Abstract
Introduction
In Gamma Knife Radiosurgery (GKRS) of suprasellar lesions, the exact localization of the visual pathways is important to avoid radiation induced optic neuropathy (RION). Reliable identification of the optic nerve, chiasm and tracts can be challenging using routine magnetic resonance imaging, especially in patients with lesions compressing the optic structures or in patients who had prior operation of suprasellar tumors. This study investigates the application of inversion recovery sequences (Fast gray and white matter acquisition T1 inversion recovery, FGATIR) to improve identification of the optic pathway.
Methods
Inversion recovery sequences were performed on 5 healthy volunteers, varying their inversion times between 400 and 500 ms, and between 800 and 1100 ms. Inversion times were optimized to either suppress or to preserve the signal of the optic structures, while increasing or suppressing the signal of processes within the surrounding cisterns. Inversion recovery sequences were performed before radiosurgery on 10 patients with suprasellar tumors that were compressing or displacing the optic structures. Signal intensities of gray and white matter, of CSF and tumors were measured and subtraction images were calculated.
Results
Compared to a standard T1-weighted sequence, delineation of the visual pathways was superior on inversion recovery images, both on images with suppression of the optic structures as well on images with suppression of its surrounding tissues, and was rated best on subtraction images.
Conclusion
For radiosurgery of suprasellar tumors, inversion recovery sequences can be of valuable benefit for accurate delineation of optic pathway and radiosurgical dose planning in order to avoid radiation-induced normal tissue effects.
Keywords: inversion recovery sequences, optic pathways, radiosurgery, suprasellar lesions
Introduction
Radiation induced optic neuropathy (RION) is a major late complication in radiosurgery of peri-optical lesions, caused by irradiation of the visual pathway. RION results in acute and irreversible, unilateral or bilateral visual loss. Onset of RION occurs between 10-20 months, with an average of 18 months after treatment; onset may range from 3 months to 9 years.12 Single doses to parts of the anterior visual pathway larger than 10 12 Gy are usually required for RION to develop.1,13 Reliable identification of the optic nerves, chiasm and optic tracts is important in preoperative planning of gamma knife radiosurgery for tumors and other lesions in the suprasellar space to avoid radiogenic damage to the optic system. Often, lesions such as pituitary adenomas, perisellar meningiomas, or craniopharyngiomas are in direct contact with or displace parts of the optic system. Due to the steep dose gradient in GKRS, a spatial variation of as little as 1 mm may differ the dose by up to more than 10 Gy, particularly in hormone secreting adenomas that require large treatment doses. Because of the high spatial selectivity of stereotactic radiosurgery, the radiation dose received by these structures can be limited to a tolerable maximum point dose below 12 Gy in most instances,1 or alternatively hypofractionated stereotactic radiosurgery may be applied, as well with careful limitation of the dose to the optic apparatus.2 To achieve this restriction, the course and extension of the optic nerve, chiasm and tract have to be defined as precisely as possible. This is usually performed on non-contrast-enhanced high-resolution T1 weighted images. However, in extensive space-occupying lesions surrounding, compressing and displacing the optic structures as in cases of prior subtotal resection or recurrence, identification is not always possible, especially in lesions that involve high-signal areas such as hemorrhages or fluid-containing cysts or areas with signal intensities similar to the optic system.
To overcome these difficulties, we investigated the possibilities of a fast gray matter acquisition T1 inversion recovery sequence (FGATIR) which acquires high-resolution 3D images.3 Due to a 180° inversion pulse and an inversion time (TI) of 400 ms, the signal of white matter (WM) structures is suppressed.4 By increasing the inversion time, we also explored the opposite approach: to nullify the signal of surrounding tumor tissue, which usually contains larger quantities of water, without affecting the signal of white matter and optic structures. A similar approach was used by Bydder and Young in an early MRI study to differentiate between tumor and brain tissue.5
Material and Methods
This prospective study has been approved by the local ethics committee. Informed consent had been given by all participants.
Imaging was performed on a 3T scanner (Philips Achieva, Best, Netherlands).
Volunteers
In 5 healthy volunteers (all males, age range 22 72 years), we applied inversion recovery (IR) sequences with TIs of 400 ms, 450 ms and 500 ms for signal suppression of the optic structures, and between 800 and 1100 ms to look for the best parameters for suppression of the surroundings without suppressing the optic nerve, chiasm and tract.
Patients
In 10 patients (7 females, 3 males, age range 34 67 years) with suprasellar lesions, 8 of them pituitary adenomas, 1 meningioma and 1 cystic craniopharyngioma, with no, modest or severe visual impairment, the above-mentioned sequences were applied in addition to the usual MRI protocol for planning of Gamma Knife radiosurgery.
Magnetic Resonance Imaging
To volunteers, we applied a high-resolution 3D turbo gradient echo T1-weighted sequence (TR 20 ms, TE 2.1 ms, flip angel 20°, reconstructed voxel size 1x1x1 mm, with an inversion prepulse of a T1 delay of 399.1 ms, optimized for tissue contrast), and the following 3D IR sequences: TI of 400, 450 and 500 ms to achieve maximal fat suppression, and TIs of 800, 900, 1000 and 1100 ms to achieve maximal suppression of the signal of the suprasellar cistern and its contents, while preserving the signal of the optic nerve, chiasm and tract. Other parameters of these sequences were: TR 5.7 ms, TE 2.6 ms, shot interval 3000 ms, flip angle 8°, reconstructed voxel size 1x1x1 mm, acquisition time 10 min 20 s. The T1-weighted and IR sequences were applied with exactly the same axial orientation to facilitate final co-registration.
In patients, the same sequences were applied, but based on the results in the control study; we only applied a TI of 400 ms for fat suppression and changed the longer TIs to 850, 900, 950 and 1000 ms.
Evaluation of data
In volunteers, signal intensities were measured from Regions of Interest (ROIs) placed in five consecutive slices into the anterior and posterior parts of the corpus callosum, in both putamina and in the anterior and posterior parts of the lateral ventricles in areas free from the choroid plexus. These ROIs were chosen, because meaningful consistent evaluation of the signal from ROIs placed in the optic nerve and chiasm failed due to the volume of these structures and large intersubject variability. In patients, the signal of the WM, both putamina and CSF was measured as described, and in addition the signal of the superior parts of the suprasellar lesion.
From these data, we calculated the Contrast Ratio (CR) as the quotient of the difference of signal from white matter and another structure as putamen, CSF or tumor, divided by the signal of the other structure.
Using the Gamma Knife workstation GammaPlan, Version 10 (Elekta, Stockholm, Sweden), images with signal suppression respective preservation of the optic structures were coregistrated and subtracted from one another (preservation minus suppression) in order to further increase the signal of these structures and to suppress the signal of its surroundings.
Finally, all patients’ images were reconstructed in the transversal, coronal and sagittal plane and were rated by two experienced neuroradiologists (PSt and RF) with respect to the delineation of the optic nerve, chiasm and tract on a 3-level scale (moderate=3, good=2 and excellent=1). Patients’ images were also compared to the T1 MPRAGE images measured during the preceeding routine diagnostic MR examination.
Results
a. Volunteers
Evaluation of the scans acquired from healthy volunteers with different TIs between 400 and 500 ms to suppress signal of the optic nerve, chiasm and tracts, showed best WM signal suppression and highest CR values at a TI of 400 ms (Table 1).
Table 1.
Change of CR of signal intensity of white matter (WM) vs. putamen or CSF in sequences with different inversion times (TI) and in standard T1-weighted sequence in 5 healthy volunteers
| TI | 400 ms | 450 ms | 500 ms | 800 ms | 900 ms | 1000 ms | 1100 ms | T1 standard |
| CR: (WM - Putamen) / Putamen | 82.9 ± 2.2 | 68.8 ± 11.8 | 16.8 ± 6.1 | 126.6 ± 20.9 | 76.5 ± 9.3 | 54.7 ± 6.3 | 41.7 ± 5.6 | 20.2 ± 1.8 |
| CR: (WM - CSF) / CSF | 87.1 ± 1.0 | 82.4 ± 5.4 | 64.2 ± 8.6 | 154.3 ± 35.3 | 321.9 ± 36.5 | 613.1 ± 65.7 | 1124.8 ± 305.9 | 228.2 ± 47.1 |
Using TIs between 800 and 1100 ms to suppress the signal of suprasellar structures with preservation of the signal of the WM showed a signal increase of WM and gray substance with increasing TI and a progressive signal drop in CSF. The CR between WM and putamen decreased and the CR between WM and CSF increased with increasing TI. Inspection of these images showed in all 5 volunteers a higher signal within the chiasm and tract as compared to more rostral, but still intracranial parts of the optic nerve.
The standard T1 weighted images showed the lowest CR between WM and putamen and the second lowest CR between WM and CSF for all sequences applying a TI in the upper range.
Based on these results in volunteers and in order to look for conditions which allow us to achieve a robust signal suppression the optic structures or of CSF and solid parts within the suprasellar space, we imaged patients with a TI of 400 ms for the suppression condition and TIs of 850, 900, 950 and 1000 ms for suppression of signal in the suprasellar space.
b. Patients
Similar to the results in volunteers, signal intensity of putamen and WM increased and signal intensity of CSF dropped with increasing TI in patients, and the course of the signal in tumors followed that of the putamen and WM (Fig. 1a). CR of WM and putamen increased and that of WM and CSF or tumor decreased with increasing TI (Table 2, Fig. 1b).
Figure 1.
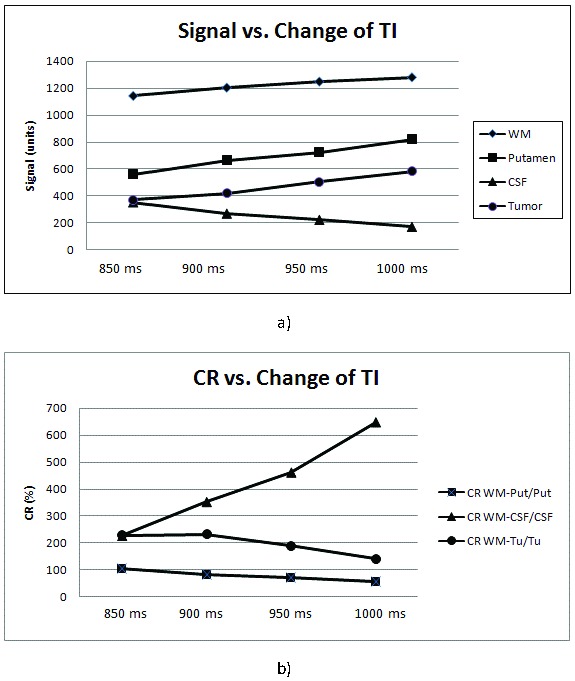
Change of signal intensity (a) and contrast ratio CR (b) with different inversion times
Table 2.
Change of CR of signal intensity of white matter (WM) vs. putamen, CSF or tumor in sequences with different inversion times (TI) and in standard T1-weighted sequence and rating of delineation quality of optic structures in 10 patients with suprasellar tumors.
| TI | 400 ms | 850 ms | 900 ms | 950 ms | 1000 ms | T1 standard |
| CR: (WM-Putamen) / Putamen | 80.5 ± 9.7 | 105.5± 22.8 | 82.3 ± 14.8 | 72.8 ± 16.8 | 56.6 ± 10.9 | 20.4 ± 4.0 |
| CR: (WM – CSF) / CSF | 86.7 ± 5.0 | 225.5 ± 17.3 | 351.0 ± 59.4 | 460.2 ± 42.3 | 646.2 ± 55.3 | 203.3 ± 31.8 |
| CR: (WM-Tumor) / Tumor | 85.5 ± 5.4 | 227.8 ± 23.2 | 230.7 ± 159.9 | 187.4 ± 154.3 | 139.8 ± 80.8 | 40.6 ± 26.3 |
| Deliniation of optic structures (rating) | 2.8 | 2.05 | 2.2 | 1.7 | 1.1 | 1.5 |
- GKRS: Gamma Knife Radiosurgery
- FGATIR: fast gray matter acquisition T1 inversion recovery sequence
- IR: inversion recovery
- CR: Contrast Ratio
- MP2RAGE: magnetization-prepared two rapid acquisition gradient echoes
- MPnRAGE: magnetization-prepared n rapid acquisition gradient echoes
Rating of the quality of delineation of optic nerves, chiasm and tracts gave best results for a TI of 950 ms, although the CR between WM and tumor was slightly higher at 900 ms as compared to 950 ms.
The sequences with fat suppression were regarded to be nearly as helpful in delineating the exact course of the optic system as the images with suppression of the surrounding tumor tissue and cystic components. In all cases, the subtraction images (image with preservation minus image with suppression of the signal of the optic structures) were regarded superior to the original images. In this way, most components of the optic structures could be delineated well in all but one patient (Fig. 2 and 3). In all instances, T1 weighted images without fat or basal cistern suppression gave inferior CR.
Figure 2.
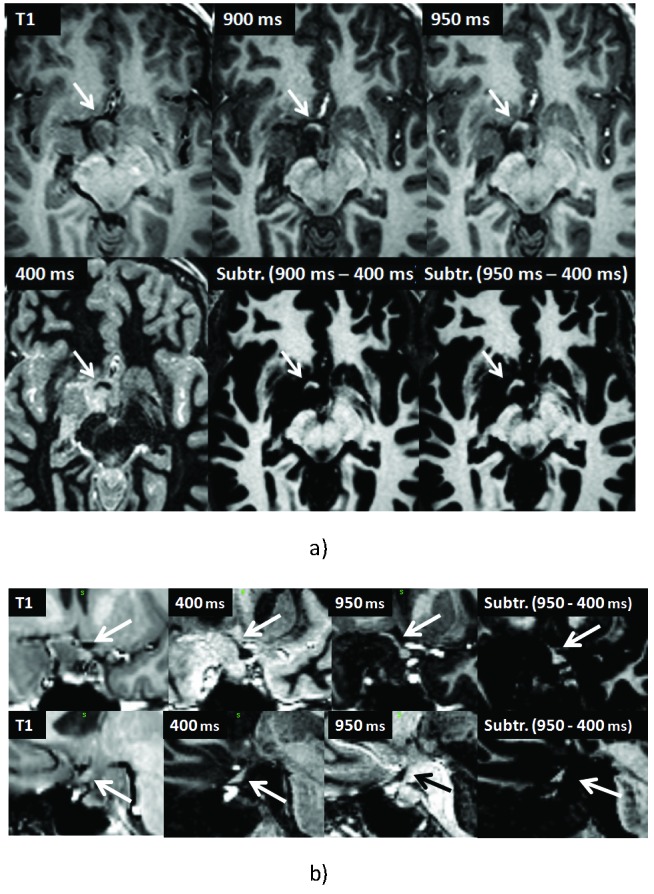
39-year-old female with pituitary adenoma displacing the optic nerve, chiasm and tracts (arrows). Transversal (a), coronal (b, upper row) and sagittal (lower row) T1-weighted and IR and subtraction images of different TIs as indicated.
Figure 3.
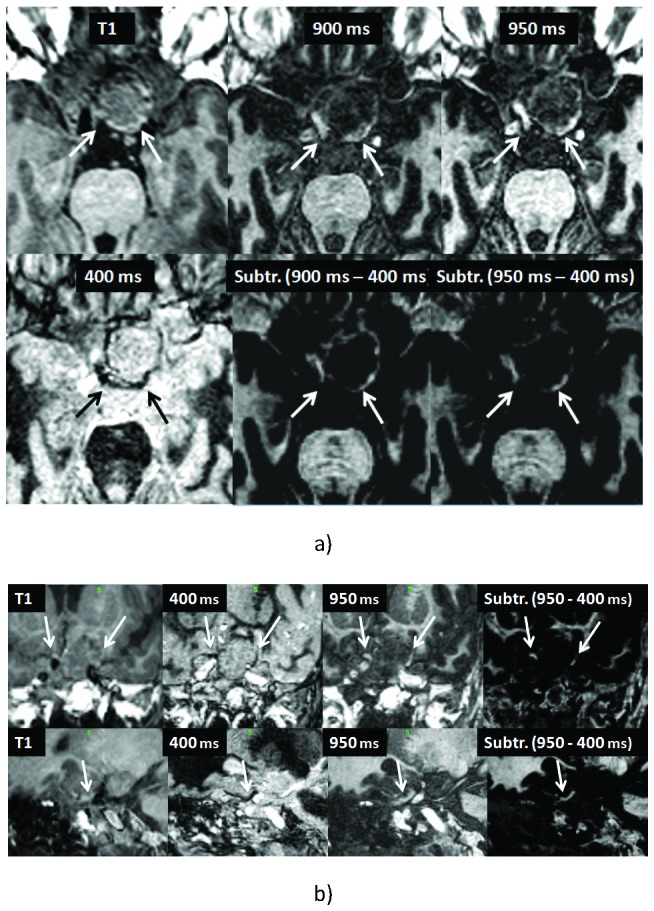
42-year-old male with pituitary adenoma displacing the optic nerve, chiasm and tracts (arrows). Transversal (a), coronal (b, upper row) and sagittal (lower row) T1-weighted and IR and subtraction images of different TIs as indicated.
Discussion
In clinical routine conventional T1-weighted sequences are applied to identify the visual system. Non contrast enhanced T1-weighted images present a high tissue contrast against surrounding structures, because of the relatively high fat concentration of the optic nerve, chiasm and tracts. Contrast enhanced T1-weighted images are used in order to distinguish contrast enhancing lesions from the non contrast enhancing optical system.
In all but one patient with suprasellar lesions, most sections of the optic nerve, chiasm and tracts could be identified on conventional T1-weighted sequences. However, the delineation of some parts of the optic system was only possible by application of IR sequences with different TIs. Those parts, which were not visible on the original T1-weighted images due to severe compression or displacement, were segments in direct neighborhood to the lesions, where exact planning of the radiation field was highly critical to avoid delivery of high doses to the optic system.
Within the group of images achieved with TIs between 850 and 1000 ms, a TI of 950 ms was rated to be superior in most instances to delineate the optic structures, although the CR between WM and tumor was slightly higher in images acquired with a TI of 900 ms. In retrospect, this discrepancy was commented by the raters to be due to the higher signal of the optic structures in the images achieved at this TI, which was regarded as an important factor in the process of delineation.
Although the sequences with different TIs worked well in 9 cases, there was one exception, where only parts of the optic structures could be identified. In this case of a craniopharyngioma, tumor compression or previous operation might have destroyed part of the nerve and tract because the patient was not totally blind. Parts of the system may have been preserved and allowed vision within one quadrant of the visual field, in spite of our failure to demonstrate nerve continuity with any of the special MR sequences.
The question remains, if in those cases, where parts of the optic nerve, chiasm or tract have been destroyed, IR sequences with a slightly longer TI than 400 ms would have been more effective in suppressing the optic system, because chronic compression might have reduced the number of intact myelin sheaths and replaced it by tissue with a higher content of water. In healthy volunteers, a TI of 400 ms gave best results for fat suppression within WM areas, thus confirming the results of the previous FGATIR study .4
Apart from these pathologic alterations, higher water content has been reported in the optic structures, which amounts to 90% within axons of the optic nerve as compared to the 33-55% of water contained in the cerebral white matter fibers.6 Another factor that may influence the signal of the these structures, mainly its increase from the nerve towards the chiasm, may be the content of cholesterol, which in animal studies had been shown to increase from the nerve to the optic tract from 2.8 to 5.0 mg per 100 mg wet weight.7 Cholesterol has been noted to be the “determinant of gray-white matter contrast in MRI of the brain”.8
As far as suppression of signal from surrounding tumor tissue and other contents of the suprasellar cistern is concerned, the TI value of 950 ms was found empirically by evaluation of the results of 10 patients. In most cases, signal suppression from the tumor increased with increasing TI and thus followed the signal change of CSF, but varied from lesion to lesion.9 Again assuming a higher water content within affected, but not completely destroyed optic structures, one could speculate that a reduction of the TI below 950 ms could improve the contrast between the reduced intensity of the lesion and the preserved signal of the optic apparatus in some cases.
The FGATIR approach to use an inversion preparation with short TI to null white matter signal in a high resolution gradient echo image permits to depict deep brain structures with high contrast.4 Due to the short TI, fat is partially suppressed within the gradient echo acquisition block. However, at the given TI and shot interval (400 and 3000 ms respectively) the GM and CSF signals are opposite to the remaining fat signal which can result in partial volume effects. In deep brain regions this effect is thanks to only amounts of fat, unless pathological conditions alter this condition.
On the other hand, using a long TI is essentially the classic MPRAGE condition were the joint effects of shot length, TI and saturation within the gradient echo acquisition block are used to null the CSF signal while maximizing WM/GM contrast. When care has been taken to acquire images in both conditions with identical parameters, apart from the different TIs, the combination of both images can further improve the ability to depict otherwise difficult to distinguish deep brain structures.
This method is basically the MP2RAGE approach10 or generalizing MPnRAGE.11 However these sequences were not available to us. The separate acquisition of multiple TI images implies a longer total scanning time, but with the benefit of improved contrast in the short TI images due to the effect that magnetization is permitted to relax. The simple image subtraction can unite the advantages of both conditions, which in our study achieved the best results to depict the optic system.
Conclusion
During the planning of Gamma Knife radiosurgery of perioptic lesions, exact localization of the visual pathways is critical in order to avoid application of high doses that may cause radiogenic damage to the optic system. In cases of large or preoperated suprasellar lesions, in which identification of the optic nerve, chiasm and tract can be challenging using conventional T1w sequences, IR sequences with different TIs can be helpful to better delineate these structures, either by suppressing the signal of the optic system or by suppressing the signal of its surroundings. Subtraction images may contribute to automatic segmentation methods of the optic pathway in future developments, not only limited to radiosurgery.
Acknowledgments
Authors’ disclosure of potential conflicts of interest
The authors reported no conflict of interest.
Author contributions
Conception and design: Herwin Speckter, Peter Stoeter
Data collection: Herwin Speckter, José Bido, Giancarlo Hernandez, Diones Rivera, Luis Suazo, Santiago Valenzuela, Rafael Fermin, Jairo Oviedo, Bernd Foerster, Cesar Gonzalez, Peter Stoeter
Data analysis and interpretation: Herwin Speckter, José Bido, Giancarlo Hernandez, Diones Rivera, Luis Suazo, Santiago Valenzuela, Rafael Fermin, Jairo Oviedo, Bernd Foerster, Cesar Gonzalez, Peter Stoeter
Manuscript writing: Herwin Speckter, Peter Stoeter
Final approval of manuscript: Herwin Speckter, José Bido, Giancarlo Hernandez, Diones Rivera, Luis Suazo, Santiago Valenzuela, Rafael Fermin, Jairo Oviedo, Bernd Foerster, Cesar Gonzalez, Peter Stoeter
References
- 1. Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J: Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76;(Suppl):28-35 [DOI] [PubMed] [Google Scholar]
- 2. Liao HI, Wang CC, Wei KC, et al. : Fractionated stereotactic radiosurgery using the Novalis system for the management of pituitary adenomas close to the optic apparatus. J Clin Neurosci. 2014;21:111-5 [DOI] [PubMed] [Google Scholar]
- 3. Zhang B, MacFadden D, Damyanovich AZ, et al. : Development of a geometrically accurate imaging protocol at 3 Tesla MRI for stereotactic radiosurgery treatment planning. Phys Med Biol. 2010;55:6601-15 [DOI] [PubMed] [Google Scholar]
- 4. Sudhyadhom A, Haq IU, Foote KD, Okun MS, Bova FJ: A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR). Neuroimage. 2009;47(Suppl):44-52 [DOI] [PubMed] [Google Scholar]
- 5. Bydder GM, Young IR: MR imaging: clinical use of the inversion recovery sequence. J Comput Assist Tomogr. 1985;9:659-75 [PubMed] [Google Scholar]
- 6. LoPachin RM, Castiglia CM, Saubermann AJ: Elemental composition and water content of myelinated axons and glial cells in rat central nervous system. Brain Res. 1991;549:253-9 [DOI] [PubMed] [Google Scholar]
- 7. Friede RL, Miyaghishi T, Hu KH: Axon calibre, neurofilaments, microtubules, sheath thickness and cholesterol in cat optic nerve fibres. J Anat. 1971;108:365-73 [PMC free article] [PubMed] [Google Scholar]
- 8. Koenig SH: Cholesterol of myelin is the determinant of gray-white contrast in MRI of brain. Magn Reson Med. 1991;20:285-91 [DOI] [PubMed] [Google Scholar]
- 9. Sheppard S, Davis PC, Kater G, Peterson JE: Inversion-recovery echo-planar MR in adult brain neoplasia. AJNR Am J Neuroradiol. 1998;19:267-73 [PMC free article] [PubMed] [Google Scholar]
- 10. Tanner M, Gambarota G, Kober T, et al. : Fluid and white matter suppression with the MP2RAGE sequence. J Magn Reson Imaging. 2012;35:1063-70 [DOI] [PubMed] [Google Scholar]
- 11. Kecskemeti S, Samsonov A, Hurley SA, Dean DC, Field A, Alexander AL: MPnRAGE: A technique to simultaneously acquire hundreds of differently contrasted MPRAGE images with applications to quantitative T1 mapping. Magn Reson Med. 2016;75:1040-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Danesh-Meyer HV: Radiation-induced optic neuropathy. J Clin Neurosci. 2008; 15:95-100. [DOI] [PubMed] [Google Scholar]
- 13. Leavitt JA, Stafford SL, Link MJ, Pollock BE. Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2013;87:524-7 [DOI] [PubMed] [Google Scholar]


