Abstract
Osteomicrobiology refers to the role of microbiota in bone health and the mechanisms by which the microbiota regulates post-natal skeletal development, bone aging and pathologic bone loss. Here, we review recent reports linking gut microbiota to changes in bone phenotype. A pro-inflammatory cytokine milieu drives bone resorption in conditions such as sex-steroid hormone deficiency. The response of the immune system to activation by the microbiome results in increased circulating osteoclastogenic cytokines in a T-cell dependent mechanism. Additionally, gut microbiota affect bone homeostasis through nutrient absorption, mediation of the IGF-1 pathway, and short chain fatty acid and metabolic products. Manipulation of microbiota through prebiotics or probiotics reduces inflammatory cytokine production, leading to changes in bone density. One mechanism of probiotic action is through upregulating tight junction proteins, increasing the strength of the gut epithelial layer and leading to less antigen presentation and less activation of intestinal immune cells. Thus, prebiotics or probiotics may represent a future therapeutic avenue for ameliorating the risk of post-menopausal bone loss in humans.
Keywords: Estrogen, Sex steroids, microbiota, Intestine, bone loss, probiotics, LGG, VSL#3™
Introduction
The gut microbiota consists of trillions of microbial organisms including bacteria, viruses, and fungi that live in close contact with human surfaces. The largest microbiota is the intestinal microbiota. These microbes live in proximity with the intestinal wall, a boundary between the self and the foreign of the host. Symbiotic gut microbes increase nutrient absorption from food, and make it difficult for pathogenic bacteria to colonize the gut. In recent years, especial attention has been made to the role that the gut microbiota, especially bacteria, play in interfacing with human pathophysiology. The sheer quantity of bacteria and their genetic material – it is estimated there may be 100 times as many bacterial genes as there are human ones – not to mention the difficulty in isolating and culturing many of these species, has made the study of microbiota formidable [1–3]. Inter-individual variation and even intra-individual variation add to the complexity of study. With the advent of more rapid and cheaper sequencing technologies, the diversity of bacterial composition within and between human hosts has come to light. The importance of microbiota has become definite; many would even call microbiota our largest organ. Specific strains have been found to have effects on immune cells [4], and new insights have been made on overall microbiota effects on systemic immune responses. Gut microbes have now been shown to modulate local intestinal as well as systemic immune responses, with oftentimes surprising associations with distant and diverse organ systems and disease processes such as hematopoiesis [5], brain microglia [6], renal ischemia [7], periodontal disease [8], and graft versus host disease [9]. In this review, we will focus on recent discoveries of how these bacteria interact with the immune system to regulate bone in health and in disease.
The recognition that both the immune system and the microbiota are critical for bone homeostasis signifies the evolution from the field of osteoimmunology to that of “Osteomicrobiology”, a term introduced by Ohlsson et al [10], to refer to investigations on the role of microbiota in bone health and the mechanisms by which the microbiota regulates postnatal skeletal development, bone aging and pathologic bone loss.
Bone as an Immunological Organ
Bone undergoes continual turnover, with a balance in formation and resorption crucial to bone health [11]. The importance of the immune system in regulating bone remodeling was recognized three decades ago [12]. A strong association between inflammatory conditions and bone loss has long been established clinically [13, 14]. The most common cause of osteoporosis is due to estrogen deficiency [15]. Postmenopausal bone loss results from a decrease in estrogen levels, leading to increases in bone resorption which is, in part, countered by increases in bone formation. In mice, menopause is modeled by surgical removal of the ovaries. The mechanism by which sex steroid deficiency leads to bone loss is primarily increased osteoclast formation, activity and lifespan driven by increased production of the inflammatory cytokines IL-1, IL-17, TNFα (TNF) and RANKL in the bone marrow (BM) [16–19]. A role for IL-1 and TNF in humans is supported by reports that menopause increases the levels of these factors [20–24], while treatment with inhibitors of IL-1 and TNF prevents the increase in bone resorption induced by estrogen deficiency [25]. The causal role of TNF in ovx-induced bone loss in mice has been demonstrated in a variety of experimental models [26–28]. Key mechanisms by which TNF stimulates bone resorption include potentiation of the activity of RANKL [29, 30] and induction of Th17 cells [31–33]. Osteoblastic cells are one major source of RANKL. Osteocytes are probably the most relevant source of RANKL in the mouse [34, 35].
Significant experimental evidence supports the notion that T cells are a pivotal source of TNF in ovx mice and postmenopausal women [36, 37]. Evidence backing a mechanistic role for T cells include the fact that ovx is unable to induce bone loss in T cell deficient nude mice [26, 29, 38, 39], in wild-type (WT) mice depleted of T cells [40], in mice treated with the costimulation inhibitor CTLA4-Ig [41], and in mice lacking the costimulatory molecule CD40L [40]. The relevance of T cells for ovariectomy induced bone loss has been confirmed by several laboratories [42, 43]. Evidence for a role of T cell produced TNF in ovx induced bone loss has been provided by studies with mice lacking the capacity to produce TNF or lacking the expression of TNF receptors [26].
One T cell lineage pivotal for the bone loss of estrogen deficiency is Th17 cells. Th17 cells are a subset of CD4+ cells which produce IL-17 and exert pro-osteoclastogenic effects. In women, there is an association with increased levels of serum IL-17 and osteoporosis [44–46]. Ovx drives differentiation of naïve CD4+ helper cells into mature Th17 cells [47], a process which is inhibited by estrogen binding to ERα and stimulated by cytokines such as TGFβ, IL-1β and TNF [32, 33, 48, 49]. In addition, ovx leads to increases in conventional CD4+ and CD8+ T cells, which produce TNF and RANKL. Silencing of IL-17R [50] or use of anti-IL17 antibodies [51] result in protection against bone loss.
Another T cell lineage relevant for bone is regulatory T cells (Tregs), a suppressive population of CD4+ T cells defined by the expression of the transcription factor FoxP3 and the ability to block conventional T cell proliferation and production of effector cytokines [52]. Defects in Treg numbers and/or activity have been implicated in several chronic inflammatory diseases. Tregs regulate OC formation [53–56] and blunt bone resorption [54, 57] through the secretion of IL-4, IL-10 and TGFβ1 [56]. Importantly, estrogen is known to directly increase the relative number of Tregs [58] while Tregs prevent ovx-induced bone loss [59].
The Microbiota Affects Bone Health
The microbiota regulates metabolism [60, 61] and affects the production of hormones critical for bone health including sex-steroids [62], vitamin D [63] and serotonin [64]. More importantly, the gut microbiota is critical for the development and the function of the immune system. A link between microbiota and bone was first recognized in 2012 [65]. In their seminal paper K. Sjogren et al demonstrated an increase in trabecular bone mass in mice raised in germ-free conditions, as compared to controls raised in conventional conditions. As this phenotype was reversed by colonization with gut flora from conventionally raised mice, the evidence was convincing that the results were not due to innate abnormalities of germ-free mice. Additional findings of this initial investigation were a lower number of CD4+ T cells in the bone marrow and lower levels of bone marrow TNF in germ-free mice. This corresponded with fewer osteoclast precursors and a higher bone mass. In keeping with the study of K. Sjogren et al, we reported that 20-week-old female germ-free C57Bl/6 mice have higher trabecular bone volume than congenic age matched mice raised in conventional conditions [66]. These initial observations have been recently confirmed in a study demonstrating that commensal gut microbiota stimulates bone resorption and inhibits bone formation, thus lowering bone mass [67].
Mechanistic studies have disclosed that the microbiota regulates bone formation by altering the production of insulin-like growth factor 1 (IGF-1), an important regulator of bone remodeling. The exact role of IGF-1 remains controversial because in one study mice raised in conventional conditions were found to have lower bone formation and lower serum and bone marrow levels of IGF-1 compared to GF mice [67]. By contrast, in a second investigation colonization of GF mice with physiologic microbiota increased liver-derived IGF-1 [61, 64]. In the short-term IGF-1 stimulated bone resorption causing bone loss [64]. However, over a longer period of time (8 months) IGF-1 stimulated bone formation leading to a net gain in bone mass [64]. Investigations have also shown that commensal microbiota induces sustained changes to in RANKL mediated osteoclastogenesis [67].
Recent work has also disclosed that regulation of bone mass by the microbiota is dependent on NOD1 and NOD2 signaling [68]. That study has indeed disclosed that cortical bone thickness in mice lacking Nod1 or Nod2 was not increased under germ-free conditions as compared to conventionally raised controls. Moreover, the expression of TNF and RANKL, which is below normal in germ-free mice, is within normal limits in Nod1−/− or Nod2−/− GF mice, indicating that the effect of the microbiota is dependent on both NOD1 and NOD2 signaling [68].
It is now clear that various factors may affect the bone density of germ-free mice. One important factor is genetic strain. Another is gender. While female germ-free C57Bl/6 mice have higher bone mass than controls, in male BALB/c mice the effect of the microbiota on bone mass is opposite [61]. Adding further complexity to this issue, bacterial colonization of adult CB6F1 mice was found to stimulate bone resorption and decrease bone mass in the first 4 weeks after colonization [69]. However, long-term colonization leads to an equalization of bone mass, indicating that duration of colonization is another critical variable that influences bone turnover and bone mass [69]. To determine whether endogenous microbiota contribute to the regulation of bone remodeling at baseline, the same investigators administered antibiotics to conventionally raised mice. The consequent elimination of the microbiota caused an increase in bone mass and a lowering of the rate of bone turnover [69]. These findings indicate that in a short-term setting the ablation of the microbiota increases bone mass, whereas reconstitution of the microbiota in former germ-free mice causes bone waste.
Adding further complexity, the results of these investigations are not in line with a series of studies conducted to analyze the effect of malnourishment on body growth and skeletal development. Stool samples from children from Malawi with severe malnutrition contained an immature microbiome. Colonization of germ-free mice with stool samples from malnourished children resulted in stunted body growth and shorter bones, whereas germ-free mice on the same diet given “mature” microbiomes of healthy children underwent normal body growth [70]. Interestingly, colonization of germ-free mice with stools from malnourished children caused an increase in cortical bone density [70]. Germ-free mice colonized with stools from healthy 6 months old children acquired a higher bone density than those colonized with stools from healthy 18 months old children [70], further suggesting that immature microbiota induces bone anabolism.
In another investigation, it was found that the reduced skeletal development of germ-free mice was partially reversed by colonization with selected lactobacilli [61]. Moreover, sialylated human milk oligosaccharides (HMOs) are significantly less abundant in the milk of mothers with severely malnourished infants [71]. HMOs are not significant nutrients for humans, but gut bacteria utilize HMOs as an energy source. Colonization of germ-free mice with stools from 6-month-old stunted Malawian infants resulted in blunted body growth. Feeding the colonized mice with HMOs produced a microbiota-related increase in body mass, and altered muscle and liver, metabolism according to a pattern suggestive of a greater capacity to exploit nutrients [71]. Among the changes induced by HMOs supplementation was an increase in bone volume [71], indicating that positive modification of the microbiota induced by a prebiotic substance stimulates bone growth. Together, these studies demonstrate that changes in the microbiota regulate independently bone growth and bone mass acquisition.
The notion that the gut microbiota plays a critical role in bone growth is strengthened by antibiotic studies in mice. Short-term administration of low doses of antibiotics at weaning resulted in elevated levels of bone mass [60, 72]. Treatment with antibiotics that specifically decrease intestinal bacteria also increases bone mass [69]. In addition, administration of a cocktail of tetracyclines were found to prevent ovariectomy induced bone loss [73]. Some antibiotic studies revealed unexpected differences on the bone response to microbiota reduction in male and female mice [72]. Moreover, an increase in bone density in response to antibiotics was more consistently observed in young animals [60, 74]. These studies clearly demonstrate that antibiotic treatment influences bone mass. Because antibiotics lower the number of bacteria, and/or alter the diversity of microbial taxa within the intestinal lumen, it can be inferred that the bacterial load and diversity within the gut are significant contributing variables to the mechanisms by which the gut microbiota regulates bone mass.
A great deal of differences exists in the microbiome of mice housed in different facilities and/or fed different types of chow [75–77]. Therefore, the diversity of the microbiome in the particular facility is a potential confounding factor to data interpretation when assessing the influences of the microbiome on bone density. For example, at sacrifice the trabecular bone volume of the conventionally raised mice used in our studies was less than 50% of that of the BALB/c male mice used by Schwarzer et al [61]. Further attesting to the relevance of local housing conditions, we found C57Bl/6 mice from Jackson Laboratory have a higher bone volume than syngeneic mice bought from Taconic Biosciences. However, following 4 weeks of co-housing in our animal facility, the bone volume of mice from Jackson Laboratory decreased to the level of that of Taconic Biosciences mice. Since mice are coprophagic, and transfer their microbiomes from mouse to mouse by this behavior, the bone density decrease observed in mice from Jackson Laboratory is perhaps due to colonization of Jackson Laboratory mice with fecal material from Taconic Biosciences mice. These observations clearly point to the critical need to account for reciprocal host-microbiome interactions in experimental approaches that investigate the microbiome and bone density.
Microbiota and the bone loss induced by sex steroid deficiency
Postmenopausal osteoporosis is the most common form of bone wasting disorder [15]. Fractures due to osteoporosis are a frequent cause of disability, particularly in the elderly. Declining estrogen levels at menopause result in a potent stimulation of bone resorption and, to a lesser extent, bone formation leading to a period of rapid bone loss [11]. This initial phase is followed by a slower but more prolonged period of bone loss that affects mostly the cortical compartment of the skeleton. Osteoporosis due to androgen deficiency is also relatively common in men [78]. However, androgens regulate bone homeostasis in men mostly via their peripheral conversion to estrogen [79] and estrogen deficiency has a dramatic effect on bone homeostasis in men [80, 81].
The role of the microbiome in the bone loss induced by sex steroid deficiency was investigated in a study employing mice raised in germ-free conditions. Mice raised in conventional conditions and germ-free mice colonized with commensal gut microbiota, were used as control groups. Sex steroid deficiency was induced pharmacologically using the GN-RH agonist Leuprolide. This investigation revealed that germ-free mice do not develop the loss of trabecular bone typically induced by sex steroid withdrawal [66]. Colonization of germ-free mice with the microbiota of conventionally raised animals made the skeleton of these animals as sensitive to sex steroid deprivation as the skeleton of conventionally raised mice, demonstrating that the protection of germ-free mice against bone loss is not due to intrinsic, irreversible immune abnormalities conferred by the germ-free status. Interestingly, Leuprolide treatment caused equal cortical bone loss in germ-free mice and control mice, indicating that cortical bone loss occurs via a microbiota-independent mechanism [66]. Leuprolide increased bone resorption in conventional and colonized mice, but not in germ-free mice. In addition, germ-free sex steroid depleted mice had lower rates of compensatory bone formation than conventional sex steroid depleted mice [66].
Assessment of cytokine production revealed that sex steroid deficiency expands TNF and IL-17 producing T cells, and increases the levels of TNF, RANKL and IL-17 protein in the bone marrow and in the small intestine of conventionally raised mice. By contrast, in germ-free mice, no increase in these cytokine levels occurred in the bone marrow, nor in tissues of the small intestine following sex steroid deficiency [66]. Overall, the data showed that the microbiota is required for sex steroid withdrawal to induce bone loss. Moreover, the data indicated that this may occur due to the gut microbiome driving the expansion of intestinal T cells and Th17 cells, which produce TNF, RANKL and IL-17.
Still unclear is whether bone loss is induced by cytokines that travel to the bone marrow but are produced by gut immune cells, or bone loss is induced by immune cells activated in the gut that home to the bone marrow. A third possibility is that foreign antigens of intestinal origin reach the bone marrow causing an immune response. Bacterial translocation, the passage of viable bacteria across the intestinal wall into the systemic circulation, is a recognized phenomenon, although very rare in healthy individuals [82]. Translocation of gut microbiota derived ligands into the circulation occurs physiologically [83], and this may be sufficient to activate bone marrow T cells. Based on preliminary cell migration studies, it appears likely that immune cells, first activated in the intestine, home to the bone marrow where they produce osteoclastogenic cytokines.
The intestinal wall is the border between the mammalian host and the luminal microbiota. The intestinal wall is in contact with the gut microbiota and constantly samples and respond to the antigenic load within the intestinal lumen [84]. Gut permeability, the passage of small molecules between the gut lumen and submucosa, is essential for health. Increased permeability allows larger molecules and potential antigenic load to enter the gut wall and initiate intestinal inflammatory responses [85–88]. Maintaining tight gut permeability is critical in the context bone formation because osteoclastogenic cytokines are produced by immune cells that reside in intestinal sub-epithelial compartments of the intestine, and any change in gut permeability is thus likely to elevate levels of osteoclastogenic cytokines and influence bone density.
This is relevant because estrogen withdrawal increases gut permeability, leading to increased bacterial translocation and increased levels of inflammatory markers [66, 89]. Specifically, sex-steroid deficiency resulted in reduced transcript levels of a number of the gap junction proteins of the Claudin family that regulate gut permeability [90, 91]. Thus, increased gut permeability after sex-steroid depletion results in a higher antigenic load entering the epithelial submucosa, thereby initiating the production of inflammatory cytokines. Therefore, any therapeutic approach that can tighten gut permeability would offer a promising therapy for preventing sex steroid depletion-induced bone loss.
Manipulation of Microbiota Changes Bone Remodeling
Since bone health is affected by the lack or pathologic alteration of the intestinal microbiota, it is logical to assume that strategies may be developed to alter the microbiota in a way to induce beneficial skeletal effects. One such strategy is nutritional supplementation with probiotics. Probiotics are defined as live microorganisms that provide certain health benefits to the host, when administered in appropriate amounts [92]. Earlier work had already revealed positive effects of probiotics on bone mass. For example, a 2004 study revealed that rats receiving Lactobacillus helveticus – fermented milk developed a higher bone density as compared to controls [93]. However, the idea that microbiota manipulation directly impacted the inflammatory response and bone phenotype was not widely accepted until several groups in Europe and in the United States independently demonstrated that probiotics could prevent the loss of bone mass induced by ovariectomy by reducing inflammatory cytokine production [94, 95]. However, the effects of probiotics in bone were found to be influenced by many factors. For example, in one study probiotics appeared to decrease intestinal inflammation and increase bone density in eugonadic male but not female mice [96]. In female mice, probiotics improved bone density under inflammatory stress but not in normal conditions [95]. In fact, treatment with Lactobacillus reuteri increased bone density in mice subjected to a dorsal surgical incision mimicking the incision carried out to perform ovx, whereas it had no effect in non-operated mice [97]. This suggests that the presence of an inflammatory state is required for probiotic to increase bone density. However, caution must be taken when generalizing these studies, as the specific strain of probiotic, the species of the animal model, and even factors such as the location of the laboratory appear to complicate phenotypic findings.
In further exploring the mechanism of probiotic effect, Li et al demonstrated that probiotics interact with the epithelial layer of the small intestine, and change regulation of tight junction proteins that form the gut epithelial layer [66]. Through treatment with either Lactobacillus rhamnosus GG (LGG) or a commercially available mix, VSL#3, which contains eight different strains, probiotics prevented ovx induced bone loss, and increased bone mass in mice without ovx (figure 1). These effects correlated with a decrease in gut permeability and intestinal and systemic inflammation. The intestinal epithelium uses tight and gap junction proteins of the claudins and Jam families to maintain a physiological barrier between the gut lumen and submucosa. Estrogen deficiency leads to decreased expression of junction proteins causing increased gut permeability and increased levels of inflammation. Probiotics act, in part, by strengthening the gut barrier (figure 2). A stronger intestinal barrier leads to less antigen presentation and less activation of intestinal immune cells. Further studies are needed to elucidate this mechanism of action of probiotics in estrogen deficient and estrogen replete animals and humans, but it appears that the link between gut inflammation and production of osteoclastogenic cytokines could be ripe for therapeutic targeting with probiotics.
Figure 1. Probiotic supplementation prevents sex-steroid induced bone loss.
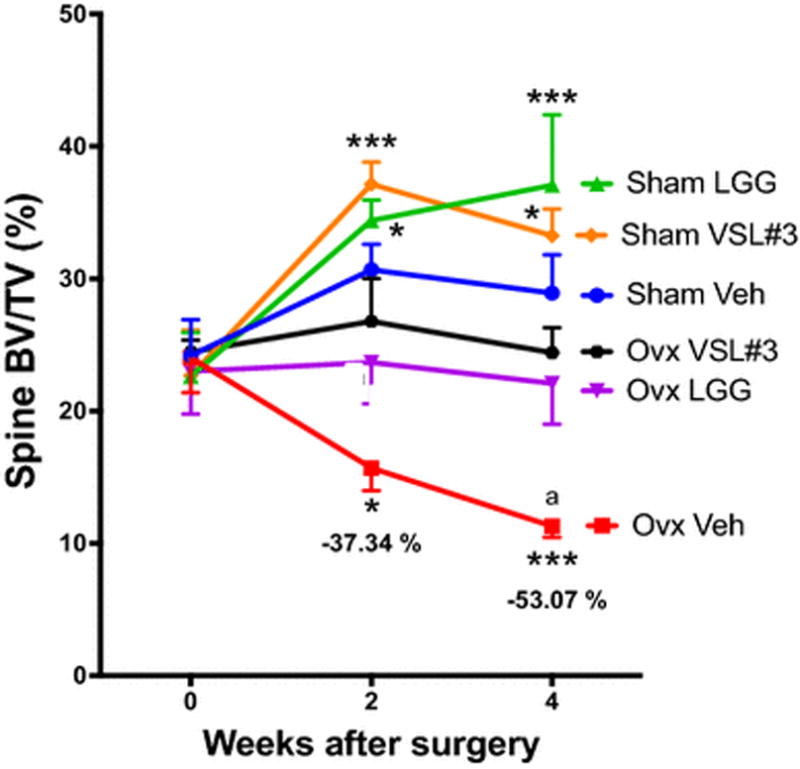
Conventional ovariectomized (ovx) and sham operated mice were supplemented twice a week with either VSL#3™, Lactobacillus rhamnosus GG (LGG), or vehicle. The figure shows in vivo prospective measurements of spinal bone volume (BV/TV), as measured by micro-computed tomography (μCT) scanning at baseline and 2 and 4 weeks after surgery. n = 10 to 14 mice per group. Data are expressed as Mean + SEM. * = p <0.05, and ***= p<0.001 compared to sham vehicle, a = p<0.0001 compared to baseline. Figure and legend reproduced with permission from JCI.
Figure 2. Diagrammatic representation of the effects of sex steroids deficiency and probiotics on gut permeability.
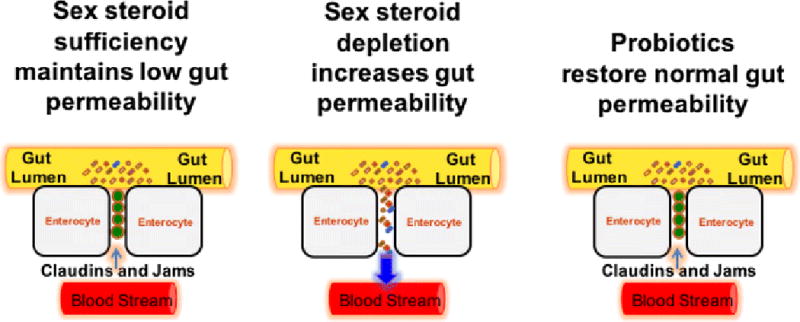
In physiologic conditions, proteins of the Jam and Claudin families seal the space between intestinal epithelial cells preventing bacteria and bacterial products from penetrating the intestinal wall and activating immune cells. Sex steroid deprivation downregulates the expression of gap junction proteins leading to increased gut permeability. The resulting increased bacterial translocation induces a local and systemic immune response that causes an increased production of osteoclastogenic cytokines. Probiotic supplementation upregulates expression of Claudins and Jams, thus restoring normal gut barrier function. Figure and legend reproduced with permission of Cold Spring Harbor.
While numerous animal studies have documented the positive skeletal effects of probiotics, little information is available in humans. Recently, a short-term prospective double-blind study has been conducted in postmenopausal women [98]. In this study 50 women were treated with placebo or the multispecies probiotic supplement GeriLact for 6 months. No changes in bone density were detected probably because of the short duration of the study. However, patients treated with probiotics had a reduction in indices of bone turnover, and serum levels of TNF and PTH [98].
The microbiota can also be manipulated via nutritional supplementation with prebiotics. Prebiotics are non-digestible fermentable food ingredients that promote the growth of beneficial microbes and/or promote beneficial changes in the activity of the microbiome [99]. Prebiotics are contained in food items such as dandelion greens, garlic, leek, banana, onion, artichoke and chicory [100]. In many cases, a significant amount of the food is needed to get enough prebiotic for activity, therefore prebiotics, such as inulin, have been developed into capsule, or shake forms [100]. Prebiotics include non-digestible oligosaccharides such as polydextrose, fructo-oligosaccharides (FOS), inulin, xylo-oligosaccharides, galacto-oligosaccharides (GOS) and soybean oligosaccharides. The mechanism of action of prebiotics is not clear but these substances are known to increase calcium absorption in healthy animals [101, 102], healthy humans [103, 104], ovx animals [105] and postmenopausal women [106]. In experimental animals FOS and inulin increase trabecular and cortical bone volume in intact rodents [107–109], and prevent ovx-induced bone loss in mice [110]. Studies in humans have revealed that prebiotics increase whole body bone mineral density in adolescent girls [104] and decrease bone loss in post-menopausal women [111]. Prebiotics act by regulating both bone formation and bone resorption. The mechanism of action of prebiotics in bone remains unknown. However, it is likely that metabolic and immunological pathway may be involved [112]. First, dietary fiber is fermented to short chain fatty acids (SCFA) in the lower gut by resident microbiota. SCFAs lower intestinal pH, which causes an increase in calcium absorption[112]. Moreover, SCFAs serve as energy sources for gut epithelial cells, and this may lead to improved gut health and gut barrier function [113].
Conclusions
In the last five years, it has become clear that the intestinal microbiota is relevant for bone health. Most critical data have been obtained in experimental animals and confirmation in humans is needed. As of yet, there is no FDA-approved treatment for osteoporosis based on manipulation of microbiota. However, advances in other areas suggest that manipulations of the microbiota may be used in the future to treat common skeletal disease. Such interventions might include simple dietary modifications that affect the gut microbiota. For example, a low-glycemic diet was found to alter microbiota composition in a way that offered protection against age-related macular degeneration in the mouse, leading to the creation of the term “gut-retina axis” [114]. Perhaps dietary modifications that alter the composition of the microbiota will be found to be useful to prevent bone loss. Prebiotics, and probiotics, are already widely in use for a variety of conditions. If clinical trials currently in progress establish their efficacy, prebiotics and probiotics might become an inexpensive means to prevent bone loss in humans.
Fecal microbial transplant (FMT), a procedure in which stool, and its associated microbiota, are taken from a healthy donor and placed into a recipient, has already become an accepted treatment for Clostridium difficile infections in humans. Research highlights putative roles of similar human microbiota manipulations in atherosclerosis, obesity, and oncologic disorders [115]. Bone diseases might become another application for simplified stool transplant procedures. Speculating on the growth of personalized medicine, perhaps in the future, patients with severe osteoporosis would have their microbiota analyzed, and means to shift the microbiota to a more bone-protective composition would be attempted through diet, prebiotics, probiotics, or fecal transplant.
Nonstandard abbreviations used
- BM
Bone marrow
- GF
Germ-Free
- Conv.R
conventionally raised
- TNF
Tumor necrosis factor α
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.Lin PW, Myers LE, Ray L, Song SC, Nasr TR, Berardinelli AJ, Kundu K, Murthy N, Hansen JM, Neish AS. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic Biol Med. 2009;47:1205–1211. doi: 10.1016/j.freeradbiomed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommer F, Backhed F. The gut microbiota–masters of host development and physiology. Nature reviews Microbiology. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 3.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josefsdottir KS, Baldridge MT, Kadmon CS, King KY. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. 2017;129:729–739. doi: 10.1182/blood-2016-03-708594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erny D, Hrabe de Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. 2017;150:7–15. doi: 10.1111/imm.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emal D, Rampanelli E, Stroo I, Butter LM, Teske GJ, Claessen N, Stokman G, Florquin S, Leemans JC, Dessing MC. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2017;28:1450–1461. doi: 10.1681/ASN.2016030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Qi J, Zhao H, He S, Zhang Y, Wei S, Zhao F. Metagenomic sequencing reveals microbiota and its functional potential associated with periodontal disease. Sci Rep. 2013;3:1843. doi: 10.1038/srep01843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varelias A, Ormerod KL, Bunting MD, Koyama M, Gartlan KH, Kuns RD, Lachner N, Locke KR, Lim CY, Henden AS, Zhang P, Clouston AD, Hasnain SZ, McGuckin MA, Blazar BR, MacDonald KP, Hugenholtz P, Hill GR. Acute graft-versus-host disease is regulated by an IL-17-sensitive microbiome. Blood. 2017;129:2172–2185. doi: 10.1182/blood-2016-08-732628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohlsson C, Sjogren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2014 doi: 10.1016/j.tem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 12.Pacifici R, Rifas L, Teitelbaum S, Slatopolsky E, McCracken R, Bergfeld M, Lee W, Avioli LV, Peck WA. Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proc Natl Acad Sci U S A. 1987;84:4616–4620. doi: 10.1073/pnas.84.13.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 14.Mbalaviele G, Novack DV, Schett G, Teitelbaum SL. Inflammatory osteolysis: a conspiracy against bone. J Clin Invest. 2017;127:2030–2039. doi: 10.1172/JCI93356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khosla S, Pacifici R. Estrogen Deficiency, PostmenopausalOsteoporosis, and Age-Related Bone Loss. In: Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA, editors. Osteoporosis. Elsvierer; Amsterdam: 2013. pp. 1113–1138. [Google Scholar]
- 16.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. Embo J. 2008;27:535–545. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacifici R, Rifas L, McCracken R, Vered I, McMurtry C, Avioli LV, Peck WA. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci U S A. 1989;86:2398–2402. doi: 10.1073/pnas.86.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, McCracken R, Avioli LV. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991;88:5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen-Solal ME, Graulet AM, Denne MA, Gueris J, Baylink D, de Vernejoul MC. Peripheral monocyte culture supernatants of menopausal women can induce bone resorption: Involvement of cytokines. Journal OF Clinical Endocrinology and Metabolism. 1993;77:1648–1653. doi: 10.1210/jcem.77.6.8263153. [DOI] [PubMed] [Google Scholar]
- 23.Beaudreuil J, Mbalaviele G, Cohen-Solal M, Morieux C, de Vernejoul MC, Orcel P. Short-term local injections of transforming growth factor-beta 1 decrease ovariectomy-stimulated osteoclastic resorption in vivo in rats. Journal of Bone & Mineral Research. 1995;10:971–977. doi: 10.1002/jbmr.5650100619. [DOI] [PubMed] [Google Scholar]
- 24.Bernard-Poenaru O, Roux C, Blanque R, Gardner C, de Vemejoul MC, Cohen-Solal ME. Bone-resorbing cytokines from peripheral blood mononuclear cells after hormone replacement therapy: a longitudinal study. Osteoporos Int. 2001;12:769–776. doi: 10.1007/s001980170054. [DOI] [PubMed] [Google Scholar]
- 25.Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. Effect of Blockade of Tumor Necrosis Factor-alpha and Interleukin-1 Action on Bone Resorption in Early Postmenopausal Women. J Bone Miner Res. 2007 doi: 10.1359/jbmr.070207. [DOI] [PubMed] [Google Scholar]
- 26.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: A key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ammann P, Rizzoli R, Bonjour JP, Bourrin S, Meyer JM, Vassalli P, Garcia I. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J Clin Invest. 1997;99:1699–1703. doi: 10.1172/JCI119333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimble RB, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997;12:935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 29.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-alpha therapy. Arthritis Res Ther. 2011;13:R126. doi: 10.1186/ar3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugita S, Kawazoe Y, Imai A, Yamada Y, Horie S, Mochizuki M. Inhibition of Th17 differentiation by anti-TNF-alpha therapy in uveitis patients with Behcet’s disease. Arthritis Res Ther. 2012;14:R99. doi: 10.1186/ar3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 36.D’Amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D, Pescarmona GP, Isaia G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Adeel S, Singh K, Vydareny KH, Kumari M, Shah E, Weitzmann MN, Tangpricha V. Bone loss in surgically ovariectomized premenopausal women is associated with T lymphocyte activation and thymic hypertrophy. J Investig Med. 2013;61:1178–1183. doi: 10.231/JIM.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y, Qian WP, Dark K, Toraldo G, Lin AS, Guldberg RE, Flavell RA, Weitzmann MN, Pacifici R. Estrogen prevents bone loss through transforming growth factor beta signaling in T cells. Proc Natl Acad Sci U S A. 2004;101:16618–16623. doi: 10.1073/pnas.0404888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li JY, Tawfeek H, Bedi B, Yang X, Adams J, Gao KY, Zayzafoon M, Weitzmann MN, Pacifici R. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci U S A. 2011;108:768–773. doi: 10.1073/pnas.1013492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grassi F, Tell G, Robbie-Ryan M, Gao Y, Terauchi M, Yang X, Romanello M, Jones DP, Weitzmann MN, Pacifici R. Oxidative stress causes bone loss in estrogen-deficient mice through enhanced bone marrow dendritic cell activation. Proc Natl Acad Sci U S A. 2007;104:15087–15092. doi: 10.1073/pnas.0703610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, Patel V, Gutkind S, Young M, Gronthos S, Le A, Wang CY, Chen W, Shi S. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE. 2008;3:e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyagi AM, Srivastava K, Kureel J, Kumar A, Raghuvanshi A, Yadav D, Maurya R, Goel A, Singh D. Premature T cell senescence in Ovx mice is inhibited by repletion of estrogen and medicarpin: a possible mechanism for alleviating bone loss. Osteoporos Int. 2012;23:1151–1161. doi: 10.1007/s00198-011-1650-x. [DOI] [PubMed] [Google Scholar]
- 44.Molnar I, Bohaty I, Somogyine-Vari E. IL-17A-mediated sRANK ligand elevation involved in postmenopausal osteoporosis. Osteoporos Int. 2014;25:783–786. doi: 10.1007/s00198-013-2548-6. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Fu Q, Ren Z, Wang Y, Wang C, Shen T, Wang G, Wu L. Changes of serum cytokines-related Th1/Th2/Th17 concentration in patients with postmenopausal osteoporosis. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2014:1–8. doi: 10.3109/09513590.2014.975683. [DOI] [PubMed] [Google Scholar]
- 46.Molnar I, Bohaty I, Somogyine-Vari E. High prevalence of increased interleukin-17A serum levels in postmenopausal estrogen deficiency. Menopause. 2014;21:749–752. doi: 10.1097/GME.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 47.Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS ONE. 2012;7:e44552. doi: 10.1371/journal.pone.0044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basu R, Hatton RD, Weaver CT. The Th17 family: flexibility follows function. Immunol Rev. 2013;252:89–103. doi: 10.1111/imr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 50.DeSelm CJ, Takahata Y, Warren J, Chappel JC, Khan T, Li X, Liu C, Choi Y, Kim YF, Zou W, Teitelbaum SL. IL-17 mediates estrogen-deficient osteoporosis in an Act1-dependent manner. Journal of cellular biochemistry. 2012;113:2895–2902. doi: 10.1002/jcb.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tyagi AM, Mansoori MN, Srivastava K, Khan MP, Kureel J, Dixit M, Shukla P, Trivedi R, Chattopadhyay N, Singh D. Enhanced immunoprotective effects by anti-IL-17 antibody translates to improved skeletal parameters under estrogen deficiency compared with anti-RANKL and anti-TNF-alpha antibodies. J Bone Miner Res. 2014;29:1981–1992. doi: 10.1002/jbmr.2228. [DOI] [PubMed] [Google Scholar]
- 52.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Zaiss MM, Axmann R, Zwerina J, Polzer K, Guckel E, Skapenko A, Schulze-Koops H, Horwood N, Cope A, Schett G. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56:4104–4112. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- 54.Kim YG, Lee CK, Nah SS, Mun SH, Yoo B, Moon HB. Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2007;357:1046–1052. doi: 10.1016/j.bbrc.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 55.Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68:744–750. doi: 10.1136/ard.2007.086066. [DOI] [PubMed] [Google Scholar]
- 56.Luo CY, Wang L, Sun C, Li DJ. Estrogen enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cellular & molecular immunology. 2011;8:50–58. doi: 10.1038/cmi.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan FL, Li X, Lu WG, Xu RS, Zhao YQ, Li CW, Li JP, Chen FH. Regulatory T cells as a potent target for controlling bone loss. Biochemical and biophysical research communications. 2010;402:173–176. doi: 10.1016/j.bbrc.2010.09.120. [DOI] [PubMed] [Google Scholar]
- 58.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, Wang B. Induction of regulatory T cells by physiological level estrogen. Journal of cellular physiology. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 59.Zaiss MM, Sarter K, Hess A, Engelke K, Bohm C, Nimmerjahn F, Voll R, Schett G, David JP. Increased bone density and resistance to ovariectomy-induced bone loss in FoxP3-transgenic mice based on impaired osteoclast differentiation. Arthritis and rheumatism. 2010;62:2328–2338. doi: 10.1002/art.27535. [DOI] [PubMed] [Google Scholar]
- 60.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, Rieusset J, Kozakova H, Vidal H, Leulier F. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 62.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 63.Charles JF, Ermann J, Aliprantis AO. The intestinal microbiome and skeletal fitness: Connecting bugs and bones. Clin Immunol. 2015 doi: 10.1016/j.clim.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016 doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Novince CM, Whittow CR, Aartun JD, Hathaway JD, Poulides N, Chavez MB, Steinkamp HM, Kirkwood KA, Huang E, Westwater C, Kirkwood KL. Commensal Gut Microbiota Immunomodulatory Actions in Bone Marrow and Liver have Catabolic Effects on Skeletal Homeostasis in Health. Scientific reports. 2017;7:5747. doi: 10.1038/s41598-017-06126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohlsson C, Nigro G, Boneca IG, Backhed F, Sansonetti P, Sjogren K. Regulation of bone mass by the gut microbiota is dependent on NOD1 and NOD2 signaling. Cell Immunol. 2017;317:55–58. doi: 10.1016/j.cellimm.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016;113:E7554–E7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, Fan YM, Henrissat B, Leyn SA, Rodionov DA, Osterman AL, Maleta KM, Newgard CB, Ashorn P, Dewey KG, Gordon JI. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351 doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Charbonneau MR, O’Donnell D, Blanton LV, Totten SM, Davis JC, Barratt MJ, Cheng J, Guruge J, Talcott M, Bain JR, Muehlbauer MJ, Ilkayeva O, Wu C, Struckmeyer T, Barile D, Mangani C, Jorgensen J, Fan YM, Maleta K, Dewey KG, Ashorn P, Newgard CB, Lebrilla C, Mills DA, Gordon JI. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell. 2016;164:859–871. doi: 10.1016/j.cell.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zarate Rodriguez JG, Rogers AB, Robine N, Loke P, Blaser MJ. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams S, Wakisaka A, Zeng QQ, Barnes J, Martin G, Wechter WJ, Liang CT. Minocycline prevents the decrease in bone mineral density and trabecular bone in ovariectomized aged rats. Bone. 1996;19:637–644. doi: 10.1016/s8756-3282(96)00302-x. [DOI] [PubMed] [Google Scholar]
- 74.Nobel YR, Cox LM, Kirigin FF, Bokulich NA, Yamanishi S, Teitler I, Chung J, Sohn J, Barber CM, Goldfarb DS, Raju K, Abubucker S, Zhou Y, Ruiz VE, Li H, Mitreva M, Alekseyenko AV, Weinstock GM, Sodergren E, Blaser MJ. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun. 2015;6:7486. doi: 10.1038/ncomms8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stappenbeck TS, Virgin HW. Accounting for reciprocal host-microbiome interactions in experimental science. Nature. 2016;534:191–199. doi: 10.1038/nature18285. [DOI] [PubMed] [Google Scholar]
- 76.Ericsson AC, Davis JW, Spollen W, Bivens N, Givan S, Hagan CE, McIntosh M, Franklin CL. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PloS one. 2015;10:e0116704. doi: 10.1371/journal.pone.0116704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev. 2016;40:117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Finkelstein JS, Klibanski A, Neer RM, Greenspan SL, Rosenthal DI, Crowley WF., Jr Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann Intern Med. 1987;106:354–361. doi: 10.7326/0003-4819-106-3-. [DOI] [PubMed] [Google Scholar]
- 79.Finkelstein JS, Lee H, Leder BZ, Burnett-Bowie SA, Goldstein DW, Hahn CW, Hirsch SC, Linker A, Perros N, Servais AB, Taylor AP, Webb ML, Youngner JM, Yu EW. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest. 2016;126:1114–1125. doi: 10.1172/JCI84137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bilezikian JP, Morishima A, Bell J, Grumbach MM. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med. 1998;339:599–603. doi: 10.1056/NEJM199808273390905. [DOI] [PubMed] [Google Scholar]
- 81.Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, Orwoll ES. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91:3908–3915. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 82.O’Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut. 1998;42:29–35. doi: 10.1136/gut.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lunz JG, 3rd, Specht SM, Murase N, Isse K, Demetris AJ. Hepatology. Vol. 46. Baltimore, Md: 2007. Gut-derived commensal bacterial products inhibit liver dendritic cell maturation by stimulating hepatic interleukin-6/signal transducer and activator of transcription 3 activity; pp. 1946–1959. [DOI] [PubMed] [Google Scholar]
- 84.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:71–78. doi: 10.1007/s12016-011-8291-x. [DOI] [PubMed] [Google Scholar]
- 86.Heyman M, Abed J, Lebreton C, Cerf-Bensussan N. Intestinal permeability in coeliac disease: insight into mechanisms and relevance to pathogenesis. Gut. 2012;61:1355–1364. doi: 10.1136/gutjnl-2011-300327. [DOI] [PubMed] [Google Scholar]
- 87.Hijazi Z, Molla AM, Al-Habashi H, Muawad WMRA, Molla AM, Sharma PN. Intestinal permeability is increased in bronchial asthma. Arch Dis Child. 2004;89:227–229. doi: 10.1136/adc.2003.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teixeira TFS, Collado MC, Ferreira CLLF, Bressan J, Peluzio MDG. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32:637–647. doi: 10.1016/j.nutres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Raehtz S, Fedorko A, McCabe L. Estrogen deficiency induced intestinal inflammation and permeability is linked with osteoporosis (488.8) The FASEB Journal. 2014;28 [Google Scholar]
- 90.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. The Journal of nutrition. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 92.Salminen S, Ouwehand A, Benno Y, Lee YK. Probiotics: how should they be defined? Trends in Food Science & Technology. 1999;10:107–110. [Google Scholar]
- 93.Narva M, Collin M, Lamberg-Allardt C, Karkkainen M, Poussa T, Vapaatalo H, Korpela R. Effects of long-term intervention with Lactobacillus helveticus-fermented milk on bone mineral density and bone mineral content in growing rats. Ann Nutr Metab. 2004;48:228–234. doi: 10.1159/000080455. [DOI] [PubMed] [Google Scholar]
- 94.Ohlsson C, Engdahl C, Fak F, Andersson A, Windahl SH, Farman HH, Moverare-Skrtic S, Islander U, Sjogren K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE. 2014;9:e92368. doi: 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229:1822–1830. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228:1793–1798. doi: 10.1002/jcp.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Collins FL, Irwin R, Bierhalter H, Schepper J, Britton RA, Parameswaran N, McCabe LR. Lactobacillus reuteri 6475 Increases Bone Density in Intact Females Only under an Inflammatory Setting. PLoS ONE. 2016;11:e0153180. doi: 10.1371/journal.pone.0153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, Keshavarz SA. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J Am Coll Nutr. 2017:1–10. doi: 10.1080/07315724.2017.1318724. [DOI] [PubMed] [Google Scholar]
- 99.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutrition research reviews. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 100.McCabe L, Britton RA, Parameswaran N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr Osteoporos Rep. 2015;13:363–371. doi: 10.1007/s11914-015-0292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weaver CM, Martin BR, Story JA, Hutchinson I, Sanders L. Novel fibers increase bone calcium content and strength beyond efficiency of large intestine fermentation. Journal of agricultural and food chemistry. 2010;58:8952–8957. doi: 10.1021/jf904086d. [DOI] [PubMed] [Google Scholar]
- 102.Legette LL, Lee W, Martin BR, Story JA, Campbell JK, Weaver CM. Prebiotics enhance magnesium absorption and inulin-based fibers exert chronic effects on calcium utilization in a postmenopausal rodent model. J Food Sci. 2012;77:H88–94. doi: 10.1111/j.1750-3841.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- 103.Griffin IJ, Davila PM, Abrams SA. Non-digestible oligosaccharides and calcium absorption in girls with adequate calcium intakes. The British journal of nutrition. 2002;87(Suppl 2):S187–191. doi: 10.1079/BJNBJN/2002536. [DOI] [PubMed] [Google Scholar]
- 104.Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, Ellis KJ. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr. 2005;82:471–476. doi: 10.1093/ajcn.82.2.471. [DOI] [PubMed] [Google Scholar]
- 105.Zafar TA, Weaver CM, Zhao Y, Martin BR, Wastney ME. Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. The Journal of nutrition. 2004;134:399–402. doi: 10.1093/jn/134.2.399. [DOI] [PubMed] [Google Scholar]
- 106.van den Heuvel EG, Schoterman MH, Muijs T. Transgalactooligosaccharides stimulate calcium absorption in postmenopausal women. The Journal of nutrition. 2000;130:2938–2942. doi: 10.1093/jn/130.12.2938. [DOI] [PubMed] [Google Scholar]
- 107.Garcia-Vieyra MI, Del Real A, Lopez MG. Agave fructans: their effect on mineral absorption and bone mineral content. J Med Food. 2014;17:1247–1255. doi: 10.1089/jmf.2013.0137. [DOI] [PubMed] [Google Scholar]
- 108.Roberfroid MB, Cumps J, Devogelaer JP. Dietary chicory inulin increases whole-body bone mineral density in growing male rats. The Journal of nutrition. 2002;132:3599–3602. doi: 10.1093/jn/132.12.3599. [DOI] [PubMed] [Google Scholar]
- 109.Takahara S, Morohashi T, Sano T, Ohta A, Yamada S, Sasa R. Fructooligosaccharide consumption enhances femoral bone volume and mineral concentrations in rats. The Journal of nutrition. 2000;130:1792–1795. doi: 10.1093/jn/130.7.1792. [DOI] [PubMed] [Google Scholar]
- 110.Chonan O, Matsumoto K, Watanuki M. Effect of galactooligosaccharides on calcium absorption and preventing bone loss in ovariectomized rats. Biosci Biotechnol Biochem. 1995;59:236–239. doi: 10.1271/bbb.59.236. [DOI] [PubMed] [Google Scholar]
- 111.Slevin MM, Allsopp PJ, Magee PJ, Bonham MP, Naughton VR, Strain JJ, Duffy ME, Wallace JM, Mc Sorley EM. Supplementation with calcium and short-chain fructo-oligosaccharides affects markers of bone turnover but not bone mineral density in postmenopausal women. The Journal of nutrition. 2014;144:297–304. doi: 10.3945/jn.113.188144. [DOI] [PubMed] [Google Scholar]
- 112.Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015;13:125–130. doi: 10.1007/s11914-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell metabolism. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rowan S, Jiang S, Korem T, Szymanski J, Chang ML, Szelog J, Cassalman C, Dasuri K, McGuire C, Nagai R, Du XL, Brownlee M, Rabbani N, Thornalley PJ, Baleja JD, Deik AA, Pierce KA, Scott JM, Clish CB, Smith DE, Weinberger A, Avnit-Sagi T, Lotan-Pompan M, Segal E, Taylor A. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci U S A. 2017;114:E4472–E4481. doi: 10.1073/pnas.1702302114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]


