Alt-Pre) | n (HC Alt-Pre, Pre) | |||||||
|
|
|||||||||
| PD17 | 13, 13***, 11** | 12, 12***, 9* | 12, 10***, 11*** | ||||||
| PD24 | 12, 10***, 11** | 12, 10***, 11* | 12, 11**, 12 | ||||||
| F | P | F | p | F | p | ||||
|
|
|||||||||
| Age | 0.47 | 0.49 | NS | 1.54 | 0.22 | NS | 0.99 | 0.32 | NS |
|
|
|||||||||
| Preexposure | 11.50 | <.001 | Pre, Alt-Pre > HC | 15.72 | <.001 | Alt-Pre > Pre > HC | 13.37 | <.001 | Alt-Pre > Pre > HC |
|
|
|||||||||
| Age x Preexposure | 0.15 | 0.86 | NS | 0.45 | 0.64 | NS | 1.52 | 0.23 | NS |
|
| |||||||||
| Experiment 1C: Single-Exposure CPFE Training day Egr-1 expression PD31 | |||||||||
|
| |||||||||
| AC | PL | IL | |||||||
| n (HC, Alt-Pre, Pre) | n (HC, Pre, Alt-Pre) | n (HC, Pre, Alt-Pre) | |||||||
|
|
|||||||||
| PD31 | 12, 12**, 11** | 12, 12*, 12*** | 12, 12**, 12*** | ||||||
| F | p | F | p | F | p | ||||
|
|
|||||||||
| Preexposure | 7.08 | < .01 | Pre, Alt-Pre > HC | 12.02 | <.001 | Pre > Alt-Pre >HC | 13.32 | < .001 | Pre > Alt-Pre >HC |
|
|
|||||||||
| OFC | dHPC | dLA | |||||||
| n (HC, Alt-Pre, Pre) | n (HC, Pre, Alt-Pre) | n (HC, Pre, Alt-Pre) | |||||||
|
|
|||||||||
| PD31 | 12, 12*, 12* | 11, 12, 12 | 11, 12, 12 | ||||||
| F | p | F | p | F | p | ||||
|
|
|||||||||
| Preexposure | 3.80 | 0.03 | Pre, Alt-Pre > HC | 2.87 | 0.069 | 3.04 | 0.059 | ||
Asterisks (next to group n) represent Newman-Keuls post hoc tests comparing experimental groups to homecage (HC) controls.
p < .05,
p < .01,
p < .001.
Main or interaction effects are described by inequality signs (> or <) and non-significant effects are listed as “NS.”
Table 3.
Statistical results (F and p values) and group numbers (n) for Experiment 3. Factorial ANOVA and Newman-Keuls post-hoc tests were used to evaluate changes in gene expression as a function of age at preexposure (using the multiple-exposure protocol). Gene expression was analyzed in PD17 and PD24 animals following immediate shock training.
| Experiment 3: Multiple-Exposure CPFE Training day Egr-1 expression PD17 and PD24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| AC | PL | IL | |||||||
| n (HC, Pre, Alt-Pre) | n (HC, Pre, Alt-Pre) | n (HC, Alt-Pre, Pre) | |||||||
|
|
|||||||||
| PD17 | 13, 11*, 11* | 11, 11, 10 | 11, 12*, 11 | ||||||
| PD24 | 12, 11***, 11*** | 11, 10***, 12*** | 11, 11***, 12*** | ||||||
|
|
|||||||||
| F | p | F | p | F | p | ||||
|
|
|||||||||
| Age | 14.73 | <.001 | PD24<PD17 | 76.03 | <.001 | PD24>PD17 | 39.66 | <.001 | PD24>PD17 |
|
|
|||||||||
| Preexposure | 25.21 | <.001 | Pre, Alt-Pre > HC | 27.56 | <.001 | Pre, Alt-Pre > HC | 25.22 | <.001 | Pre, Alt-Pre > HC |
|
|
|||||||||
| Age x Preexposure | 4.01 | 0.02 | Pre 24, Alt Pre 24 > PD 17 >HC | 26.45 | <.001 | Pre 24 > Alt Pre 24 > PD 17, HC | 15.45 | <.001 | Pre 24 > Alt Pre 24 > PD 17, HC |
|
|
|||||||||
| OFC | dHPC | LA | |||||||
| n (HC, Pre, Alt-Pre) | n (HC, Pre, Alt-Pre) | n (HC, Alt-Pre, Pre) | |||||||
|
|
|||||||||
| PD17 | 12, 11, 11 | 12, 10, 12 | 12, 11*, 11* | ||||||
| PD24 | 11, 12***, 12*** | 12, 11, 11 | 12, 12*, 11* | ||||||
|
|
|||||||||
| F | p | F | p | F | p | ||||
|
|
|||||||||
| Age | 35.11 | <.001 | PD24>PD17 | 1.91 | 0.17 | NS | 1.27 | 0.26 | NS |
|
|
|||||||||
| Preexposure | 20.19 | <.001 | Pre, Alt-Pre > HC | 1.51 | 0.23 | NS | 4.29 | 0.02 | Pre, Alt-Pre > HC |
|
|
|||||||||
| Age x Preexposure | 9.55 | <.001 | Pre 24, Alt Pre 24 > PD 17 >HC | 0.67 | 0.51 | NS | 0.35 | 0.7 | NS |
Asterisks (next to group n) represent Newman-Keuls post hoc tests comparing experimental groups to homecage (HC) controls.
p < .05,
p < .01,
p < .001.
Main or interaction effects are described by inequality signs (> or <) and non-significant effects are listed as “NS.”
3. Results
3.1 Experiment 1: Egr-1 expression in developing rats following a single context exposure or immediate foot-shock training
Animals for Experiment 1A and 1B were littermates randomly assigned to begin conditioning on PD17 or PD24 and sacrificed following preexposure or foot-shock training or retained for behavioral testing. Animals for Experiment 1C were a separate set of littermates that began conditioning on PD31 and sacrificed following foot-shock training. Statistical analyses for behavioral results (section 3.1.1), gene expression for PD17 and PD24 following preexposure (section 3.1.2) or training (section 3.1.3) and PD31 gene expression following training (section 3.1.4) are presented separately in the following sections.
3.1.1 Behavioral Results
Behavior subjects were 25 and 22 animals from 20 litters preexposed on PD17 or PD24, respectively, to either the training context (Pre) or the alternate context (Alt-Pre). This yielded a four-group experimental design (PD17 Pre, PD17 Alt-Pre, PD24 Pre and PD24 Alt-Pre). Three total outliers were removed (PD17 Alt-Pre n=1; PD17 Pre n=1; PD24 Alt-Pre n=1) and statistical analyses were carried out on the remaining 44 animals with final group sizes as follows: (PD17 Alt-Pre n=12; PD17 Pre n=10; PD24 Alt-Pre n=11; PD24 Pre n=11). In addition, 25 animals from 10 litters were preexposed at PD31. One case of same-sex littermate oversampling was averaged in the PD31-Alt-Pre group and two outliers were removed (PD31 Pre n=1, PD31 Alt-Pre n=1) leaving 11 final animals in the PD31 Alt-Pre and PD31 Pre groups.
Figure 2a shows the behavioral results for Experiment 1. Comparing PD17 and PD24 animals, a 2 (Age: PD17 v PD24) x 2 (Preexposure condition: Pre v Alt-Pre) factorial ANOVA revealed a significant effect of age [F(1,40) = 7.04, p = .01], preexposure condition [F(1,40) = 15.68, p < .001] and a age x preexposure condition interaction [F(1,40) = 10.75, p < .01]. Newman-Keuls post-hoc analyses revealed that group PD24 Pre froze significantly more during retention testing than group PD24 Alt-Pre, PD17 Pre and the PD17 Alt-Pre groups (all p’s < .001) however these groups did not differ (p’s > .62). In addition, one-way ANOVA (PD 31 Preexposure condition: Pre v Alt-Pre) revealed a significant effect of group [F(1, 20) = 56.90, p < .001] and confirmed the presence of the CPFE at PD31 in the Pre group relative to the Alt-Pre group. These results replicate previous findings from our lab (Asok et al., 2013; Jablonski et al., 2012; Murawski & Stanton, 2011; Schiffino et al., 2011) that PD24 and PD31 but not PD17 rats display 24hr retention of contextual fear conditioning when trained on the “single preexposure” CPFE protocol.
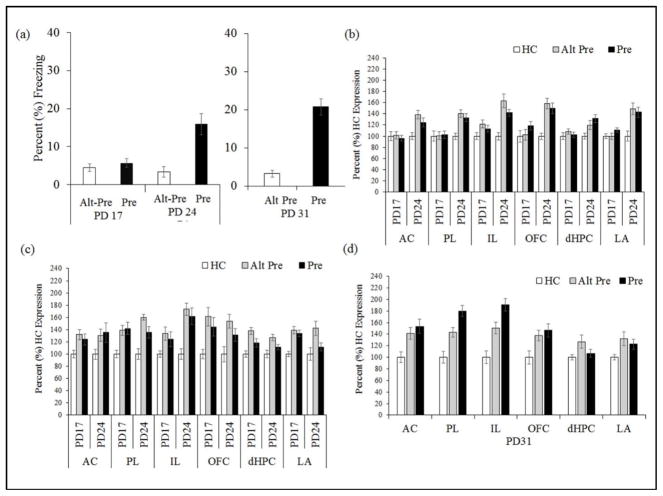
Figure 2.
(a) Mean (±SEM) percent test freezing 24hr after immediate shock training in rats preexposed on PD17, PD24 or PD31 using the single-exposure CPFE protocol. The CPFE was evident in animals preexposed on PD24-31 but not PD17, as reflected in higher Pre group freezing relative to Alt-Pre controls. (b) Mean (±SEM) percent expression of Egr-1 mRNA relative to homecage (HC) controls following context preexposure. Pre and Alt-Pre rats preexposed on PD24 displayed significantly elevated gene expression in all regions relative to HC controls and to rats preexposed on PD17. These increases were absent in rats preexposed on PD17. (c) Following immediate foot-shock training, Pre and Alt-Pre expression was increased similarly between PD17 and PD24 rats in all regions except the IL where PD24 expression was elevated relative to PD17 expression, and LA where only Alt-Pre exceeded HC in PD24 rats. (d) Mean (±SEM) percent expression of Egr-1 mRNA in PD31 rats following immediate foot-shock training. Learning-related increases in gene expression (Pre > Alt-Pre) in the mPFC were evident in the PL and IL but no other regions.
3.1.2 Preexposure Day Egr-1 (Experiment 1A)
Experiment 1A assessed changes in Egr-1 expression in the mPFC, dHPC and LA following the preexposure day of the CPFE to determine the potential contribution of this gene expression to the developmental emergence of the CPFE. Subjects for Experiment 1A began the behavioral protocol on either PD17 or PD24. Using 13 litters, tissue was collected from 39 animals preexposed on PD17 and 37 animals preexposed on PD24. HC, Alt-Pre and Pre groups were initially comprised of 13 animals and, after outliers were removed (see in situ hybridization and image analysis above), final group sizes ranged from 10–13 animals (see Table 1, Exp. 1A).
Figure 2b shows the results of Experiment 1A. In general, novel context exposure increased gene expression in all regions on PD24 but not PD17. This was true for both Context A (Pre) and Context B (Alt-Pre) which did not differ but were both elevated over home-cage (HC) control levels on PD24 but not PD17. In all regions (see Table 1, Exp. 1a), separate 2 (Age: PD17 v PD24) x 3 (Preexposure condition: HC, Alt-Pre, Pre) factorial ANOVAs revealed significant main effects of age, preexposure condition and significant age x preexposure condition interactions (except in the dHPC where the interaction was only trending; p = .0502). Newman-Keul’s post-hoc test revealed that regardless of preexposure condition, PD 24 animals had significantly elevated Egr-1 expression following context preexposure relative to PD 17 animals (p’s < .01). Further examination of the interaction effects revealed that for the mPFC (AC, PL and IL) and the LA both group Pre and Alt Pre showed significantly increased Egr-1 expression relative to group HC when animals were preexposed at PD 24 (p’s < .02) but there was no increase observed at PD17 (p’s > .05). Trends were similar in the dHPC but the interaction failed to reach significance [F(2, 62) = 3.14, p = .0502]; there was a dHPC main effect of age (p <.01) and preexposure condition (p < .02) such that groups Pre and Alt-Pre were significantly elevated above HC (p’ < .03) and PD 24 had increased expression relative to PD 17 animals (p < .01). Together, these results confirm that exposure to a novel context significantly increases relative Egr-1 expression in all regions on PD24 but does not drive gene expression on PD17.
3.1.3 Training Day Egr-1 (Experiment 1B)
Experiment 1B assessed ontogenetic differences in Egr-1 expression following the training day of the CPFE in PD17 and PD24 rats (Figure 2c). Tissue was collected from 39 animals (13 litters) and 36 animals (12 litters) from the PD17 and PD24 age groups, respectively. After outliers were removed (see method section), statistics were run on each region separately with final group sizes ranging from 9–13 animals (see Table 1, Exp. 1B).
In contrast to Experiment 1A, 2 (Age: PD17 v PD24) x 3 (Preexposure condition: HC, Alt-Pre, Pre) followed by Newman-Keul’s post-hoc test did not reveal any main effects of age or age x preexposure interactions in any region (p’s > .22; see Table 1) except in the IL where there was a main effect of age (p < .01) reflecting increased expression in the PD24 animals relative to PD17. There was a significant main effect of preexposure condition in all regions, regardless of age, such that groups Pre and Alt-Pre had increased Egr-1 expression relative to HC (p’s < .02). These results confirm that immediate footshock training can drive Egr-1 in the mPFC, dHPC and LA in both PD17 and PD24 animals although only PD24 animals acquire the CPFE.
3.1.4 Training day Egr-1 (Experiment 1C)
In Experiment 1B, PD24 animals did not show the learning-related changes in mPFC gene expression following foot-shock training as previously observed at PD31 (Asok et al., 2013). Historically these animals have not differed in their levels of conditioning (Jablonski et al., 2012; Robinson-Drummer & Stanton, 2015; Schiffino et al., 2011) so these results were surprising. Because we have observed this effect using the single-exposure protocol on PD31 in only a single study (Asok et al., 2013), Experiment 1C sought to assess the replicability of this effect by reexamining PD31 gene expression following the single-exposure protocol. Tissue was collected on the training day from 39 animals from 10 litters. After outliers were removed (see method section), statistics were run on each region separately with final group sizes ranging from 11–13 (see Table 1, Exp. 1C).
Figure 2d shows the results of Experiment 1C. One-way ANOVA (Preexposure condition: HC, Alt-Pre, Pre) revealed a significant main effect of Preexposure condition in all four prefrontal regions (Table 1, Exp. 1C). Newman-Keul’s test showed both experimental groups were significantly increased (all p’s < .05) relative to HC. In the AC and OFC, Newman Keuls post-hoc test showed no significant difference between Pre and Alt-Pre gene expression (p’s > .41). In contrast, in both the PL and IL, Pre group expression was significantly elevated above Alt-Pre and HC, replicating the learning-related change in PD31 rats previously reported with the single-exposure protocol by our lab (Asok et al., 2013; Chakraborty et al., 2016).
In the LA, there was a marginally significant main effect of group with a trending increase in both Pre and Alt-Pre gene expression above HC. Although not significant, this trend is similar to previous findings from our lab using both single-exposure (Asok et al., 2013) and multiple-exposure (Schreiber et al., 2014) protocols. Similarly, a trending group effect was observed in the dHPC, driven by a marginally increased Alt-Pre group (but not Pre group) relative to HC. Again, this trend replicates our previous report (Asok et al., 2013) and these results together with Experiments 1A and 1B suggest Egr-1 expression induced by novel context exposure and foot-shock training during the CPFE develops between PD17 and PD31.
3.2 Experiment 2: Training day Egr-1 expression in PD24 rats following single- or multiple-context preexposure
The behavioral results of Experiment 1 revealed relatively low conditioning (~15%) in the PD24 animals (Figure 2a) relative to our historical data (~25–30%; Jablonski et al., 2012; Robinson-Drummer & Stanton, 2015; Schiffino et al., 2011). The lack of learning-related expression (Asok et al., 2013; Chakraborty et al., 2016; Schreiber et al., 2014) in the PD24 group may reflect a true ontogenetic difference between young and older rats however it is also possible that weak conditioning in the PD24 animals simply did not drive learning-related Egr-1 expression. In order to address this possibility, Experiment 2 manipulated the amount of exposure received on the preexposure day in order to strengthen conditioning on the training day. The effect of single- vs. multiple-preexposure protocols on behavior and training-day gene expression was examined in PD24 rats.
3.2.1 Behavioral Results
Behavioral subjects were 36 animals from 15 litters (the remaining littermates were used during conditioning for in situ hybridization; see following section). On the preexposure day, animals received either the single-exposure (SE) protocol (previous study) or the multiple-exposure (ME) CPFE protocol which has been described previously (see section 2.3.1 Preexposure; Dokovna et al., 2013; Robinson-Drummer et al., 2016; Robinson-Drummer et al., 2017; Rudy & Wright-Hardesty, 2005). This yielded a four-group design (SE Pre, SE Alt-Pre, ME Pre and ME Alt-Pre). Four outliers were removed (ME Alt-Pre n=1; ME Pre n=1; SE Alt-Pre n=1; SE Pre n=1) and final group sizes as follows: (ME Alt-Pre n=4; ME Pre n=5; SE Alt-Pre n=12; SE Pre n=11).
Figure 3a shows the behavioral results for Experiment 2. A 2 (Exposures: Single v Multiple) x 2 (Preexposure condition: Pre v Alt Pre) factorial ANOVA revealed a significant effect of preexposure condition [F(1,27) = 10.53, p < .01], but no main effect of exposure [F(1,27) = .46, p = .50] or exposure x preexposure condition interaction [F(1,27) = .05, p = .82]. Newman-Keuls post-hoc analyses revealed significantly higher freezing in group Pre (regardless of number of exposures) relative to the Alt-Pre groups (p’s < .01). The increased freezing in the Pre groups relative to the Alt-Pre groups indicates the presence of the CPFE however the additional exposure in the ME Pre did not increase overall conditioned freezing over the SE Pre group. That the SE Pre group froze more than its counterpart in Experiment 1 (~25% vs. 15%) reflects cross-experiment variation (sampling error) that we have occasionally seen in our previous studies.
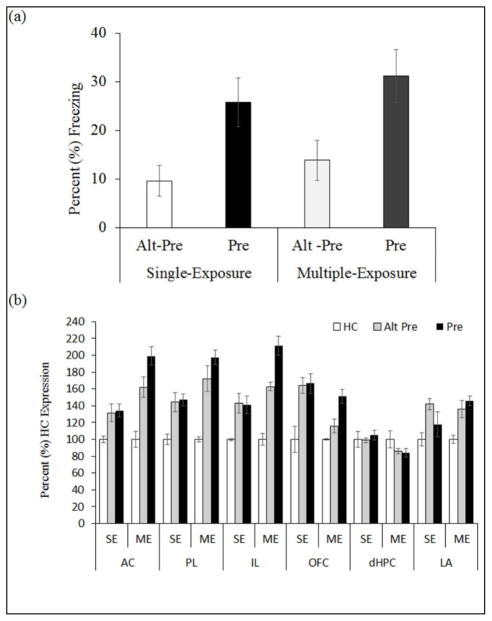
Figure 3.
(a) Mean (±SEM) percent test freezing 24hr after immediate shock training in rats preexposed on PD24 using the single- or multiple-exposure CPFE protocol. A significant CPFE was evident using both protocols with no difference observed between single-exposure and multiple-exposure freezing and both groups were significantly elevated over Alt-Pre controls. (b) Mean (±SEM) percent expression of Egr-1 mRNA relative to HC controls following immediate shock training. Immediate shock training increased Egr-1 expression in Pre and Alt-Pre rats relative to HC controls using both the single-exposure and multiple-exposure protocols. However a learning-related increase (Pre> Alt-Pre) in the AC and IL was observed using the multiple-exposure protocol only.
3.2.2 Training day Egr-1 (Experiment 2)
The effect of single vs. multiple context preexposure on Egr-1 expression following immediate foot shock on the training day in PD24 rats is shown in Figure 3b. Subjects began the behavioral protocol on PD24 (59 animals from 14 litters). The HC group was comprised equally of animals given single- and multiple-exposures on the preexposure day (for final group sizes see Table 2). In general, immediate-shock training elevated Egr-1 expression over HC control levels in all exposure groups and in all regions except dHPC (Figure 2b), however learning-related changes were observed in some prefrontal sub-regions following the multiple-exposure but not single-exposure protocol.
Table 2.
Statistical results (F and p values) and group numbers (n) for Experiment 2. Factorial ANOVA and Newman-Keuls post-hoc tests were used to evaluate changes in PD24 gene expression following immediate shock training as a function of context exposures on the preexposure day (single vs multiple-exposures).
| Experiment 2: Single v Multiple- Exposure CPFE Training day Egr-1 expression PD24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| AC | PL | IL | |||||||
| n (HC, Pre, Alt-Pre) | n (HC, Pre, Alt-Pre) | n (HC, Alt-Pre, Pre) | |||||||
|
|
|||||||||
| SE PD 24 | 3, 10, 8 | 3, 10***, 8*** | 3, 10, 7 | ||||||
| ME PD 24 | 4, 8**, 8*** | 3, 8***, 7*** | 3, 7**, 8*** | ||||||
| F | p | F | p | F | p | ||||
|
|
|||||||||
| Exposure | 10.07 | <.01 | ME>SE | 5.72 | 0.02 | ME>SE | 8.77 | <.01 | ME>SE |
|
|
|||||||||
| Pre Condition | 12.55 | <.001 | Pre, Alt-Pre >HC | 12.27 | <.001 | Pre, Alt-Pre > HC | 15.70 | <.001 | Pre > Alt-Pre > HC |
|
|
|||||||||
| Exposure x Pre Condition | 3.28 | 0.049 | ME Pre > all others | 1.57 | 0.22 | NS | 4.74 | 0.02 | ME Pre > all others |
|
| |||||||||
| OFC | dHPC | dLA | |||||||
| n (HC, Pre, Alt-Pre) | n (HC, Pre, Alt-Pre) | n (HC, Alt-Pre, Pre) | |||||||
|
|
|||||||||
| SE PD 24 | 3.9***, 7*** | 5, 9, 6 | 4, 10***, 5*** | ||||||
| ME PD 24 | 3.8 ***, 8*** | 3, 7, 8 | 5, 8***, 9*** | ||||||
| F | p | F | p | F | p | ||||
|
|
|||||||||
| Exposure | 5.38 | 0.03 | SE>ME | 6.23 | 0.02 | SE>ME | 0.94 | 0.34 | NS |
|
|
|||||||||
| Pre Condition | 11.43 | <.001 | Pre > Alt-Pre > HC | 0.82 | 0.45 | NS | 9.03 | <.001 | Pre, Alt-Pre > HC |
|
|
|||||||||
| Exposure x Pre Condition | 2.66 | 0.09 | NS | 1.54 | 0.23 | NS | 2.2 | 0.13 | NS |
Asterisks (next to group n) represent Newman-Keuls post hoc tests comparing experimental groups to homecage (HC) controls.
p < .05,
p < .01,
p < .001.
Main or interaction effects are described by inequality signs (> or <) and non-significant effects are listed as “NS.”
Separate 2 (Exposure: Single v Multiple) x 3 (Pre condition: HC, Pre, Alt-Pre) factorial ANOVAs (see Table 2) revealed a main effect of exposure (p’s < .02) in the AC, PL, IL, OFCand dHPC [but not the LA (p = .34)] such that, regardless of preexposure condition, animals in the group ME showed increased Egr-1 expression relative to group SE except in the OFC and dHPC where this pattern was reversed. Additionally, a significant main effect of preexposure condition (p’s < .001) was found in all mPFC subregions and the LA [but not the dHPC (p = .45)] such that groups Pre and Alt-Pre, regardless of exposure, had significantly elevated Egr-1 above HC. The AC and IL also had significant exposure x pre condition interaction (p’s < .05). Both single and multiple preexposures increased Egr-1 expression in groups Pre and Alt-Pre relative HC, however group multiple-exposure Pre (ME Pre) had gene expression significantly elevated above all other experimental groups which did not differ from each other (p’s > .88). A similar non-significant trend was also observed in the PL (Figure 3b).
Experiment 2 revealed learning-related Egr-1 expression on the training day in the AC and IL mPFC (but not the dHPC or LA) following the multiple-exposure but not the single-exposure protocol. This result was not found in PD 31 rats as both the single-exposure (Asok et al., 2013) and multiple-exposure (Schreiber et al., 2014) protocol produced learning-related increases in some mPFC sub-regions. These results suggests that more context training is needed at PD 24 to achieve similar patterns of mPFC activation as that observed at older ages.
3.3 Experiment 3: Training day Egr-1 expression in PD17 and PD24 rats following the multiple-exposure preexposure day protocol
The different patterns of mPFC gene expression following the single- vs. multiple-exposure protocols was interesting in light of no behavioral differences observed across these protocols. To replicate this finding and examine if this same change would be observed in PD17 rats, gene expression was again measured following immediate foot-shock training using a multiple-exposure protocol comparing PD17 and PD24 animals.
3.3.1 Behavioral Results
Behavior subjects were 36 animals from 14 litters (the remaining littermates were used during conditioning for in situ hybridization; see the following section). Animals were assigned to begin the CPFE protocol on either PD17 or PD24. Two animals were removed as statistical outliers (PD17 Pre n = 1; PD24 Alt-Pre n = 1) and the final group sizes as follows: (PD17 Pre n = 11; PD17 Alt-Pre n = 6; PD24 Pre n = 12; PD24 Alt-Pre n = 5).
Figure 4a shows the behavioral results for Experiment 3. Similar to Experiment 1, 2(Age: PD17 v PD24) x 2 (Preexposure Condtition: Pre v Alt-Pre) factorial ANOVA revealed a significant effect of age [F(1,30) = 4.39, p = .04], preexposure condition [F(1,30) = 9.98, p < .01] and a age x preexposure condition interaction [F(1,30) = 8.97, p < .01]. Again, Newman-Keuls post-hoc analyses revealed significantly higher freezing in PD24 Pre relative to groups PD24 Alt-Pre, PD17 Pre and the PD17 Alt-Pre groups (all p’s < .01) however these other groups did not differ (p’s > .52). Like Experiment 1, PD17 animals failed to show a CPFE that is readily observed using the same parameters at PD24, even with stronger conditioning (i.e. multiple-exposures).
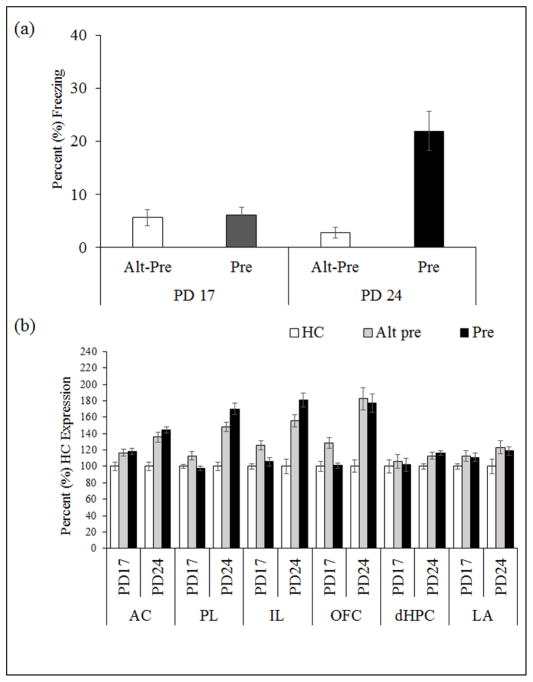
Figure 4.
(a) Mean (±SEM) percent test freezing 24hr after immediate shock training in rats preexposed on PD17 or PD24 using the multiple-exposure CPFE protocol. The CPFE (Pre > Alt-Pre) was evident in animals preexposed on PD24 but not PD17. (b) Mean (±SEM) percent expression of Egr-1 mRNA relative to HC controls following immediate shock training. Learning-related increases (Pre > Alt-Pre) in gene expression were evident in the PL and IL subdivisions of mPFC on PD24 but not on PD 17.
3.3.2 Training day Egr-1 (Experiment 3)
Gene expression in Experiment 3 was examined on the training day 24hr following the same multiple-exposure protocol used in Experiment 2. For both PD17 and PD24, 38 animals from 14 litters were sacrificed on the training day and final group sizes ranged from 10–13 animals (see Table 3).
Figure 4b shows the Egr-1 results of Experiment 3. Separate 2 (Age: PD17 v PD24) x 3 (Pre condition: HC, Pre, Alt-Pre) revealed no effects or interactions (Table 3) in the dHPC (p’s > .17). In the LA, there was a main effect of preexposure condition (p < .02) such, that regardless of age, groups Pre and Alt-Pre were elevated above HC. Separate 2×3 ANOVA for the mPFC subregions revealed significant main effects age, preexposure condition and significant age x preexposure condition interactions (p’s < .03). Generally, groups Pre and Alt-Pre were significantly elevated above HC for both ages (p’s < .001). In the OFC, this effect was driven by significant increases in PD24 Pre and Alt-Pre above HC; for PD17, Pre and Alt-Pre were not significantly elevated above HC. In the AC, both PD24 and PD17 experimental groups were elevated above HC but PD 24 expression was higher than PD17 expression. For both the PL and the IL PD24 Egr-1 expression was elevated in groups Pre and Alt-Pre above HC and PD17 with no significant increase observed in PD17 groups. Additionally, in both the PL and IL at PD24 group Pre was elevated above group Alt-Pre and HC.
For two prefrontal (PL and IL) subregions, PD24 animals showed the learning-related increase in Pre-group gene expression (relative to Alt-Pre and both PD17 groups) observed in the AC and IL of Experiment 2 and in previous reports (Asok et al., 2013; Chakraborty et al., 2016; Schreiber et al., 2014). However, even with increased context exposure, PD17 animals did not show learning-related increases in Egr-1 expression and expression levels actually seemed lower in this experiment relative to that observed in Experiment 1. This may suggest that much of the gene expression observed in PD17 animals on the training day following the single-exposure protocol may be due to novelty of the fear chambers which was reduced with multiple-exposures. Although the positive effect in the AC did not replicate between Experiments 2 and 3, the positive effect at PD24 in the IL does replicate Experiment 2 while the effect in PL, which was only a trend in Experiment 2, was significant in the current experiment. The multiple-exposure protocol did not change the ontogeny of contextual fear conditioning which was absent at PD17 and robust at PD24 nor did it produce learning-related changes in gene expression observed at older ages (Experiment 2 and 3; Asok et al., 2013; Chakraborty et al., 2016; Schreiber et al., 2014).
4. Discussion
The current study examined developmental changes in Egr-1 expression during contextual fear conditioning, using the CPFE, in infant and juvenile rats. Experiment 1 revealed that context preexposure increased Egr-1 expression in the prefrontal cortex, hippocampus and lateral nucleus of the amygdala of the Pre and Alt-Pre groups relative to HC baseline levels in PD24 rats but not PD17 rats. In contrast, Egr-1 expression following immediate foot-shock training was similar between ages. Importantly, increased exposures to the training context on the preexposure day altered expression in PD24 animals such that an increase in expression reflective of the preexposure condition between Pre and Alt-Pre animals was observed in the mPFC on the training day. In contrast, increased context preexposure did not produce this pattern of Egr-1 expression in PD17 rats. Furthermore, development of mPFC gene expression patterns seemed to continue through PD31. Together, these results illustrate a clear maturation of Egr-1 expression that is both age- and experience-dependent.
An alternative explanation to the current results could be that reduced gene expression observed in PD17 rats is due to pre-weaning housing conditions somehow suppressing gene expression. However, similar patterns of gene expression between PD17 and PD24 rats following training (Experiment 1B and 3) suggests that Egr-1 is inducible in preweanling rats. Similarly, other studies have found that basal levels of Egr-1 in pre-weaning (PD17) and post-weaning (PD24) rats are similar (Travaglia, Bisaz, Cruz, & Alberini, 2016) and no difference in amygdalar or hippocampal Egr-1 level changes between PD17 and PD24 rats following tone fear conditioning (Deal, Erickson, Shiers, & Burman, 2016). However, manipulations which hold weaning conditions constant across ages would better control for this possible effect in future studies.
4.1 Circuit maturation and the ontogeny of context learning/memory in developing rats
Previous work in adult animals has shown significant increases in Egr-1 mRNA expression in response to novel context exposure (Chakraborty et al., 2016; Hall, Thomas, & Everitt, 2000) however developmental studies of this change are lacking. Although a cross-study meta-analysis was not performed, the current report, in conjunction with previous studies from our lab (Asok et al., 2013; Chakraborty et al., 2016; Schreiber et al., 2014), reveals a gradual increase in prefrontal Egr-1 expression in response to context exposure between infancy and adulthood. Following context preexposure, adult levels of prefrontal Egr-1 increased by over 200% in some regions relative to homecage controls (Chakraborty et al., 2016) while adolescent expression (PD31) for all but one group did not increase above 150% (Asok et al., 2013). For PD24 rats (Experiment 1A) IL and OFC expression increased by approximately 45–65% and AC and PL expression changes were even smaller, increasing by only 30–40%. Most interestingly, PD17 rats (Experiment 1A) showed no increase in Egr-1 expression on the preexposure day. The gradual increase in Egr-1 likely reflects both structural and functional maturation of the mPFC and its connections as the mPFC shows significant ontogenetic changes through young adulthood in the rat (Ferguson & Gao, 2014; Van Eden & Uylings, 1985; Zhang, 2004). Activity in mPFC becomes integral for memory retrieval with increased time between memory encoding and retrieval (Frankland & Bontempi, 2005; Wiltgen & Tanaka, 2013) and long-term memory consolidation processes, which function to preserve context memories in adult animals, do not maintain remote memories in developing rodents (for behavioral studies see Akers, Arruda-Carvalho, Josselyn, & Frankland, 2012; Robinson-Drummer & Stanton, 2015; Rudy & Wright-Hardesty, 2005). Reduced mPFC activity at the time of acquisition may result in insufficient cortical reactivation by the mPFC at the time of retrieval. Likewise, immaturity of molecular activity in the mPFC would likely impair context learning and/or consolidation in infant rats as prefrontal function on the preexposure day is necessary for successful performance of the CPFE in PD31 rats (N. A. Heroux et al., 2017; Robinson-Drummer et al., 2017).
The prelimbic and infralimbic cortices are thought to control fear expression and inhibition, respectively, however more recent research has also postulated a role for the mPFC in contextual fear learning (Giustino & Maren, 2015; Maren, Phan, & Liberzon, 2013; Sierra-Mercado, Padilla-Coreano, & Quirk, 2011; Zelikowsky et al., 2013; Zelikowsky, Hersman, Chawla, Barnes, & Fanselow, 2014). Following context preexposure, the two regions regions respond to context exploration suggesting that they both participate in the initial encoding or consolidation of the context representation. Recent electrophysiological research has found evidence of context-specific activity in mPFC neuronal firing which may stabilize hippocampal context representations (Kyd & Bilkey, 2003, 2005) and track multiple exposures to the same context (Hyman, Ma, Balaguer-Ballester, Durstewitz, & Seamans, 2012). That the gene expression in the prelimbic and infralimbic cortices following footshock training were reflective of preexposure conditioning may indicate a temporal tracking of contextual experiences that functions to facilitate proper fear expression or inhibition when multiple context memories have been acquired (i.e. a neutral memory following preexposure vs a fear memory during training). Previous data from our lab shows that the fear association in the CPFE can still proceed without mPFC activity however 24hr retention is impaired (N. A. Heroux et al., 2017) which suggests that if the mPFC is participating in disambiguating the context meaning following CPFE training, it is doing so through consolidation processes functioning to alter memories after the context-shock association happens. Elucidating whether Egr-1 in the prefrontal cortex serves these learning functions requires further experimentation.
In contrast to the prefrontal cortex, Egr-1 expression in the hippocampus was much smaller and more stable than in mPFC across ages in response to context exposure. Increases varied by about 25–50%, regardless of age, between PD24 (Experiment 1A) and older ages (Asok et al., 2013; Chakraborty et al., 2016) while PD17 animals showed no increase (Experiment 1A). These results parallel the ontogeny of the CPFE in that PD17 animals historically do not show the effect while a fully developed CPFE is observed beginning at PD24 (Jablonski et al., 2012; Pugh & Rudy, 1996; Robinson-Drummer & Stanton, 2015; Schiffino et al., 2011). These results are of interest because a previous study which reduced hippocampal Egr-1 using antisense during the CPFE in adult rats showed no effect of preexposure day infusions (Lee, 2010). It is possible that reducing Egr-1 activity in the hippocampus is insufficient to reduce context learning due to compensatory mechanisms in the mPFC. Using adult animals, (Zelikowsky et al., 2013) showed that the mPFC can compensate for hippocampal damage during contextual fear conditioning, whereas if both regions are compromised contextual conditioning is lost. This suggests that boosting mPFC activity (alone or in conjunction with the hippocampus) in PD17 animals may alleviate learning and/or consolidation deficits on the preexposure day. This is a fruitful direction for future research.
Changes in amygdalar response to context exposure varied according to age at exposure. Exposure at PD24 (Experiment 1A) and PD31 (Asok et al., 2013) increased Egr-1 expression while exposure at PD17 (Experiment 1A) and adulthood (Chakraborty et al., 2016) did not. These results may reflect changes in communication between regions like the mPFC and amygdala during this developmental window. The post-weanling period is associated with dynamic changes in prefrontal-amygdala physiology, structure and functional connectivity (Arruda-Carvalho, Wu, Cummings, & Clem, 2017; Bouwmeester, Smits, & Van Ree, 2002; Bouwmeester, Wolterink, & van Ree, 2002; Ehrlich, Ryan, Hazra, Guo, & Rainnie, 2013; Ehrlich, Ryan, & Rainnie, 2012; Ferguson & Gao, 2014; Van Eden & Uylings, 1985). Increases in experience-driven molecular activity in the BLA could reflect increased amygdalar activity resulting from overactive mPFC excitatory afferents until local pruning reduces this activity in adulthood. Some evidence of an mPFC-amygdala maturational cycle has been observed in developing mice (Arruda-Carvalho et al., 2017) however whether a similar developmental profile exists in the rat remains to be determined.
4.2 Weanlings and juveniles display age- and training-dependent Egr-1 expression
The learning-related differences between Pre and Alt-Pre groups observed at older ages (Asok et al., 2013; Chakraborty et al., 2016) was not observed following single-exposure to the training context at either PD17 or PD24 (Experiment 1B). This finding in PD24 rats was surprising when considering that PD24 and PD31 rats show equivalent levels of context fear (Jablonski et al., 2012; Robinson-Drummer & Stanton, 2015; Schiffino et al., 2011). Interestingly, additional exposures using the multiple-exposure protocol in PD24 rats (Experiment 2 and 3) produced learning-related increases in the prefrontal cortex similar to that observed in PD31 and adult animals while PD17 animals actually showed reduced expression in the Pre group with additional preexposure (Experiment 3). The relative increase at PD24 was slightly blunted relative to older animals in previous work (Asok et al., 2013; Chakraborty et al., 2016; Schreiber et al., 2014) which suggests that, like the preexposure day, training day Egr-1 expression is still maturing at this age. In addition, these results suggest that to achieve similar patterns of relative cortical activation, younger animals need increased exposure to (or time sampling) the training context on the preexposure day. Paradoxically, if learning related changes in Egr-1 expression are necessary for successful conditioning in the CPFE then we would predict similar levels of expression in the single- and multiple- exposure task at PD24. However, the current finding of equivalent fear conditioning across single-exposure and multiple-exposure protocols on P24, even though only multiple-exposure produces learning-related changes in prefrontal Egr-1 expression (Exps. 1 and 2) on the training day, suggests this pattern of expression is not required for the CPFE, at least in PD24 animals. Studies that knock down Egr-1 expression in mPFC during the single- and multiple-exposure protocols at PD24 are needed to resolve these issues.
Few studies have explored the ontogenetic emergence of immediate early gene expression using infant and adolescent rats. Deal et al., (2016) measured Egr-1 mRNA expression in several brain regions during fear conditioning. Interestingly, following acquisition of tone fear conditioning they found no increase, relative to homecage controls, in amygdalar or hippocampal Egr-1 expression between rats trained on PD17 or PD24. These results are in contrast to results from standard contextual fear conditioning (sCFC) in adults (Chakraborty et al., 2016; Malkani & Rosen, 2001) and adolescents (Schreiber et al., 2014) where the LA of the amygdala showed increased expression following both delayed and immediate foot-shock training. Furthermore, during the CPFE in juveniles (Experiments 1B–2), adolescents (Experiment 1C; Asok et al., 2013; Schreiber et al., 2014) and adults (Chakraborty et al., 2016) training with an immediate foot-shock increased Egr-1 expression in both preexposure conditions relative to homecage. There are several reasons why these results may not agree, including Deal’s use of tone fear conditioning with background contextual conditioning and examination of Egr-1 expression in the entire basolateral region of the amygdala instead of the dorsal lateral nucleus as in the present study. Further experimentation will be necessary to discern the causes of these conflicting findings.
4.3 Egr-1: A molecular component of contextual fear acquisition and consolidation?
Although Egr-1 induction has been closely tied to plasticity-related neuronal activity during learning (Alberini, 2009, 2009; Veyrac et al., 2014), its role in contextual fear learning is still poorly understood, particularly in the hippocampus. In animals that can acquire the CPFE, hippocampal activity varied as a function of conditioning phase. In PD24 (Experiment 1) and older rats (Asok et al., 2013; Chakraborty et al., 2016), Egr-1 expression increased following novel context exposure but was inconsistent or nonexistent following immediate-shock training. These differences suggest that plasticity-related activity in the hippocampus is necessary to learn the context but not for context memory retrieval or context-shock association. Previous reports using pharmacological manipulations have drawn similar conclusions. D-AP5, an NMDA-receptor antagonist, administered prior to preexposure but not prior to foot-shock training significantly impaired learning during the CPFE (Matus-Amat et al., 2007) suggesting that NMDA-dependent plasticity in the hippocampus is only necessary to acquire the context representation. Although there is some evidence that hippocampal Egr-1 is important for context memory reconsolidation following foot-shock (Lee, 2010), the current results support a role for hippocampal Egr-1 in context learning only.
IEGs participate in the consolidation of memories partly through the maintenance of post-training synaptic plasticity. Egr-1 knockout mice exhibit increased decay of long-term potentiation (LTP) and long-term memory deficits while early LTP and short-term memory is preserved (Jones et al., 2001). When considering the fear circuit, impaired learning and memory is associated with reduced IEG expression (including Egr-1) in the amygdala (Maddox, Monsey, & Schafe, 2011; Malkani & Rosen, 2001; Malkani et al., 2004), hippocampus (Farina & Commins, 2016; Lee, 2010; Lee, Everitt, & Thomas, 2004) and mPFC (Farina & Commins, 2016). Taken together, these studies illustrate a functional role for Egr-1 activity during conditioning across several different paradigms and in several regions. The current results suggest this role for Egr-1 expression particularly on the preexposure day when Egr-1 in the mPFC or dHPC may be facilitating plasticity-dependent consolidation of the context representation. Again, experiments that address the causal role of Egr-1 (i.e. knock down of Egr-1 expression) are required to address this issue. Other plasticity-related genes (e.g. Arc, c-Fos, BDNF, etc) may also show protracted expression in response to conditioning across development, a hypothesis we have begun to investigate (Heroux et al., 2018)
4.4 Conclusion
The role of Egr-1 as a “marker” for neuronal plasticity is less debated than its function as a component of associative learning and memory consolidation/reconsolidation (Alberini, 2009, 2011; Davis, Bozon, & Laroche, 2003; Okuno, 2011; Rosen, 2004), however its role in learning across development is still poorly understood. Here, we examined the ontogenetic profile of Egr-1 expression in infant and juvenile rodents using the CPFE and found significant maturation of gene expression that was region- and training-phase specific. Our findings suggest that differences in regional expression likely reflect ongoing development of cortical and subcortical structures necessary for conditioning. They also indicate the emergence of novelty-induced activity in regions crucial for contextual conditioning. In addition, we’ve found that learning-related expression of mPFC Egr-1 can be dissociated from the ontogeny of context-shock association during the CPFE however this IEG may nevertheless serve as a molecular component of other psychological processes necessary for learning. Assessment of the causal role of Egr-1 within specific brain regions in the ontogeny of learning is a fruitful area for further experimentation.
Highlights.
The Context Preexposure Facilitation Effect (CPFE) develops between PD17 and PD24.
Prefrontal (mPFC), hippocampal (dHPC) and amygdalar (LA) Egr-1 activity was probed.
Context preexposure (CxtP) increased Egr-1 in all regions on PD24 but not on PD17.
Only multiple CxtP produced training-related mPFC Egr-1 expression, only on PD24.
Ontogeny of fear more related to Egr-1 expression after CxtP than after training.
Acknowledgments
These experiments were supported by NIH grant 1-R01-HD075066-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akers KG, Arruda-Carvalho M, Josselyn SA, Frankland PW. Ontogeny of contextual fear memory formation, specificity, and persistence in mice. Learn Mem. 2012;19(12):598–604. doi: 10.1101/lm.027581.112. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89(1):121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav Neurosci. 2011;5:12. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Wu WC, Cummings KA, Clem RL. Optogenetic Examination of Prefrontal-Amygdala Synaptic Development. J Neurosci. 2017;37(11):2976–2985. doi: 10.1523/JNEUROSCI.3097-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Schreiber WB, Jablonski SA, Rosen JB, Stanton ME. Egr-1 increases in the prefrontal cortex following training in the context preexposure facilitation effect (CPFE) paradigm. Neurobiol Learn Mem. 2013;106:145–153. doi: 10.1016/j.nlm.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester H, Smits K, Van Ree JM. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J Comp Neurol. 2002;450(3):241–255. doi: 10.1002/cne.10321. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Wolterink G, van Ree JM. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J Comp Neurol. 2002;442(3):239–249. doi: 10.1002/cne.10084. [DOI] [PubMed] [Google Scholar]
- Chakraborty T, Asok A, Stanton ME, Rosen JB. Variants of contextual fear conditioning induce differential patterns of Egr-1 activity within the young adult prefrontal cortex. Behav Brain Res. 2016;302:122–130. doi: 10.1016/j.bbr.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SD, Liang KC. Roles of hippocampal GABA(A) and muscarinic receptors in consolidation of context memory and context-shock association in contextual fear conditioning: a double dissociation study. Neurobiol Learn Mem. 2012;98(1):17–24. doi: 10.1016/j.nlm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behavioural Brain Research. 2003;142(1–2):17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- Deal AL, Erickson KJ, Shiers SI, Burman MA. Limbic system development underlies the emergence of classical fear conditioning during the third and fourth weeks of life in the rat. Behav Neurosci. 2016;130(2):212–230. doi: 10.1037/bne0000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokovna LB, Jablonski SA, Stanton ME. Neonatal alcohol exposure impairs contextual fear conditioning in juvenile rats by disrupting cholinergic function. Behav Brain Res. 2013;248:114–120. doi: 10.1016/j.bbr.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Hazra R, Guo JD, Rainnie DG. Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol. 2013;110(4):926–941. doi: 10.1152/jn.01105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Rainnie DG. Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J Physiol. 2012;590(19):4819–4838. doi: 10.1113/jphysiol.2012.237453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Associative vs topographical accounts of the immediate shock-freezing deficit in rats: Implications for the response selection rules governing species-specific defensive reactions. Learning and Motivation. 1986;17(1):16–39. doi: https://doi.org/10.1016/0023-9690(86)90018-4. [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learning & Behavior. 1990;18(3):264–270. doi: 10.3758/bf03205285. [DOI] [Google Scholar]
- Farina FR, Commins S. Differential expression of immediate early genes Zif268 and c-Fos in the hippocampus and prefrontal cortex following spatial learning and glutamate receptor antagonism. Behav Brain Res. 2016;307:194–198. doi: 10.1016/j.bbr.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Ferguson BR, Gao WJ. Development of thalamocortical connections between the mediodorsal thalamus and the prefrontal cortex and its implication in cognition. Front Hum Neurosci. 2014;8:1027. doi: 10.3389/fnhum.2014.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6(2):119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front Behav Neurosci. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. 2000. [DOI] [PubMed] [Google Scholar]
- Heroux NA, Osborne BF, Kawan M, Miller LA, Buban KN, Rosen JB, Stanton ME. Differential expression of the immediate early genes c-Fos, Arc, Egr-1, and Npas4 during long-term memory formation in the context preexposure facilitation effect (CPFE) Neurobiology of Learning and Memory. 2018;147:128–138. doi: 10.1016/j.nlm.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heroux NA, Robinson-Drummer PA, Sanders HR, Rosen JB, Stanton ME. Differential involvement of the medial prefrontal cortex across variants of contextual fear conditioning. Learn Mem. 2017;24(8):322–330. doi: 10.1101/lm.045286.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2012;109(13):5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Dev Psychobiol. 2012;54(7):714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, … Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4(3):289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Prefrontal cortex lesions modify the spatial properties of hippocampal place cells. Cereb Cortex. 2003;13(5):444–451. doi: 10.1093/cercor/13.5.444. [DOI] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Hippocampal place cells show increased sensitivity to changes in the local environment following prefrontal cortex lesions. Cereb Cortex. 2005;15(6):720–731. doi: 10.1093/cercor/bhh173. [DOI] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the updating of hippocampal memory content. Front Behav Neurosci. 2010;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304(5672):839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Maddox SA, Monsey MS, Schafe GE. Early growth response gene 1 (Egr-1) is required for new and reactivated fear memories in the lateral amygdala. Learn Mem. 2011;18(1):24–38. doi: 10.1101/lm.1980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. Specific induction of early growth response gene 1 in the lateral nucleus of the amygdala following contextual fear conditioning in rats. Neuroscience. 2000;97(4):693–702. doi: 10.1016/s0306-4522(00)00058-0. [DOI] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. N-Methyl-D-aspartate receptor antagonism blocks contextual fear conditioning and differentially regulates early growth response-1 messenger RNA expression in the amygdala: implications for a functional amygdaloid circuit of fear. Neuroscience. 2001;102(4):853–861. doi: 10.1016/s0306-4522(00)00531-5. [DOI] [PubMed] [Google Scholar]
- Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) antisense oligodeoxynucleotide infused into the amygdala disrupts fear conditioning. Learn Mem. 2004;11(5):617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14(6):417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24(10):2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121(4):721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4-9. Behav Brain Res. 2010;212(2):133–142. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Effects of dose and period of neonatal alcohol exposure on the context preexposure facilitation effect. Alcohol Clin Exp Res. 2011;35(6):1160–1170. doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H. Regulation and function of immediate-early genes in the brain: beyond neuronal activity markers. Neurosci Res. 2011;69(3):175–186. doi: 10.1016/j.neures.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 7. Boston, MA: Academic Press; 2013. [Google Scholar]
- Pugh CR, Rudy JW. A developmental analysis of contextual fear conditioning. Dev Psychobiol. 1996;29(2):87–100. doi: 10.1002/(SICI)1098-2302(199603)29:2<87::AID-DEV1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Dokovna LB, Heroux NA, Stanton ME. Cholinergic mechanisms of the context preexposure facilitation effect in adolescent rats. Behav Neurosci. 2016;130(2):196–205. doi: 10.1037/bne0000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Heroux NA, Stanton ME. Antagonism of muscarinic acetylcholine receptors in medial prefrontal cortex disrupts the context preexposure facilitation effect. Neurobiol Learn Mem. 2017;143:27–35. doi: 10.1016/j.nlm.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Stanton ME. Using the context preexposure facilitation effect to study long-term context memory in preweanling, juvenile, adolescent, and adult rats. Physiol Behav. 2015;148:22–28. doi: 10.1016/j.physbeh.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3(1):23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16(10):573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: Implications for consolidation, infantile amnesia, and hippocampal system function. Behavioral Neuroscience. 1994;108(2):227–234. doi: 10.1037/0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience. 1999;113(5):867–880. doi: 10.1037/0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Wright-Hardesty K. The temporal dynamics of retention of a context memory: something is missing. Learn Mem. 2005;12(2):172–177. doi: 10.1101/lm.84005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem. 2011;95(2):190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber WB, Asok A, Jablonski SA, Rosen JB, Stanton ME. Egr-1 mRNA expression patterns in the prefrontal cortex, hippocampus, and amygdala during variants of contextual fear conditioning in adolescent rats. Brain Res. 2014;1576:63–72. doi: 10.1016/j.brainres.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglia A, Bisaz R, Cruz E, Alberini CM. Developmental changes in plasticity, synaptic, glia and connectivity protein levels in rat dorsal hippocampus. Neurobiol Learn Mem. 2016;135:125–138. doi: 10.1016/j.nlm.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241(3):253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Besnard A, Caboche J, Davis S, Laroche S. The Transcription Factor Zif268/Egr1, Brain Plasticity, and Memory. Progress in Molecular Biology and Translational Science. 2014;122:89–129. doi: 10.1016/B978-0-12-420170-5.00004-0. doi: http://dx.doi.org/10.1016/B978-0-12-420170-5.00004-0. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Tanaka KZ. Systems consolidation and the content of memory. Neurobiol Learn Mem. 2013;106:365–371. doi: 10.1016/j.nlm.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, Fanselow MS. Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci U S A. 2013;110(24):9938–9943. doi: 10.1073/pnas.1301691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J Neurosci. 2014;34(25):8462–8466. doi: 10.1523/JNEUROSCI.3624-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW. Maturation of layer V pyramidal neurons in the rat prefrontal cortex: intrinsic properties and synaptic function. J Neurophysiol. 2004;91(3):1171–1182. doi: 10.1152/jn.00855.2003. [DOI] [PubMed] [Google Scholar]