Abstract
Anaplastic large cell lymphoma (ALCL) limited to the skin is a distinct disease that is designated primary cutaneous ALCL (pcALCL). It has an indolent course with a significantly better prognosis compared to systemic ALCL (sALCL). Anaplastic lymphoma kinase (ALK) expression in lesions of cutaneous ALCL is classically considered to be a marker for skin involvement by sALCL. However recent reports of patients with ALK-positive pcALCL challenge this concept and raise prognostic and therapeutic dilemmas. Herein we report a case of ALK-positive pcALCL in a 45 year-old woman who was treated with local radiotherapy. We review previously reported cases in the literature to better characterize this rare variant. Overall the rates of cutaneous recurrence, systemic dissemination and disease related mortality in ALK-positive pcALCL do not differ from those previously reported in pcALCL. ALK-positive pcALCL is diagnosed at younger age and has a better disease course in children compared to adults with lower incidences of skin recurrence and progression to systemic disease. We conclude that ALK-positivity in cutaneous ALCL does not necessarily imply systemic disease. ALK-positive pcALCL has an excellent prognosis and should be treated by excision and/or radiotherapy. However patients must remain under close long-term follow-up as recurrence and progression to systemic disease may occur.
Introduction
Anaplastic large cell lymphoma (ALCL) is a CD30+ lymphoproliferative disorder that has two distinct forms with different disease courses: primary cutaneous ALCL (pcALCL) which has an excellent prognosis, and systemic ALCL (sALCL), a potentially aggressive lymphoma that primarily involves the lymph nodes and carries a less favorable course. sALCL is further divided into ALK-positive and ALK-negative subtypes, depending on the expression of the anaplastic lymphoma kinase (ALK) fusion protein. pcALCL is generally found to be ALK-negative and ALK expression in skin lesions of ALCL evokes high suspicion for secondary skin involvement by sALCL.1 Recently, ALK-positive pcALCL has been reported in both children and adults2–18 with heterogenous courses described.
Case Report
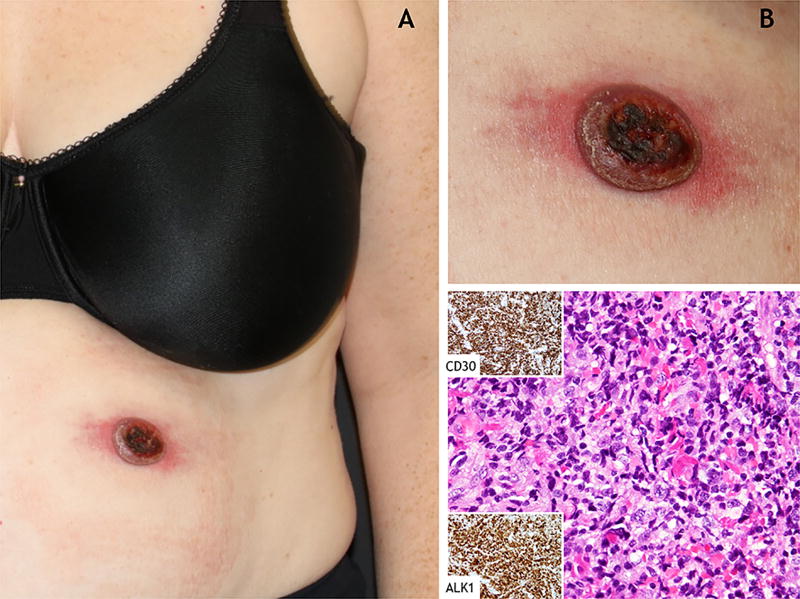
A 45 year-old woman presented to our multidisciplinary cutaneous lymphoma clinic with a one-month-history of a rapidly growing ulcerated nodule on her left abdomen. The patient was in otherwise good health without any significant past medical history. Complete review of systems was negative. Physical examination revealed a round 3-cm-in-diameter ulcerated red nodule with surrounding erythema (Figure 1(a–b)). No palpable lymphadenopathy was identified. Histopathological evaluation of lesional biopsies revealed dense dermal infiltrate of large atypical lymphocytes, positive for CD30, ALK-1 and TIA (Figure 1(c)). Complete staging was performed to assess for systemic disease. Laboratory tests were unremarkable. Positron emission tomography–computed tomography (PET-CT) demonstrated a fluoro-deoxyglucose (FDG) avid cutaneous abdominal mass corresponding to the biopsied tumor, hypermetabolic bilateral axillary lymph nodes (2 on 1.1 cm in size), and diffuse heterogeneous uptake by the bone marrow. An ultrasound-guided-biopsy showed reactive follicular hyperplasia of an axillary lymph node with negative ALK staining, and lymph node flow cytometry revealed no abnormal lymphocyte populations. The patient was diagnosed with ALK-positive pcALCL and was treated by local radiation to her left abdominal skin with a complete response. A follow-up PET-CT performed 3.5 months after the initial imaging study revealed resolution of the hypermetabolic lymphadenopathy and the cutaneous mass, and no bone marrow FDG uptake was seen.
Figure 1.
Clinical and histopathological findings in a 45 year-old woman with ALK-positive primary cutaneous anaplastic large cell lymphoma. (a–b) An ulcerated red nodule with peripheral erythema on the left upper abdomen. (c) Underlying histological features show dense dermal infiltrate of large atypical lymphocytes. The atypical lymphocytes stained positive for CD30 and showed nuclear and cytoplasmic staining for ALK-1 (Hematoxylin and eosin, original magnifications ×40).
Discussion
PcALCL is generally conceptualized as part of the cutaneous CD30+ T-cell lymphoproliferative disorder spectrum. It is most commonly diagnosed in the sixth decade; however it can also occur in childhood or adolescence. The majority of cases present with a rapidly growing solitary ulcerated nodule that can undergo partial or complete spontaneous regression. Overall, pcALCL carries a very favorable prognosis with a 5-year overall survival (OS) of 90%.19 Cutaneous recurrences are not uncommon and occur in up to 40% of treated patients, however progression to systemic disease is rare, affecting only 12–16%.1,20,21 Overexpression of the ALK fusion protein has been postulated to have a role in the malignant transformation of sALCL.22 In sALCL, ALK expression is found mainly in pediatric and young age groups19 and is of prognostic significance as ALK-positive sALCL has a 5-year OS of 70% compared to an OS of 49% in ALK-negative sALCL.19 ALK expression can also aid in therapeutic decision making: crizotinib is an ALK tyrosine kinase inhibitor that can be used as a treatment in ALK-positive sALCL.23
Following the diagnosis of ALK-positive pcALCL in the presented case, and in a pediatric patient that had been recently reported by our institution,8 we searched the literature for ALK-positive pcALCL cases in order to better characterize the course and prognosis of this rare variant. Including our cases, we identified 9 pediatric patients7,8,13,14,18 and 12 adults2–6,9–12,15–17 who were diagnosed with ALK-positive pcALCL (Table 1). Complete staging was performed in 15/21 of cases. In one case, no staging procedures were done and a disseminated disease was diagnosed after 1.5 months17 – we excluded this case from the following analyses as it was most probably sALCL rather than pcALCL.
Table 1.
Clinical features, treatment and outcome in ALK-positive primary cutaneous anaplastic large cell lymphoma (pcALCL) cases
| Reference | Age | Gender | Localization | Staging tests performed |
Initial treatment | Local/distant Skin recurrence (time to recurrence, months) |
Systemic dissemination (time to dissemination, months) |
Treatment of recurrence / dissemination |
Outcome | Follow up period (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pediatric cases (< 18 years old) | ||||||||||
| Tokuyama et al13 | 5 | F | Single (arm) | CT, PET-CT, US, LNB, OMB, LP | Dexamethasone, cyclophosphamide, methotrexate, ifosfamide, cytarabine, etoposide and doxorubicin | No | No | N/A | DF | 28 |
| Fauconneau et al18 | 17 | F | NR | CT, PET-CT, OMB | Radiotherapy | No | No | N/A | DF | 48 |
| Pulitzer et al.8 | 11 | F | Multifocal (trunk) | PET-CT, OMB, LP | Methotrexate, cyclophosphamide, cytarabine, etoposide and vinblastine, doxorubicine | No | No | N/A | DF | 36 |
| Oschlies et al.7 | 9.1 | NR | Single (leg) | CT, OMB, LP, bone scan | Excision | No | No | N/A | DF | 96 |
| 7.5 | NR | Single (neck) | CT, OMB, LP, bone scan | Excision | No | No | N/A | DF | 28 | |
| 10 | NR | Single (leg) | CT, OMB, LP, bone scan | Excision | No | No | N/A | DF | 96 | |
| 11.9 | NR | Single (leg) | CT, OMB, LP, bone scan | Radiation | No | No | N/A | DF | 62 | |
| 13.8 | NR | Single (leg) | CT, OMB, bone scan | Excision, radiation | No | No | N/A | DF | 12 | |
| Rannan-Eliya et al14 | 14 | F | Single (trunk) | NR | Excision | No | Yes (10m) | dexamethasone, cyclophosphamide, methotrexate, ifosfamide, cytarabine, etoposide and doxorubicin | DOD | 10 |
| Adult cases (> 18 years old) | ||||||||||
| Xue et al10 | 21 | F | Single (groin) | CT, OMB | Excision, CHOP | No | No | N/A | DF | 12 |
| Paolina et al.9 | 62 | M | Single (trunk) | CT, OMB | Excision | Local (0.5m) | No | Excision, radiation | DF | 12 |
| Quintanilla-Martinez15 | 27 | M | Single (NR) | NR | Excision | No | No | N/A | DF | 12 |
| Kumaran et al.12 | 65 | M | Single (Trunk) | CT, OMB | NR | NR | NR | NR | NR | NR |
| Chan et al.2 | 33 | M | Multifocal (head, trunk, leg) | CT, OMB | CHOP | Distant (4m, 16m) | Yes (18m) | Excision, ifosfamide, carboplatin, and etoposide, autologous stem cell transplantation | DF | 31 |
| Chao-Lo et al.11 | 47 | F | Multiple (arm) | CT | CHOP | Recurrence type not defined (12m) | No | Radiation | DF | 18 |
| Kadin et al.6 | 57 | M | Single (leg) | NR | Excision | Distant (16m, 60m, 92m, 105m) | No | Excision, radiation | DF | 156 |
| Campo et al16 | 26 | M | Single (leg) | NR | NR | No | No | N/A | DF | 9 |
| Sasaki et al5 and Hosoi et al.3 | 54 | F | Single (head) | NR | Spontaneous regression | Distant (1m, 30m) | Yes (96m) | Excision, radiation for recurrences, CHOP for first dissemination, ESHAP for 2nd dissemination | DOD | 71 |
| Aoki et al17* | 22 | F | Single (arm) | NR | Cisplatin, etoposide | Local and distant (18m) | Yes (1.5m) | Cisplatin, etoposide | DF | 84 |
| Su et al.4 | 57 | F | Multifocal (trunk) | CT, OMB | CHOP | No | No | N/A | DF | 13 |
| Current case | 45 | F | Single (trunk) | PET-CT, LNB | Radiotherapy | No | No | N/A | DF | 8 |
Most probably sALCL.
M male; F female; CT: computerized tomography; PET-CT: positron emission tomography CT;US: ultrasound; OMB: osteomedulary biopsy; LP: lumbar puncture; LNB: lymph node biopsy; DF disease free; NR: not reported; CHOP cyclophosphamide, adriamycin, vincristine, prednisone; N/A not applicable; DOD death of disease
The median age at diagnosis was 23.5 years (range 5–65) which is noticeably younger than that reported for pcALCL. Overall, the disease course and prognosis in the ALK-positive pcALCL cases reflected the literature on pcALCL20,21: 15.8% presented with more than one lesion, skin recurrence occurred in 27.8% and extracutaneous dissemination developed in 16.7%. Two patients (10%) expired due to systemic disease. Differences in disease course could be appreciated between pediatric and adult ALK-positive pcALCL patients. None of the pediatric patients who had complete staging at diagnosis recurred or progressed to systemic disease, whether treated with excision/radiation (6 cases) or with systemic chemotherapy (2 cases). A single pediatric case did progress (11%) and expired due to disease, however he had no staging workup done14. Among the adult cases, the rates of cutaneous recurrence and progression to systemic disease were 55.6% and 22.2%, respectively, and one adult died due to disease (11%). The rates of skin recurrence and disease progression and the differences between adult and pediatric cases remained similar when analyzing only the 15 cases that had a complete staging. The median reported follow-up for the reviewed cases was 36 months (10–96 months) for the pediatric cases, and 13 months (9–156 months) for the adults. The relatively short follow-up period (8 months) is a limitation of our case report.
Patients with ALK-positive pcALCL present diagnostic and prognostic challenges and pose questions regarding the ideal treatment and monitoring in such rare cases. It is unclear whether these cases are actually sALCL first manifesting in skin or true pcALCL with ALK overexpression. Summarizing the limited ALK-positive pcALCL reports in the literature, the low rate of systemic dissemination supports the later option. In addition we found that while the prognosis of ALK-positive pcALCL is excellent in pediatric patients, it is heterogenous in adults with an overall similar indolent course and prognosis compared to what had been previously reported in pcALCL.
Reccomendations and conclusions
Complete staging is mandatory in all pcALCL cases and lymphadenopathy per radiology studies alone cannot substitute for a lymph node biopsy if such involvement is suspected, as was demonstrated by the current case. Once the diagnosis of ALK-positive pcALCL is confirmed, it should be treated with skin-directed therapy. Close long-term monitoring is required as extracutaneous dissemination of the skin disease has been reported at 10, 18 and 96 months following the diagnosis of ALK-positive pcALCL .
In conclusion, ALK-positivity in cutaneous ALCL is not an unequivocal indicator of systemic disease. ALK-positive pcALCL has an excellent prognosis particularly in children, and should be treated by excision and/or radiotherapy. Patients must remain under close long-term follow-up as skin recurrence and progression to systemic disease are possible, mostly in adults.
Acknowledgments
Funding sources: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Shamir Geller is a recipient of a supplemental grant from the American Physicians and Friends For Medicine in Israel (APF).
Footnotes
Conflict of interest disclosures: None.
References
- 1.Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024–35. doi: 10.1182/blood-2011-05-351346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan DV, Summers P, Tuttle M, et al. Anaplastic lymphoma kinase expression in a recurrent primary cutaneous anaplastic large cell lymphoma with eventual systemic involvement. J Am Acad Dermatol. 2011;65:671–3. doi: 10.1016/j.jaad.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosoi M, Ichikawa M, Imai Y, Kurokawa M. A case of anaplastic large cell lymphoma, ALK positive, primary presented in the skin and relapsed with systemic involvement and leukocytosis after years of follow-up period. Int J Hematol. 2010;92:667–8. doi: 10.1007/s12185-010-0708-4. [DOI] [PubMed] [Google Scholar]
- 4.Su LD, Schnitzer B, Ross CW, et al. The t(2;5)-associated p80 NPM/ALK fusion protein in nodal and cutaneous CD30+ lymphoproliferative disorders. J Cutan Pathol. 1997;24:597–603. doi: 10.1111/j.1600-0560.1997.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki K, Sugaya M, Fujita H, et al. A case of primary cutaneous anaplastic large cell lymphoma with variant anaplastic lymphoma kinase translocation. Br J Dermatol. 2004;150:1202–7. doi: 10.1111/j.1365-2133.2004.05987.x. [DOI] [PubMed] [Google Scholar]
- 6.Kadin ME, Pinkus JL, Pinkus GS, et al. Primary cutaneous ALCL with phosphorylated/activated cytoplasmic ALK and novel phenotype: EMA/MUC1+, cutaneous lymphocyte antigen negative. Am J Surg Pathol. 2008;32:1421–6. doi: 10.1097/PAS.0b013e3181648d6d. [DOI] [PubMed] [Google Scholar]
- 7.Oschlies I, Lisfeld J, Lamant L, et al. ALK-positive anaplastic large cell lymphoma limited to the skin: clinical, histopathological and molecular analysis of 6 pediatric cases. A report from the ALCL99 study. Haematologica. 2013;98:50–6. doi: 10.3324/haematol.2012.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pulitzer M, Ogunrinade O, Lin O, Steinherz P. ALK-positive (2p23 rearranged) anaplastic large cell lymphoma with localization to the skin in a pediatric patient. J Cutan Pathol. 2015;42:182–7. doi: 10.1111/cup.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paolino G, Didona D, Gianno F, Garelli V, Soda G, Cantisani C. Primary Cutaneous Alk Positive Anaplastic Large Cell Lymphoma in a Melanoma Patient. Austin J Cancer Clin Res. 2015;2:1029. [Google Scholar]
- 10.Xue D, Li X, Ren Y, Liu Q, Yen Y, Xue L. Primary Cutaneous Anaplastic Large Cell Lymphoma With Positive ALK Expression and a Rapidly Progressive Cutaneous Nodule. Int J Surg Pathol. 2015;23:333–5. doi: 10.1177/1066896915568993. [DOI] [PubMed] [Google Scholar]
- 11.Chao-Lo MPG, King-Ismael D, Lopez RA. Primary cutaneous CD30+ anaplastic large cell lymphoma: report of a rare case. J Dermatol Case Rep. 2008;2:31–4. doi: 10.3315/jdcr.2008.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumaran MS, Jithendriya M, Nagaraj P, Tirumalae R, Jayaseelan E. Anaplastic lymphoma kinase-positive primary cutaneous anaplastic large cell lymphoma--is it a new variant? Indian J Dermatol Venereol Leprol. 2012;78:354–7. doi: 10.4103/0378-6323.95454. [DOI] [PubMed] [Google Scholar]
- 13.Tokuyama M, Kurashige Y, Ota T, et al. Pediatric case of anaplastic lymphoma kinase-positive anaplastic large cell lymphoma forming a solitary skin tumor on the forearm. J Dermatol. 2017;44:465–467. doi: 10.1111/1346-8138.13688. [DOI] [PubMed] [Google Scholar]
- 14.Rannan-Eliya YF, Pulford K, Johnson R, et al. Isolated cutaneous anaplastic large cell lymphoma progressing to severe systemic disease with myocardial involvement and central nervous system infiltration. Pediatr Blood Cancer. 2008;50:879–81. doi: 10.1002/pbc.21357. [DOI] [PubMed] [Google Scholar]
- 15.Quintanilla-Martinez L, Jansen PM, Kinney MC, Swerdlow SH, Willemze R. Non-mycosis fungoides cutaneous T-cell lymphomas: report of the 2011 Society for Hematopathology/European Association for Haematopathology workshop. Am J Clin Pathol. 2013;139:491–514. doi: 10.1309/AJCP83AOQTMLOJTM. [DOI] [PubMed] [Google Scholar]
- 16.Campo E, Chott A, Kinney MC, et al. Update on extranodal lymphomas. Conclusions of the Workshop held by the EAHP and the SH in Thessaloniki, Greece. Histopathology. 2006;48:481–504. doi: 10.1111/j.1365-2559.2006.02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki M, Niimi Y, Takezaki S, Azuma A, Seike M, Kawana S. CD30+ lymphoproliferative disorder: primary cutaneous anaplastic large cell lymphoma followed by lymphomatoid papulosis. Br J Dermatol. 2001;145:123–6. doi: 10.1046/j.1365-2133.2001.04295.x. [DOI] [PubMed] [Google Scholar]
- 18.Fauconneau A, Pham-Ledard A, Cappellen D, et al. Assessment of diagnostic criteria between primary cutaneous anaplastic large-cell lymphoma and CD30-rich transformed mycosis fungoides; a study of 66 cases. Br J Dermatol. 2015;172:1547–54. doi: 10.1111/bjd.13690. [DOI] [PubMed] [Google Scholar]
- 19.Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 20.Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653–61. [PubMed] [Google Scholar]
- 21.Liu HL, Hoppe RT, Kohler S, Harvell JD, Reddy S, Kim YH. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049–58. doi: 10.1016/s0190-9622(03)02484-8. [DOI] [PubMed] [Google Scholar]
- 22.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–4. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 23.Richly H, Kim TM, Schuler M, et al. Ceritinib in patients with advanced anaplastic lymphoma kinase-rearranged anaplastic large-cell lymphoma. Blood. 2015;126:1257–1258. doi: 10.1182/blood-2014-12-617779. [DOI] [PMC free article] [PubMed] [Google Scholar]