Abstract
Purpose
The purpose of this study was to evaluate the role of stereotactic radiosurgery in the treatment of primary tumors of the spine and spinal cord.
Methods
An Institutional Review Board approved retrospective analysis of 30 patients with primary spine tumors treated at a single institution was performed. Post-treatment pain, neurological, and radiographic responses were the endpoints.
Results
Nine patients were treated for benign tumors, and 21 patients were treated for malignant tumors. The median dose delivered was 16 Gy in one fraction. Median follow up was 13.13 months (range, 1 month-84 months). Pain relief was 88% initially. Pain recurred in five patients with a median time to recurrence of 5 months (range, 3.6 months – 80 months). Neurological improvement was achieved in 65% of patients. Three patients experienced a recurrence in their neurological deficit (at 3.6 months, 1.6 years, and 3.7 years after SRS). Radiographic control was achieved in 77% of sites treated with SRS. Thirteen of the treated sites recurred with a median time of 9.9 months. Two long-term toxicities were observed (asymptomatic radio-necrosis of the erector spinae muscle and foot drop).
Conclusions
Our results suggest that SRS is a safe and effective treatment option for primary tumors of the spine and spinal cord.
Keywords: primary spine tumor, stereotactic radiosurgery, stereotactic body radiation therapy
Introduction
Primary tumors of the spine are remarkably rare. They occur far less frequently than metastatic spinal tumors [1-4] and are often intradural or intramedullary, making them anatomically distinct from metastases which are primarily extradural. The treatment goals of primary spine tumors also differ from metastases. Since metastases are often indicators of late stage disease, the primary reason for attempting to achieve local control (LC) is to palliate symptoms.[5] In contrast, achieving LC in a primary spine tumor, may not only alleviate symptoms, but may lead to substantial improvements in overall survival (OS). Despite the clear distinctions between primary tumors of the spine, and spinal metastases, the radio-therapeutic approach to the treatment of these two categories of tumors is very similar.
The current standard treatment for primary spine tumors depends mainly on the pathology of the tumor. When feasible, surgical resection is typically the treatment of choice.[6-8] En bloc resection is usually preferred, but extent of surgery can vary. Intra-lesional resections have been attributed as a cause of increased risk of leptomeningeal/cerebrospinal fluid (CSF) dissemination, and positive margins resulting in a relatively high rate of recurrence.[9-12] When more invasive resections are pursued and negative margins are achieved, recurrence may be reduced. However, complete resection is sometimes difficult to achieve in an attempt to sparing of nerves and other critical adjacent structures. For patients who are not surgical candidates, or for patients where adjuvant therapy is indicated, radiation therapy is often utilized. Standard fractionated external beam radiation therapy (EBRT) is used in both cases, but because of the spinal cord dose constraints, sufficient therapeutic radiation dose could not be delivered. Indeed, most of the primary tumors require high radiation doses above the known spinal cord tolerance dose. To limit potential toxicities, guidelines recommend a biologically equivalent dose (BED 2 Gy equivalents fractions) of 50 Gy,[13] However, for certain primary spine tumor histologies such as sarcomas, a dose of 60Gy is recommended at the minimum to prevent tumor recurrence and the likelihood of local failure following EBRT remains relatively high.[14]
Stereotactic radiosurgery (SRS) has been used to successfully palliate spinal metastases, even for tumors of radio-resistant histologies.[15-20] This success with spinal metastases has led to the widespread extrapolation of SRS to the treatment of primary spine tumors.[21, 22] Extensive literature is available on the responses of metastases, however due to the rarity of primary spine tumors, the available literature regarding their response to SRS is scarce. Given the differences in morphology, location, and treatment goals of these two categories of tumors, it is important that the responses of primary spine tumors are clearly distinguished. Therefore, we reviewed our institutional experience to demonstrate the role of SRS in the treatment of primary spine tumors.
Methods
Thirty patients (81 vertebral levels) with pathologically confirmed primary spine tumors were treated with SRS at our institution between June 2001 and December 2013. Each patient was discussed in a multidisciplinary spine tumor board. Recommendations regarding whether a patient was a suitable candidate for spine SRS were made according to consensus opinions. Indications for SRS included pain, neurological compromise, and imaging progression. An Institutional Review Board approved retrospective analysis was performed using electronic medical records of clinical exams, as well as Computed Tomography (CT) and Magnetic Resonance Imaging (MRI). Post treatment pain control, neurologic improvement, and radiographic tumor control were the primary endpoints of this study.
During this period, the Novalis system (BrainLAB, Inc, Munich, Germany) was utilized for spine SRS. Patient immobilization was achieved with the aid of vacuum bags (Elekta BodyFIX, Stockholm, Sweden). A contrast-enhanced simulation computed tomography (CT) with a slice-thickness of 3 mm was performed with infrared fiducial markers (ExacTrac, BrainLAB). These images were fused with diagnostic magnetic resonance images (MRI) in the treatment planning system in order to define the target volume (TV) consisting of the gross tumor and the involved vertebral body. An illustration of this can be seen in Figure 1. No expansion margin was added to the defined target volume. T2-weighted magnetic resonance images were used to delineate the spinal cord 6 mm above and 6 mm below the defined TV. A spinal cord planning organ at risk volume was not constructed. Multiple coplanar intensity modulated radiation beams were used to optimize the radiation dose to the target volume and minimize the dose to surrounding tissue. Single doses ranging from 10 Gy in 1 fraction to 24 Gy in 1 fraction (median, 16 Gy in 1 fraction) were delivered. All doses were prescribed to the 90% isodose line. The primary dose constraint for plan selection was to achieve the objective of 10 Gy to 10% of the partial volume of the spinal cord and a maximum point dose of 14 Gy. When this constraint was not achievable, under-dosage to the GTV was accepted to meet the above dosimetric objective. Prescribed dose did not vary on the basis of the presumed radio-resistance of the histopathology. This procedure has been detailed in previous reports.[15, 23, 24]
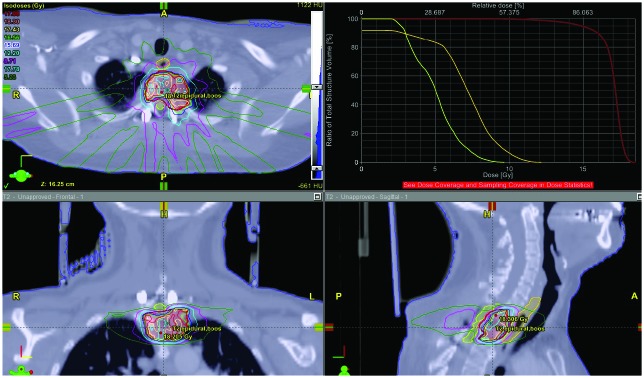
Figure 1.
Treatment planning images and a dose volume histogram of a T1-T2 meningiothelial meningioma after a subtotal resection. The tumor was dumbbell shaped and resulted in significant epidural compression.
CT and MRI were used to evaluate the radiographic response using the updated Response Evaluation Criteria in Solid Tumors.[25] Initial follow-up imagining was completed at approximately two to three months after SRS followed by additional imaging completed at 6-month intervals or as clinically indicated. Clinical follow up typically coincided with imaging follow up, unless patients had a specific issue or concern, at which point they were seen immediately, The 0-10 Numerical Rating Pain Scale (NRPS) was used to quantify pain response. Several methods were used to assess neurological function including the 0-5 point Medical Research Council (MRC) scale for motor strength, the pin prick test for numbness, the Romberg evaluation for balance, and testing of the cranial nerves.[26] As a secondary end-point, adverse events related to SRS including the occurrence and progression of vertebral compression fractures were also recorded using criteria detailed in a previous report.[27] Radiographic and clinical responses occurring within approximately 6 months after SRS were attributed to SRS and included in our response rates. Tumor progression and toxicity observed at any point during the follow-up period were reported, regardless of whether or not they occurred within the first 6 months following SRS.
Results
Overall median follow up (calculated from time of each patient’s first SRS treatment) was 13.13 months (range, 1 month-84 months). Clinical follow up was available in 27 (90%) of the patients. Sixty-percent of the patients were males and 73% were Caucasian, with a median age of 58 (Table 1). Sixty-four percent of tumors/treatment sites were surgically resected prior to SRS. Thirty-three percent of tumors/treatment sites received EBRT prior to receiving SRS (Table 2). Twenty-one patients were treated for malignant tumors and nine patients for benign tumors, including 9 sarcoma, 7 chordoma, 2 ependymomas, 2 glioma, 3 schwannoma, 4 meningioma, 1 hemangioma, 1 fibroma, and 1 desmoid tumor. Median tumor volume was 32.0 cc (average: 53.87, range: 0.74-228).
Table 1.
Patient Characteristics
| Total number of Patients | 30 |
| Sex, number. (%) | |
| - Males | 18 (60.0%) |
| - Females | 12 (40.0%) |
| Age, years | |
| - Median (range) | 58 (13-79) |
| Ethnicity, number. (%) | |
| - African American | 4 (13.3%) |
| - Caucasian | 22 (73.3%) |
| - Other | 4 (13.3%) |
| Benign tumor, number | 9 patients (9 sites) |
| Malignant tumor, number | 21 patients (36 sites) |
Table 2.
Treatment Site and Stereotactic Radiosurgery Characteristics
| Number of spines Treated | 81 |
| Spine Levels Treated, % of spines | |
| - Cervical | 19.8% |
| - Thoracic | 21.0% |
| - Lumbar | 25.9% |
| - Sacral | 33.3% |
| Median Tumor Volume (range), cm3 | 39.1 (0.74 - 228.0) |
| Median Radiosurgery Dose* (range), Gy | 16 (10-24) |
| Newly Diagnosed or Recurrent Tumor | |
| - Recurrent Tumor, % of sites | 88.9% |
| - Newly Diagnosed, % of sites | 8.9% |
| - Insufficient information to determine | 2.2% |
| Prior Local Treatment, % of sites | |
| - Surgical Resection | 64.4% |
| - EBRT | 33.3% |
| Indications for Stereotactic Radiosurgery | |
| - Pain | 19 patients (63.3%), 28 sites |
| - Neurological Deficit(s) | 19 patients (63.3%), 23 sites |
| - Asymptomatic/Imaging Progression Only | 4 patients (13.3%), 7 sites |
| Follow-Up (clinical, radiographic, or both) | |
| - Number of Patients (%) | 27 (90%) |
| - Median Duration (range) | 13.13 m (35 d – 7 y) |
| - Radiographic, % of Tx sites | 77.8% |
| - Pain, % of corresponding Tx sites | 89.3% |
| - Neurological, % of corresponding Tx sites | 87.0% |
| Survival | |
| - Deceased | 9 patients (30.0%) |
| - Median Survival | 3 y |
Of the 19 patients (and 28 treatment sites) presenting with pain, 18 patients (and 25 treatment sites) were evaluable for pain follow up. Pain relief was experienced by all evaluable patients presenting with pain (67% partial relief, and 33% complete relief). In terms of treatment sites, pain progressed after SRS in two of the treated sites (8%), was stable in one treatment site, and pain relief was experienced in 88% of the treated sites (36% complete relief, 52% partial relief) (Table 3). Pain recurred in five of the treatment sites with a median time to recurrence of 5 months (range, 3.6 months 5.8 years).
Table 3.
Stereotactic Radiosurgery Response Rates
| Response | Total Response | Complete (CR) | Partial (PR) | Stable | Progressed |
| Pain | 88% | 36% | 52% | 4% | 8% |
| Neurologic | 65% | 15% | 50% | 25% | 10% |
| Radiographic | 77% (Stable + PR) | 0% | 23% | 54% | 23% |
Of the 19 patients with neurological deficits with 23 sites at presentation, follow up was available to evaluate 16 patients with 20 treatment sites. The neurological deficit remained stable in 5 (31%) of the evaluable patients that presented with a deficit, and improved in 11 patients (69%). In terms of treatment sites, neurological deficits progressed in two treatment sites (10%), stable in five treatment sites (25%), and improved in 65% (15% complete response, and 50% partial response) (Table 3). Deficits recurred in three patients (at 3.6 months, 1.6 years, and 3.7 years after SRS).
Radiographic follow-up was available for 35 (78%) of treated spines. Local tumor progression occurred in 23% of treated spines. Local radiographic control was achieved in 77% of treated sites (23% partial response, 54% stable) (Table 3). Thirteen of the treated sites recurred with a median time to recurrence of 9.9 months (range 3 months – 3.2 years). There were two cases of long-term toxicity that we believed was related to SRS; one case of radionecrosis of the erector spinae muscle (patient received 16 Gy for S1 desmoid tumor) and one case of foot drop (patient received 10 Gy for an L1-L2 glioma). Sixteen cases of vertebral compression fracture were observed, none of which were attributed to SRS.
DISCUSSION
Due to the rarity of primary spine tumors, the available literature on SRS in the treatment of these tumors is scarce. In an effort to help address this, we evaluated the role of SRS in the treatment of primary spine tumors at our institution. We update our previously published report,[22] and demonstrate that SRS is a safe and effective treatment option for primary tumors of the spine.
SRS appears to provide good clinical response with pain, neurological improvement and tumor control. Pain control was the main role of SRS with response rate of 88% suggests SRS is effective in achieving pain relief in patients with primary spine tumors. Neurological improvement was also observed in 65%; this is an improvement from our institution’s initial study that reported a neurological improvement in 56% of patients.[22] LC in our series was 77% with longer followup period. Chang et al from the Korea Cancer Center Hospital (KCCH) reported the only other primary spine SRS series, and observed a mean local progression-free survival of 56 months (95% CI, 41–72 months) for chordoma patients, and 73 months (95% CI, 49–97 months) for sarcoma patients.[21] Their study reported response rates for two different groups. One group included tumors that were previously treated with a different modality, then subsequently treated with SRS due to local failure. The second group included patients treated with SRS as the primary treatment. Progression-free survival was 40 months and 90 months for the recurrent group, and primary treatment group respectively; they found that tumors treated for recurrence had a 10-fold greater risk for local progression after treatment with SRS. The variation in the local control rates observed among our series and the KCCH series may be due to the differing histologies analyzed in the cohorts.
Local control may also be affected by location. A prior report on benign spine SRS suggests that the location of intradural tumors makes a partial or complete response following SRS relatively unlikely.[28, 29] In patients with intradural tumors, if significant cord compression is present, surgery may be indicated and SRS should instead be considered as an adjuvant treatment. Though SRS is at best an adjuvant treatment in certain intradural tumors, recent studies have shown that the efficacy of SRS may be comparable to that of surgery.[30] Bate et al reported their experience of 69 lesions after SRS alone (70% of lesions), or surgery followed by SRS (30%). Treatment with surgery and adjuvant SRS failed to show any clinically significant advantage over treatment with SRS alone as the definitive treatment.[30] It is worth noting that this study focused primarily on spinal metastases, and studies comparing definitive treatment with SRS to surgery and adjuvant SRS for primary spine tumors will be needed. However, given the relatively high control rates observed at our institution, and at the KCCH, SRS is worth considering as a possible definitive treatment for the properly selected patient. This would be a significant benefit to patients, who would have a treatment option that achieves excellent local control, and is non-invasive, eliminating the need for lengthy hospital stays and post-surgical rehabilitation.
The most notable toxicities observed in this series include one patient who developed a progression of proximal lower extremity weakness, and lower back pain following treatment with SRS; the progressive symptoms in this patient were attributed to their radiographic tumor progression in untreated sites. After SRS treatment of 16 Gy in 1 fraction to an L5-S1 desmoid tumor, this patient developed radionecrosis of his adjacent erector spinae muscle. This was asymptomatic, and the patient remained symptom free for the duration of the follow up period. Prior to SRS this patient had surgical resection and EBRT of 60 Gy in 30 fractions to the lumbrosarcral spine and paraspinal region, followed by 15 Gy SRS to L5-S1 two months later. This patients’ extensive history of radiation therapy likely increased the risk of toxicity. Another patient developed a gait abnormality with foot drop. The patient was treated to an adjacent dermatome, recieving10 Gy SRS to conus glioma lesion in two separate treatments to L1-L and to T11-T12 level. This patient also had prior surgical resection followed by EBRT of 60 Gy in 48 fractions to L1-L2 spine 10 years before SRS.
Limitations of this study include those that are inherent in retrospective analyses. We found 2 cases of long-term toxicity, but may need even longer followup to make definitive conclusions on the potential toxicities although the probability of serious toxicities such as radiation myelopathy following spine SRS is relatively low.[31] Another limitation of our study is the small sample size, which is difficult to overcome due to the low incidence of primary spine tumors with diverse tumor types. Though larger studies are needed, to the best of our knowledge, our series is one of the largest to date focusing on the response of primary spine and spinal cord tumors to SRS, treated at a single institution with treatment uniformity and consistent reporting of clinical responses.
CONCLUSION
The results of pain relief, neurological improvement, and local control rates suggest that SRS is effective in the treatment of primary spine tumors. SRS can be used as a definitive treatment or as an adjuvant to surgical resection. Though we are encouraged by these results, studies with larger cohorts of patients, and prospective clinical trials would help to better establish a definitive treatment regimen with substantial advantages over other treatments. In addition to considering the individual patient’s goals, functional status, and histology, we caution that healthcare providers use their clinical acumen, prior experiences, and the existing literature when determining the best plan of treatment.
Acknowledgements
The study was funded by SRS Education and Research Grant, Grant Number M60287 of the Department of Radiation Oncology, Henry Ford Hospital.
Authors’ disclosure of potential conflicts of interest
Dr. Ian Lee serves as a consultant for Medtronic and received a speaker honorarium from Varian Medical Systems, Inc. The remaining authors have nothing to disclose.
Author contributions
Conception and design: Erinma Elibe, BS, David Boyce-Fappiano, BSc, Samuel Ryu, MD, M. Salim Siddiqui, MD, PhD, Ian Lee, MD, Jack Rock, MD, Farzan Siddiqui, MD, PhD
Data collection: Erinma Elibe, BS, David Boyce-Fappiano, BSc Samuel Ryu, MD, M. Salim Siddiqui, MD, PhD, Ian Lee, MD, Jack Rock, MD, Farzan Siddiqui, MD, PhD
Data analysis and interpretation: Erinma Elibe, BS, David Boyce-Fappiano, BSc, Farzan Siddiqui, MD, PhD
Manuscript writing: Erinma Elibe, BS, David Boyce-Fappiano, BSc, Farzan Siddiqui, MD, PhD
Final approval of manuscript: Erinma Elibe, BS, David Boyce-Fappiano, BSc, Samuel Ryu, MD, M. Salim Siddiqui, MD, PhD, Ian Lee, MD, Jack Rock, MD, Farzan Siddiqui, MD, PhD
References
- 1. Schellinger KA, Propp JM, Villano JL, McCarthy BJ: Descriptive epidemiology of primary spinal cord tumors. Journal of neuro-oncology 2008, 87(2):173-179. [DOI] [PubMed] [Google Scholar]
- 2. Ropper AE, Cahill KS, Hanna JW, McCarthy EF, Gokaslan ZL, Chi JH: Primary vertebral tumors: a review of epidemiologic, histological, and imaging findings, Part I: benign tumors. Neurosurgery 2011, 69(6):1171-1180. [DOI] [PubMed] [Google Scholar]
- 3. Ropper AE, Cahill KS, Hanna JW, McCarthy EF, Gokaslan ZL, Chi JH: Primary vertebral tumors: a review of epidemiologic, histological and imaging findings, part II: locally aggressive and malignant tumors. Neurosurgery 2012, 70(1):211-219; discussion 219. [DOI] [PubMed] [Google Scholar]
- 4. Duong LM, McCarthy BJ, McLendon RE, Dolecek TA, Kruchko C, Douglas LL, Ajani UA: Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004-2007. Cancer 2012, 118(17):4220-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong DA, Fornasier VL, MacNab I: Spinal metastases: the obvious, the occult, and the impostors. Spine 1990, 15(1):1-4. [PubMed] [Google Scholar]
- 6. Mukherjee D, Chaichana KL, Gokaslan ZL, Aaronson O, Cheng JS, McGirt MJ: Survival of patients with malignant primary osseous spinal neoplasms: results from the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2003. Journal of neurosurgery Spine 2011, 14(2):143-150. [DOI] [PubMed] [Google Scholar]
- 7. Engelhard HH, Villano JL, Porter KR, Stewart AK, Barua M, Barker FG, Newton HB: Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equina. Journal of neurosurgery Spine 2010, 13(1):67-77. [DOI] [PubMed] [Google Scholar]
- 8. Mukherjee D, Chaichana KL, Adogwa O, Gokaslan Z, Aaronson O, Cheng JS, McGirt MJ: Association of extent of local tumor invasion and survival in patients with malignant primary osseous spinal neoplasms from the surveillance, epidemiology, and end results (SEER) database. World neurosurgery 2011, 76(6):580-585. [DOI] [PubMed] [Google Scholar]
- 9. Clarke MJ, Mendel E, Vrionis FD: Primary spine tumors: diagnosis and treatment. Cancer control : journal of the Moffitt Cancer Center 2014, 21(2):114-123. [DOI] [PubMed] [Google Scholar]
- 10. Clarke MJ, Dasenbrock H, Bydon A, Sciubba DM, McGirt MJ, Hsieh PC, Yassari R, Gokaslan ZL, Wolinsky JP: Posterior-only approach for en bloc sacrectomy: clinical outcomes in 36 consecutive patients. Neurosurgery 2012, 71(2):357-364; discussion 364. [DOI] [PubMed] [Google Scholar]
- 11. Tehranzadeh J, Tao C, Browning CA: Percutaneous needle biopsy of the spine. Acta radiologica (Stockholm, Sweden : 1987) 2007, 48(8):860-868. [DOI] [PubMed] [Google Scholar]
- 12. Chi JH, Sciubba DM, Rhines LD, Gokaslan ZL: Surgery for primary vertebral tumors: en bloc versus intralesional resection. Neurosurgery clinics of North America 2008, 19(1):111-117. [DOI] [PubMed] [Google Scholar]
- 13. Schultheiss TE, Kun LE, Ang KK, Stephens LC: Radiation response of the central nervous system. International journal of radiation oncology, biology, physics 1995, 31(5):1093-1112. [DOI] [PubMed] [Google Scholar]
- 14. Boriani S, Saravanja D, Yamada Y, Varga PP, Biagini R, Fisher CG: Challenges of local recurrence and cure in low grade malignant tumors of the spine. Spine 2009, 34(22 Suppl):S48-57. [DOI] [PubMed] [Google Scholar]
- 15. Jin JY, Chen Q, Jin R, Rock J, Anderson J, Li S, Movsas B, Ryu S: Technical and clinical experience with spine radiosurgery: a new technology for management of localized spine metastases. Technology in cancer research & treatment 2007, 6(2):127-133. [DOI] [PubMed] [Google Scholar]
- 16. Ryu S, Jin R, Jin JY, Chen Q, Rock J, Anderson J, Movsas B: Pain control by image-guided radiosurgery for solitary spinal metastasis. Journal of pain and symptom management 2008, 35(3):292-298. [DOI] [PubMed] [Google Scholar]
- 17. Ryu S, Rock J, Rosenblum M, Kim JH: Patterns of failure after single-dose radiosurgery for spinal metastasis. Journal of neurosurgery 2004, 101 Suppl 3:402-405. [PubMed] [Google Scholar]
- 18. Schipani S, Wen W, Jin JY, Kim JK, Ryu S: Spine radiosurgery: a dosimetric analysis in 124 patients who received 18 Gy. International journal of radiation oncology, biology, physics 2012, 84(5):e571-576. [DOI] [PubMed] [Google Scholar]
- 19. Gerszten PC, Burton SA, Ozhasoglu C, Welch WC: Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine 2007, 32(2):193-199. [DOI] [PubMed] [Google Scholar]
- 20. Guckenberger M, Mantel F, Gerszten PC, Flickinger JC, Sahgal A, Letourneau D, Grills IS, Jawad M, Fahim DK, Shin JH, Winey B, Sheehan J, Kersh R: Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: a multi-institutional analysis. Radiation oncology (London, England) 2014, 9(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang UK, Lee DH, Kim MS: Stereotactic radiosurgery for primary malignant spinal tumors. Neurological research 2014, 36(6):597-606. [DOI] [PubMed] [Google Scholar]
- 22. Ryu S, Biondo A, Rock J, Gates M, Abdalhak M: Stereotactic Radiosurgery of Primary Spine and Spinal Cord Tumors. Journal of Radiosurgery and SBRT 2013, 2:127-133. [PMC free article] [PubMed] [Google Scholar]
- 23. Rock JP, Ryu S, Yin FF: Novalis radiosurgery for metastatic spine tumors. Neurosurgery clinics of North America 2004, 15(4):503-509. [DOI] [PubMed] [Google Scholar]
- 24. Ryu S, Fang Yin F, Rock J, Zhu J, Chu A, Kagan E, Rogers L, Ajlouni M, Rosenblum M, Kim JH: Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer 2003, 97(8):2013-2018. [DOI] [PubMed] [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J: New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer (Oxford, England : 1990) 2009, 45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 26. Ropper AH, Samuels MA, Klein JP: Approach to the patient with neurologic disease. In: Adams and Victor’s Principles of Neurology. 10th edn. Edited by Ropper AH, Samuels MA, Klein JP. New York: McGraw-Hill; 2014. [Google Scholar]
- 27. Boyce-Fappiano D, Elibe E, Schultz L, Ryu S, Siddiqui MS, Chetty I, Lee I, Rock J, Movsas B, Siddiqui F: Analysis of the Factors Contributing to Vertebral Compression Fractures After Spine Stereotactic Radiosurgery. International journal of radiation oncology, biology, physics 2017, 97(2):236-245. [DOI] [PubMed] [Google Scholar]
- 28. Dodd RL, Ryu MR, Kamnerdsupaphon P, Gibbs IC, Chang SD, Jr., Adler JR, Jr.: CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery 2006, 58(4):674-685; discussion 674-685. [DOI] [PubMed] [Google Scholar]
- 29. Saraceni C, Ashman JB, Harrop JS: Extracranial radiosurgery--applications in the management of benign intradural spinal neoplasms. Neurosurgical review 2009, 32(2):133-140; discussion 140-131. [DOI] [PubMed] [Google Scholar]
- 30. Bate BG, Khan NR, Kimball BY, Gabrick K, Weaver J: Stereotactic radiosurgery for spinal metastases with or without separation surgery. Journal of neurosurgery Spine 2015:1-7. [DOI] [PubMed] [Google Scholar]
- 31. Sahgal A, Weinberg V, Ma L, Chang E, Chao S, Muacevic A, Gorgulho A, Soltys S, Gerszten PC, Ryu S, Angelov L, Gibbs I, Wong CS, Larson DA: Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. International journal of radiation oncology, biology, physics 2013, 85(2):341-347. [DOI] [PubMed] [Google Scholar]
- 32. Boyce-Fappiano D, Elibe E, Ryu S, Siddiqui MSU, Wen N, Lee I, et al. Stereotactic radiosurgery for primary and metastatic sarcomas of the spine. Int J Radiat Oncol Biol Phys 2015;93(3)(Suppl):E632. [Google Scholar]