Abstract
Objective
To examine if oculomotor and electrophysiological measures improve the clinical performance of the typical concussion protocol for classifying collegiate athletes with a history of concussion.
Design
Cross-sectional.
Setting
University Athletic Medicine and Research Facility.
Participants
Forty-five varsity collegiate athletes.
Independent Variables
Collegiate varsity athletes with or without a history of a diagnosed concussion.
Main Outcome Measures
Multivariate receiver operating curve and area under the curve (AUC) analyses tested the clinical performance of the typical concussion protocol (symptoms, postural control, neuropsychological abilities). We examined differences in clinical performance between this protocol and after adding reflexive saccade and event-related potential (ERP) indices. Hypotheses were formed after data collection.
Results
Significant AUCs were demonstrated for the typical concussion protocol (Model 1: AUC = 0.75, p = .007), after adding reflexive saccade eye excursion gain (Model 2: AUC = 0.80, p = .001), and ERPs (Model 3: AUC = 0.79, p = .002). The AUC for reflexive saccades and ERPs was significant (Model 4: AUC = 0.70, p = .030). Model 2’s increased clinical performance compared to Model 1 was non-significant, X2(2) = 1.871, p = .171.
Conclusion
All four models demonstrated adequate sensitivity and specificity for classifying athletes with a prior concussion. Adding reflexive saccades and ERPs did not significantly increase clinical performance of the typical concussion protocol. Future research should determine the clinical utility of saccades and ERPs for acute post-concussion assessments.
Keywords: sports-related concussion, electrophysiology, oculomotors, neuropsychology, postural control
1.0. Introduction
Concussion and mild traumatic brain injury (mTBI) are associated with oculomotor challenges1–6 and altered electrophysiological brain responses during cognitive tasks.7–11 However, neither are included as standard measures in baseline clinical sports-concussion assessment protocols. The typical baseline concussion testing and return-to-play protocols include symptomatology, balance functioning, and neuropsychological performance.12–14 For example, a battery including traditional neuropsychological tests, postural control, and symptomatology correctly identified approximately 96% of concussed college athletes within 24 hours.15 McCrea et al16 reported that college athletes returned to baseline levels of symptoms, postural control, and neuropsychological functioning within 7-days following concussion. Therefore, typical concussion protocols demonstrated successful clinical utility in diagnosing concussions in the acute phase and managing functional recovery on these measures. However, these measures are subjective, vulnerable to errors in self-reporting, and might not predict long-term outcomes following concussion. It is critical to identify objective measures that demonstrate excellent sensitivity and specificity for classifying athletes with a history concussion.
There is growing evidence that objective oculomotor and electrophysiological tests may improve the diagnostic accuracy of the typical concussion protocol. Athletes with a prior concussion demonstrated residual oculomotor impairments, which are unlikely to manifest on typical concussion protocols. For example, patients with mTBI generated impaired memory guided saccades (gain, errors) within 2-days following injury compared to controls1 and impaired smooth pursuit eye movements from 3–16 days2 through 12 months3 following injury. Near point fixation disparity greater than or equal to 15 cm demonstrated a significant cut-off value (AUC of 0.71) for identifying college ice-hockey athletes with a history of concussion.6
Concussions are also related to altered electrophysiological responses underlying cognitive processing. Event-related potential (ERP) components are segments of the ongoing electroencephalogram (EEG) that are time-locked to the onset of a stimulus, such as those displayed during cognitive tasks. During an oddball task, college football athletes an average of 4-years post-concussion generated larger P300 ERPs (reflecting increased memory-related attentional resources17) and delayed latencies (i.e., timing) than non-concussed teammates.10 However, the two groups of athletes did not perform differently on neuropsychological tests, a finding indicated in other similar research.11
The current study investigated whether objective oculomotor measures and working-memory related ERPs improve the clinical performance of a typical concussion assessment protocol for classifying varsity collegiate athletes with a history of concussion. The measures within the typical concussion assessment protocol included those recommended for concussion management12: a symptom checklist, a test of postural control, and four traditional neuropsychological assessments. Two functional measures of oculomotor performance (reflexive saccades, smooth pursuit eye movements) were administered because of the relationship between concussion history and oculomotor deficits.1–2 ERPs were recorded because they are sensitive to subtle changes in neurocognitive brain activity associated with prior concussion.10
2.0. Materials and Methods
2.1. Subjects
The current study was a cross-sectional investigation of 45 NCAA participating varsity men’s football and female soccer athletes (18–23 years) who completed testing prior to the their primary athletic season. Additional inclusion criteria included: 1) at least 18 years of age, 2) reported normal/corrected-normal vision (static visual acuity < 20/40), 3) capable of moving head left and right, 4) reported no consumption of alcohol in the past 24 hours, and 5) no reported orthopedic injuries.
The current study focused on the clinical performance of a novel protocol for examining athletes with a prior history of concussion (not an acute concussion). This included previously concussed athletes at least nine months post-concussion. Athletes’ concussion history was initially self-reported7,8,10,18 and confirmed through athletic medical records. Athletes reported the number of prior concussion(s) diagnosed by a health care provider and the approximate date of those concussions. Athletes with a concussion history (n = 21) experienced their most recent concussion an average of 4.11 years prior to testing (0.81–12.48; SD = 3.87). Additional sample demographics are reported in Table 1.
Table 1.
Sample Demographics (Mean ± SD)
| Conc. History | No Conc. History | |
|---|---|---|
| Participants | 21 | 24 |
| Age (years) | 20.17 ± 1.55 | 20.03 ± 1.53 |
| Gender (Males, Females) | (19, 2) | (19, 5) |
| Number of concussions | 1.52 ± 0.87 | --- |
| Time since most recent concussion (years) | 4.11 ± 3.87 | --- |
Group means and standard deviations for demographic variables.
2.2. Measures
Athletes indicated the severity that they were currently experiencing 16-symptoms (e.g., headaches, photophobia, nausea) on a five-point Likert scale (0 = “not experienced at all”, 4 = “a severe problem”) (Rivermead Post Concussion Symptoms Questionnaire, RPSQ).19 Symptom values were summed to derive each athlete’s current total symptom score.15
The BESS included three standing balance conditions (feet together, non-dominant-leg only, and feet tandem with non-dominant leg in back). Each condition was performed on a firm surface and foam-pad. Instructions and scoring methods corresponded to recommended guidelines.20 Composite scores reflected the total number of errors committed on the six tests.
A trained researcher administered three traditional paper-and-pencil neuropsychological tests: the Trail-Making Tests (TMT) A and B,21 the Wechsler Adult Intelligence Scale-IV Letter-Number Sequencing subtest (LNS),22 and the Color-word Interference-Inhibition (CWI) subtest of the Delis-Kaplan Executive Function System.23 These tests were collectively chosen to assess task-switching and processing speed,24 attention and working memory,22 and response-inhibition/self-control.25 In depth-description of these assessments are provided elsewhere.10
Reflexive saccades and smooth pursuit eye movements were examined as objective, quantitative measures of oculomotor functioning.26 Oculomotor tests, including saccades and smooth pursuit eye movements are stable measures both within and between sessions in healthy individuals.27 During both tests, participants were first screened for normal ocular range of motion (congruence), restrictions, or palsies, and then seated 4 feet (+/− 2 inches) from a light-emitting diode (LED) bar in a dark room. Participants wore 2D video eye goggles (videonystagmography ICS Chartr 200; GN Otometrics, Schaumburg, IL, USA). The position of pupil changes over time was calculated (calibration) prior to beginning each task. Athletes were instructed to keep their head in the primary position (looking directly forward) as they followed a red target with their eyes.
Saccadic eye movements for each eye were recorded while participants followed the target presented in random locations on the LED bar. Sixty total target presentations were randomly positioned between subtended arcs of 5 and 30 degrees in the horizontal direction. Intervals between target presentations randomly varied between 1.5 and 2 seconds. The primary outcome measure included the average of right and left eye gain (eye velocity/target velocity) of accepted saccades. Saccade testing lasted 80 seconds and was repeated when necessary (i.e., abnormal performance and/or less than 50% of saccades accepted) to obtain best performance.26
To record smooth pursuit eye movements, the target moved in a sinusoidal pattern from left and right of center with a maximum subtended arc of 30 degrees. The procedure included frequency sweeps from 0.2 to 0.7 Hz that were repeated up to three times to record participant’s best performance. Each frequency cycle lasted 50 seconds. Outcome measures included the average of the leftward and rightward congruency of eye movements with the target (i.e., “gain”). Catch-up saccades (i.e., corrective eye movements) were removed to calculate the gain value.26
ERPs were recorded from a high-density Ag/AgCl 256-electrode channel net while participants performed a 2-back working memory task. Participants were seated 1 meter from a Dell 15.5″ computer screen in a darkened room. Participants viewed individual English letters with a visual angle of 1.16 x 1.16 degrees, which were presented one at a time for 1000 ms (E-prime 2.0; Psychology Software Tools, Inc, Pittsburgh, PA). Time between trials ranged from 1600–2200 ms. Participants pressed two different buttons (counterbalanced between participants) to indicate if the current letter matched or mismatched the letter presented two letters previously. Participants completed 100 total trials (50 Match randomly intermixed with 50 Mismatch). Behavioral accuracy (%) and response time (ms) were recorded for each trial and averaged within condition.
Net Station 4.4.2 software and a 250 Hz sampling rate were used to record the ERPs. Electrode impedances were maintained below 60 kΩ. EEG signals were bandpass filtered offline from 0.3–30Hz and segmented to 900 ms post-stimulus onset with a 200 ms baseline correction. Only correct trials were submitted to analyses. Eye blinks were classified as a voltage shift greater than 150 uV at any electrode during any trial. Trials with eye blinks were removed from analyses. Trials were averaged within each condition (Match, Mismatch) and re-referenced to an average reference.
The ERP measures of interest in the present study included the amplitude (μV) and latency (ms) of the P300 component recorded during Match trials. Research suggests that the individuals with a prior concussion or mild TBI demonstrated altered P300 amplitudes and latencies during attention10 and working memory tasks.9,11 Using a temporal principal components analysis, the amplitude of the P300 was determined to be the mean positive deflection within the 284–680 ms time window recorded over a cluster of parietal scalp electrodes.10 The latency of the P300 was calculated as the temporal occurrence (ms) of the peak positive deflection during this temporal window.
2.3. Data Analysis
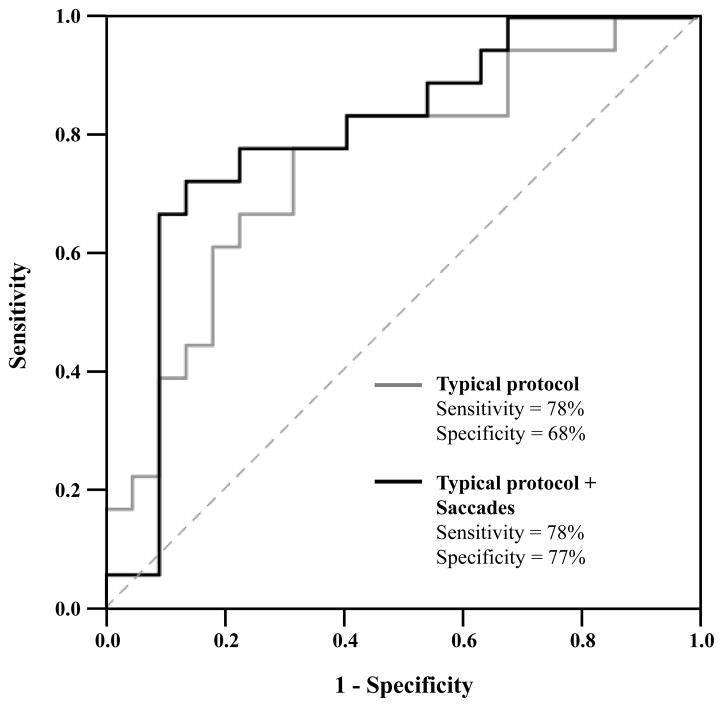
Statistical analyses were performed using SPSS version 23 (IBM, Chicago, IL, USA). Differences between concussion history and control groups on all outcome measures were first examined using independent samples t-tests (see Table 2). Second, we used receiver operating characteristics (ROC) curve and area under the curve (AUC) analyses to determine the clinical performance of each individual measure. As shown in Figure 1, the ROC curve is plotted with sensitivity (e.g., % Hit Rate) on the y-axis and false alarm rate (i.e., 1-specificity) on the x-axis. False alarm rate includes the percentage of athletes whose group membership was incorrectly classified. Specificity refers to the percentage of non-concussed athletes correctly classified. The goal of ROC analysis is to maximize the AUC, such that sensitivity is high but false alarm rates are low. Well-performing measures are those that are plotted within the upper-left space of the graph (i.e., high Hit Rate, low False Alarm Rate) and subsequently have a larger AUC.28
Table 2.
Between-groups Comparison of Symptoms, Postural Control, Neuropsychological Performance, Oculomotor Measures, and ERP Responses (Mean ± SD)
| Conc. History | No Conc. History | t-value | p-value | |
|---|---|---|---|---|
| RPSQ | 1.71 ± 3.33 | 2.63 ± 5.01 | 0.70 | .487 |
| BESS | 19.00 ± 6.33 | 21.08 ± 8.37 | 0.95 | .349 |
| TMT A | 18.43 ± 5.14 | 18.86 ± 6.28 | 0.25 | .803 |
| TMT B | 49.68 ± 21.19 | 43.57 ± 16.30 | −1.09 | .281 |
| CWI Errors | 11.05 ± 1.99 | 9.88 ± 2.85 | −1.58 | .122 |
| WAIS-LNS | 10.76 ± 2.82 | 10.29 ± 2.79 | −0.56 | .578 |
| Saccades Gain1 | 84.61 ± 7.57 | 88.61 ± 7.45 | 1.68 | .102 |
| SP-0.7 Hz2 | 0.75 ± 0.12 | 0.72 ± 0.14 | −0.70 | .489 |
| SP-0.6 Hz1 | 0.85 ± 0.10 | 0.79 ± 0.13 | −1.60 | .117 |
| SP-0.5 Hz3 | 0.89 ± 0.08 | 0.89 ± 0.08 | −0.01 | .989 |
| SP-0.4 Hz1 | 0.94 ± 0.04 | 0.90 ± 0.11 | −1.38 | .180 |
| SP-0.3 Hz3 | 0.93 ± 0.06 | 0.94 ± 0.09 | 0.25 | .801 |
| SP-0.2 Hz4 | 0.93 ± 0.07 | 0.96 ± 0.10 | 0.90 | .374 |
| P300 Amplitude | 3.79 ± 1.50 | 3.49 ± 2.03 | −0.57 | .569 |
| P300 Latency | 443.54 ± 73.04 | 438.47 ± 78.84 | −0.22 | .825 |
RPSQ = Rivermead Post Concussion Symptoms Questionnaire; BESS = Balance Error Scoring System; TMT A = Trail-Making Test A; TMT B = Trail-Making Test B, CWI Errors = D-KEFS Color Word Interference Subtest (number of errors scaled score); WAIS-LNS = WAIS-IV Letter Number Sequencing (scaled score); SP = Smooth Pursuit Eye Movements.
5 subjects missing because of unrecordable eye movements;
4 subjects missing because of unrecordable eye movements;
3 subjects missing because of unrecordable eye movements;
2 subjects missing because of unrecordable eye movements. Eye movements deemed unrecordable may be due to excessive eye noise in the recording (e.g., eye makeup or eye lashes) or excessive eye blinks.
Figure 1.
The typical concussion assessment protocol (solid gray line) demonstrated a significant area under the curve (AUC) for classifying athletes with a history of concussion (AUC = 0.75, p = .007). Adding reflexive saccade eye excursion gain to the protocol demonstrated a larger AUC (solid black line; AUC = 0.80, p = .001).
Third, we examined the AUC for a typical concussion assessment protocol,15 including concussion-like symptoms (RPSQ), postural control (BESS errors), and neuropsychological performance (TMT A, TMT B, CWI, LNS). We first used binary logistic regression to derive probability estimates for each participant. Specifically, that the participant’s combined performance on these measures predicted their group membership (concussion history, no concussion history). These probability estimates were used to fit a multivariate ROC, which determined the AUC of the typical concussion protocol for correctly classifying varsity college athletes with a prior concussion (Model 1: Typical Concussion Protocol). We also examined the AUC after adding saccades gain (Model 2: Typical Concussion Protocol + Saccades) and P300 ERP amplitude and P300 ERP latency (Model 3: Typical Concussion Protocol + Saccades + ERP). Hierarchical binary logistic regression examined the extent to which these latter two models predicted group membership more accurately than the typical concussion protocol (Model 1). Fourth, the same multivariate ROC approach examined the combined AUC for the saccades and ERP outcomes (Model 4: Saccades + ERP).
3.0 Results
As reported in Table 2, individuals with and without a history of concussion did not significantly differ on all measures (p-values > .05). The results of the individual ROCs curves are reported in Table 3. No individual measure demonstrated an AUC significantly different from chance-levels (p-values > .05). We included reflexive saccades into the multivariate ROCs (but not smooth pursuit eye movements) because it was the only oculomotor measure that demonstrated adequate sensitivity. Table 4 illustrates the clinical performance of the four multivariate ROCs. The typical concussion protocol (Model 1) demonstrated a large AUC (AUC = 0.75, 95% CI = 0.60–0.91, p = .007). As shown in Figure 1, the inclusion of saccade gain (Model 2) resulted in a larger AUC (AUC = 0.80, 95% CI = 0.66–0.94, p = .001) with high sensitivity and specificity. Model 2 did not predict group membership significantly greater than Model 1, X2(2) = 1.871, p = .171. The inclusion of P300 amplitude and latency (Model 3) also resulted in a significant AUC (AUC = 0.79, 95% CI = 0.64–0.94, p = .002). However, including these ERP outcomes did not significantly improve Model 2’s predictability of group membership, X2(2) = 0.085, p = .959. The multivariate ROC including only the oculomotor and ERP outcomes (Model 4) was also significant (AUC = 0.70, 95% CI = 0.53–0.87, p = .030).
Table 3.
Results of Individual ROC Analyses
| Measure | AUC (SE) | Sensitivity | Specificity | 95% CI | p-value |
|---|---|---|---|---|---|
| RPSQ | 0.47 (0.09) | 0.43 | 0.54 | (0.30, 0.64) | .759 |
| BESS | 0.44 (0.09) | 0.57 | 0.46 | (0.27, 0.61) | .488 |
| TMT A | 0.51 (0.09) | 0.52 | 0.54 | (0.34, 0.68) | .927 |
| TMT B | 0.57 (0.09) | 0.57 | 0.58 | (0.40, 0.74) | .400 |
| CWI Errors | 0.37 (0.08) | 0.81 | 0.28 | (0.21, 0.54) | .136 |
| WAIS-LNS | 0.44 (0.09) | 0.48 | 0.26 | (0.26, 0.61) | .460 |
| Saccades Gain1 | 0.67 (0.09) | 0.83 | 0.59 | (0.50, 0.85) | .061 |
| SP-0.7 Hz2 | 0.42 (0.09) | 0.42 | 0.46 | (0.24, 0.59) | .353 |
| SP-0.6 Hz1 | 0.31 (0.09) | 0.47 | 0.33 | (0.14, 0.48) | .038^ |
| SP-0.5 Hz3 | 0.47 (0.09) | 0.57 | 0.48 | (0.29, 0.65) | .753 |
| SP-0.4 Hz1 | 0.39 (0.09) | 0.47 | 0.43 | (0.21, 0.57) | .288 |
| SP-0.3 Hz3 | 0.52 (0.09) | 0.58 | 0.39 | (0.34, 0.70) | .820 |
| SP-0.2 Hz4 | 0.55 (0.09) | 0.65 | 0.48 | (0.34, 0.72) | .592 |
| P300-Amplitude | 0.56 (0.09) | 0.57 | 0.63 | (0.39, 0.73) | .495 |
| P300-Latency | 0.52 (0.09) | 0.38 | 0.50 | (0.34, 0.69) | .856 |
AUC = Area under the curve; SE = Standard Error; CI = Confidence Interval.
Five subjects missing because of unrecordable eye movements;
4 subjects missing because of unrecordable eye movements;
3 subjects missing because of unrecordable eye movements;
2 subjects missing because of unrecordable eye movements;
Significantly below the diagonal reference line. Eye movements deemed unrecordable may be due to excessive eye noise in the recording (e.g., eye makeup or eye lashes) or excessive eye blinks.
Table 4.
Results of Multivariate ROC analyses
| Multivariate ROC | AUC (SE) | Sensitivity | Specificity | 95% CI | p-value |
|---|---|---|---|---|---|
| Model 1: Typical Concussion Protocol | 0.75 (0.08) | 0.78 | 0.68 | (0.60, 0.91) | .007** |
|
| |||||
| Model 2: Typical Concussion Protocol + Saccades | 0.80 (0.07) | 0.78 | 0.77 | (0.65, 0.94) | .001** |
| Model 3: Typical Concussion Protocol + Saccades + ERP | 0.79 (0.07) | 0.78 | 0.77 | (0.64, 0.94) | .002** |
| Model 4: Saccades + ERP | 0.70 (0.09) | 0.78 | 0.68 | (0.53, 0.87) | .030* |
Typical Concussion Protocol= RPSQ + BESS + TMT A + TMT B + CWI Errors + WAIS-IV-LNS. Saccades from five subjects missing because of unrecordable eye excursions.
Listwise deletion was used to compare nested models (n = 40).
p < .05;
p < .01
We further explored if variability in post-concussion duration limited the clinical performance of the reflexive saccades and ERP measures. Time elapsed since concussion did not significantly correlate with reflexive saccade gain, P300 amplitude or P300 latency (ps > .05). In post hoc multivariate ROC analyses, the sample was limited to athletes whose most recent concussion occurred in the last four years (n = 16; M = 2.08 years, SD = 0.98). The AUCs for this less heterogeneous sample did not change for Model 2, (AUC = 0.80, 95% CI = 0.65–0.96, p = .003) or Model 3 (AUC = 0.79, 95% CI = 0.64–0.95, p = .004). The clinical performance of Model 4 declined and was no longer significant, (AUC = 0.69, 95% CI = 0.50, 0.88, p = .065).
4.0 Discussion
Our primary objective was to examine if oculomotor and ERP outcomes improve the clinical performance of the typical concussion protocol for classifying athletes with a history of concussion. The typical concussion protocol demonstrated a significant AUC with adequate sensitivity (0.78) and specificity (0.68). A combined model of reflexive saccades and P300 amplitude and latency also yielded a significant AUC but was not significantly different from the typical protocol.
It is recommended that oculomotor screening be incorporated into concussion assessment protocols.29 However, there is currently limited research using objective oculomotor assessment with videonystagmography (VNG) equipment in concussed athletes. Altered antisaccades and memory-guided saccades gain were reported in acute concussed athletes compared to healthy subjects.4 During concurrent fMRI, these oculomotor deficits were accompanied by increased blood oxygen level dependent (BOLD) signal change in the cerebellum, primary motor cortex, visual cortex, and subregions of the frontal and temporal cortices.4 To our knowledge, this is the first study to examine the clinical performance of objective oculomotor measures for classifying athletes with a history of concussion.
The inclusion of reflexive saccade gain did not significantly improve the clinical performance of the typical concussion protocol in the long-term post-concussion period. Further, access to objective oculomotor assessment tools (i.e., VNG) is rare for clinicians assessing concussions; thus, screening measures such as the Vestibular/Ocular Motor Screening (VOMS)5,29 may provide a more efficient alternative towards identifying college athletes with a history of concussion. The VOMS evaluates the extent to which five qualitative oculomotor tests provoke concussion-like symptoms. A multivariate ROC including three of these measures demonstrated a large AUC (0.89) for identifying those athletes with a concussion within 21 days.5 Importantly, the VOMS demonstrated high internal consistency, a low false-positive rate,5,30 and is quick to administer. These are all particularly valuable criteria for athletic trainers and clinicians coordinating large-scale baseline concussion testing. Future research should examine the clinical performance of the VOMS for classifying athletes with a history of concussion. Given the clinical utility of the VOMS in the acute post-concussion phase,5 it may be used to refer athletes for further in-depth oculomotor testing,29 such as that using VNG.
Research indicated that athletes with a history of mTBI generated aberrant neuroelectrical responses during cognitive tasks.7,10,11 However, the ERP measures in the current study did not demonstrate significant clinical utility for classifying athletes with a history of concussion nor improve the clinical performance of the typical protocol. The discrepancy between prior findings and the current study may be due to task-related differences. For instance, we chose to focus on the P300 component elicited during a working memory task, given that memory deficits are often commonly reported following concussion.31 Even though fMRI research reported that athletes with a prior concussion generate altered functional brain activity underlying working memory,32,33 the extent to which a concussion influences the P300 ERP component elicited during working memory tasks may be more tenuous. Gosselin et al34 reported that individuals with a post-acute mTBI did not generate altered P300 amplitude or latencies during working memory but showed decreased brain activity in the dorsolateral prefrontal cortex. This suggests that fMRI may be a more sensitive index of altered neural correlates underlying working memory.
Research reported that college athletes return to baseline levels of performance on symptoms, postural control, and neuropsychological performance within 7–10 days following concussion.16,31,35 To the authors’ knowledge, the current study is the first to report adequate clinical utility of this combined protocol in the post-acute period following concussion. The present study’s results may be limited to traditional neuropsychological tests, which may be more sensitive to a prior concussion than computerized neurocognitive batteries.15 The small sample size and heterogeneity of the concussion history group limits our results and could have lead to an inadequate classification rate. Although we established that duration post-concussion did not influence our findings, researchers should recruit large enough sample sizes to examine changes in oculomotor and electrophysiological measures based on number of prior concussions in gender-matched sports.
The concussion history and non-concussion history groups did not significantly differ on any single outcome measure. Although individual measures may provide limited clinical utility as single measures, they may be more useful for identifying college athletes with a history of concussion when included in a parallel protocol. Our findings suggest that such a multivariate clinical protocol successfully identified varsity collegiate athletes with long-term changes in functional outcomes associated with a history of concussion. Therefore, multivariate ROC analyses are advantageous to univariate between-groups comparisons because of their ability to identify the combined clinical utility of multiple measures for classifying previously concussed athletes.
A protocol with ERP and reflexive saccades also demonstrated significant clinical performance. Although not significant, reflexive saccade eye excursions increased the clinical performance of the typical concussion protocol. Taken together, these findings suggest that reflexive saccades and ERP outcomes demonstrate significant clinical performance for classifying varsity athletes with a prior concussion, but do not improve the clinical performance of the typical protocol. In the acute post-concussion phase, these measures may demonstrate greater sensitivity and specificity. However, the large amount of training and resources necessary to include VNG and/or electrophysiology testing may limit their clinical utility in the acute post-concussion period. Future research is warranted to investigate the clinical performance of quantitative, objective oculomotor and ERP indices in the acute post-concussion phase. This research is necessary for establishing the performance of objective oculomotor and electrophysiological testing for concussion assessment and management.
Acknowledgments
The authors graciously thank Andrea Meinders for her years of data collection and data processing efforts.
Sources of Funding
This study was partially supported by the National Institutes of Health, NIHR01 HD073202, and the Nebraska Tobacco Settlement Biomedical Enhancement funds.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.Heitger MH, Anderson TJ, Jones RD. Saccade sequences as markers for cerebral dysfunction following mild closed head injury. Prog Brain Res. 2002;140:433–448. doi: 10.1016/S0079-6123(02)40067-2. [DOI] [PubMed] [Google Scholar]
- 2.Heitger MH, Anderson TJ, Jones RD, et al. Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain. 2004;127:575–590. doi: 10.1093/brain/awh066. [DOI] [PubMed] [Google Scholar]
- 3.Heitger MH, Jones RD, Dalrymple-Alford JC, et al. Motor deficits and recovery during the first year following mild closed head injury. Brain Inj. 2006;20(8):807–824. doi: 10.1080/02699050600676354. [DOI] [PubMed] [Google Scholar]
- 4.Johnson B, Zhang K, Hallett M, et al. Functional neuroimaging of acute oculomotor deficits in concussed athletes. Brain Imaging Behav. 2015;9(3):564–573. doi: 10.1007/s11682-014-9316-x. [DOI] [PubMed] [Google Scholar]
- 5.Mucha A, Collins MW, Elbin RJ, et al. A brief vestibular/ocular motor screening (VOMS) assessment to evaluate concussions: Preliminary findings. Am J Sports Med. 2014;42(10):2476–2486. doi: 10.1177/0363546514543775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poltavski DV, Biberdorf D. Screening for lifetime concussion in athletes: Importance of oculomotor measures. Brain Inj. 2014;28(4):475–485. doi: 10.3109/02699052.2014.888771. [DOI] [PubMed] [Google Scholar]
- 7.Broglio SP, Pontifex MB, O’Connor P, et al. The persistent effects of concussion on neuroelectric indices of attention. J Neurotrauma. 2009;26:1463–1470. doi: 10.1089/neu.2008.0766. [DOI] [PubMed] [Google Scholar]
- 8.De Beaumont L, Théoret H, Mongeon D, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- 9.Gosselin N, Bottari C, Chen JK, et al. Evaluating the cognitive consequences of mild traumatic brain injury and concussion by using electrophysiology. Neurosurgical Focus. 2012;33(6):1–7. doi: 10.3171/2012.10.FOCUS12253. [DOI] [PubMed] [Google Scholar]
- 10.Ledwidge PS, Molfese DL. Long-term effects of concussion on electrophysiological indices of attention in varsity college athletes: An event-related potential and standardized low-resolution brain electromagnetic tomography approach. J Neurotrauma. 2016;33:2081–2090. doi: 10.1089/neu.2015.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozen LJ, Itier RJ, Preston FF, et al. Long-term working memory deficits after concussion: Electrophysiological evidence. Brain Inj. 2013;27(11):1244–1255. doi: 10.3109/02699052.2013.804207. [DOI] [PubMed] [Google Scholar]
- 12.Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers’ Association Position Statement: Management of sport concussion. J Athl Train. 2014;49(2):245–265. doi: 10.4085/1062-6050-49.1.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine Position Statement: Concussion in sport. Clin J Sport Med. 2013;23(1):1–18. doi: 10.1097/JSM.0b013e31827f5f93. [DOI] [PubMed] [Google Scholar]
- 14.McCrory P, Meeuwisse W, Aubry M, et al. Consensus statement on Concussion in Sport—The 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 15.Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60(6):1050–1058. doi: 10.1227/01.NEU.0000255479.90999.C0. [DOI] [PubMed] [Google Scholar]
- 16.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players. JAMA. 2003;290(19):2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 17.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore RD, Broglio SP, Hillman CH. Sport-related concussion and sensory function in young adults. J Athl Train. 2014;49(1):36–41. doi: 10.4085/1062-6050-49.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King NS, Crawford S, Wenden FJ, et al. The Rivermead post concussion symptoms questionnaire—a measure of symptoms commonly experience after head injury and its reliability. J Neurol. 1995;242:587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 20.Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263–273. [PMC free article] [PubMed] [Google Scholar]
- 21.Reitan RM, Wolfson D. The Halstead–Reitan Neuropsychological Test Battery. Tuczon, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV) San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- 23.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 24.Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, et al. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15(3):438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- 25.Dimoska-Di Marco A, McDonald S, Kelly M, et al. A meta-analysis of response inhibition and Stroop interference control deficits in adults with traumatic brain injury (TBI) J Clin Exp Neuropsychol. 2011;33(4):471–485. doi: 10.1080/13803395.2010.533158. [DOI] [PubMed] [Google Scholar]
- 26.Shepard NT, Schubert MC. Background and technique of ocular motility testing. In: Jacobson GP, Shepard JT, editors. Balance Function Assessment and Management. 2. San Diego, CA: Plural Publishing; 2015. pp. 209–223. [Google Scholar]
- 27.Ettinger U, Kumari V, Crawford TJ, et al. Reliability of smooth pursuit, fixation, and saccadic eye movements. Psychophysiology. 2003;40:620–628. doi: 10.1111/1469-8986.00063. [DOI] [PubMed] [Google Scholar]
- 28.Turner RG, Nielsen DW. Application of clinical decision analysis to audiological tests. Ear Hear. 1984;5(3):125–133. doi: 10.1097/00003446-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Kontos AP, McAllister-Deitrick J, Collins MW, et al. Review of vestibular and oculomotor screening and concussion rehabilitation. J Athl Train. 2017;52(3):256–261. doi: 10.4085/1062-6050-51.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kontos AP, Sufrinko A, Elbin RJ, et al. Reliability and associated risk factors for performance on the vestibular/ocular motor screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. 2016;44(6):1400–1406. doi: 10.1177/0363546516632754. [DOI] [PubMed] [Google Scholar]
- 31.Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: A meta-analysis. J Int Neuropsychol Soc. 2005;11:345–357. doi: 10.1017/s1355617705050411. [DOI] [PubMed] [Google Scholar]
- 32.Keightley ML, Saluja RS, Chen JK, et al. A functional magnetic resonance imaging study of working memory in youth after sports-related concussion: Is it still working? J Neurotrauma. 2014;31:437–451. doi: 10.1089/neu.2013.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slobounov S, Zhang K, Pennell D, et al. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: A functional MRI study. Exp Brain Res. 2010;202(2):341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gosselin N, Bottari C, Chen JK, et al. Electrophysiology and functional MRI in post-acute mild traumatic brain injury. J Neurotrauma. 2011;28:329–341. doi: 10.1089/neu.2010.1493. [DOI] [PubMed] [Google Scholar]
- 35.Field M, Collins MW, Lovell MR, et al. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546–553. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]