Summary
Objective
This multicenter, randomized, controlled, open‐label trial examined weight‐related quality of life, control over eating behaviour and sexual function after 26 weeks of treatment with either 32 mg naltrexone sustained release (SR)/360 mg bupropion SR plus a comprehensive lifestyle intervention program (NB + CLI, N = 153) or usual care (UC, N = 89), which included minimal lifestyle intervention.
Methods
Impact of Weight on Quality of Life‐Lite, Binge Eating Scale and Arizona Sexual Experiences Scale were assessed at baseline (BL) and weeks 16 and 26.
Results
NB + CLI and UC participants lost 9.46 and 0.94% respectively of initial body weight at week 26 (P < 0.0001). NB + CLI participants had greater improvements in Impact of Weight on Quality of Life‐Lite total score than UC participants (P < 0.0001). In participants with moderate/severe Binge Eating Scale scores at BL, 91% of NB + CLI and 18% of UC participants experienced categorical improvements. In participants with Arizona Sexual Experiences Scale‐defined sexual dysfunction at BL, 58% of NB + CLI and 19% of UC participants no longer met dysfunction criteria at week 26. The most frequent adverse events leading to discontinuation before week 26 in NB + CLI included nausea (10.5%); anxiety (3.3%); and headache, hypertension, insomnia and palpitations (1.3% each).
Conclusion
Compared with UC, participants treated with NB + CLI experienced greater improvements in weight‐related quality of life, control over eating behaviour, and sexual function.
Keywords: Contrave®, Mysimba®, IWQOL‐Lite, Binge Eating Scale
Introduction
Obesity is an important public health issue not only because of its prevalence (over two‐thirds of US and half of European adults are overweight or have obesity) 1, 2 but also because of the global impact it has on an individual's physical health and quality of life. In studying weight loss intervention therapies, patient‐reported outcome (PRO) data can complement weight data by providing information related to the patient experience and perspective on additional benefits or limitations of an intervention.
Quality of life, control over eating behaviour and issues related to sexual function are three topics of relevance to a population with overweight or obesity. Studies have demonstrated that as body mass index (BMI) increases, weight‐related quality of life scores diminish; similarly, reductions in weight have been shown to improve these outcomes in patients with obesity 3, 4, 5. Binge eating and control over eating behaviour are additional issues of importance to individuals with overweight or obesity. While the lifetime prevalence of binge eating disorder in the USA is estimated at 2.6% 6, estimates of up to approximately 30% have been reported for binge eating disorder (BED) in individuals seeking weight loss treatment, and evidence suggests that treatment with weight loss medications and participation in behavioural weight loss programs can increase control of eating and reduce binge eating symptoms 7, 8. Even in individuals who do not have BED, enhanced control over eating behaviour may be an important contributor to the efficacy of medications for weight management, so further information about patients' perspective of control may be informative. Obesity is also associated with greater impairment in sexual function 4, 9, 10, 11, and bupropion, when used as an antidepressant, has been reported to either have pro‐sexual effects or blunt anti‐sexual effects of other antidepressants 12; therefore, this study included the Impact of Weight on Quality of Life‐Lite (IWQOL‐Lite), Binge Eating Scale (BES) and Arizona Sexual Experiences Scale (ASEX) as pilot measures to determine if treatment affected weight‐related quality of life, self‐reported binge eating and eating control behaviours and sexual function.
The sustained release combination of naltrexone and bupropion (NB; Contrave®, Mysimba®) is approved in numerous countries, including the USA (2014) and European Union (2015), for the chronic treatment of obesity, as an adjunct to lifestyle modification. Evidence suggests that NB may reduce hunger through activation of pro‐opiomelanocortin neurons in the hypothalamus by bupropion while naltrexone blocks the negative feedback loop on pro‐opiomelanocortin neurons to maintain their activation 13. NB is believed to reduce cravings through effects on dopaminergic and opioid pathways that have been associated with feelings of pleasure while eating 14. NB has been evaluated in four pivotal, placebo‐controlled, double‐blind randomized phase 3 clinical trials 15, 16, 17, 18. In each of these trials, NB was safe, generally well tolerated and associated with significant weight loss and improvements in various secondary measures of cardiovascular risk as well as measures of weight‐related quality of life 19.
The purpose of the current clinical trial was to compare results from PROs assessing weight‐related quality of life, eating control behaviour and sexual function in participants treated with NB plus comprehensive lifestyle intervention (NB + CLI; treatment consistent with prescribing instructions) to outcomes in participants treated with general weight loss advice that a patient might receive from their physician [usual care (UC)]. This open‐label trial design approximated a real‐world setting, including two treatment options a patient would be likely to receive when seeking treatment for obesity.
Methods
Study design
This was a phase 3b, multicenter, randomized, open‐label, controlled trial designed to assess body weight and numerous PROs at 26 weeks in participants with obesity, or overweight with dyslipidemia and/or controlled hypertension who were treated with either 32 mg naltrexone sustained release (SR)/360 mg bupropion SR (NB) in conjunction with a CLI program, or UC (diet and exercise education from the study site). The trial had a total treatment duration of up to 78 weeks, including a 52‐week uncontrolled period after the initial 26‐week controlled period, where UC participants were switched to NB + CLI treatment and NB + CLI participants remained in the same treatment group. The collection of PROs was limited to the 26‐week controlled treatment period; therefore, the focus of this report is limited to the 26‐week controlled period. Weight loss, vital signs and obesity‐related risk factor data throughout the duration of this trial has been previously published 20.
Participants and eligibility criteria
Adult male and female participants, aged 18–60 years, had an initial BMI of 30–45 kg m−2 (obese) or 27–45 kg m−2 (overweight) with dyslipidemia and/or controlled hypertension were eligible for study inclusion. Key exclusion criteria, which was largely consistent with product labelling, included type 1 or 2 diabetes mellitus; myocardial infarction within 6 months prior to screening; angina pectoris grade III/IV (per the Canadian Cardiovascular Society grading scheme); clinical history of large vessel cortical strokes, including ischemic or hemorrhagic strokes (transient ischemic attack was not exclusionary); history (within 20 years prior to screening) of seizures, cranial trauma, bulimia, anorexia nervosa or other conditions that predispose participants to seizures; chronic use or positive screen for opioids; psychiatric conditions including mania, psychosis, acute depressive illness or suicide risk; and regular use of tobacco products. In contrast to phase 3 studies, patients with chronic depression were not excluded, even if they were currently taking antidepressant medication.
Lifestyle intervention programs
Comprehensive lifestyle intervention was a commercially available telephone‐based and internet‐based program that included a progressive nutrition and exercise program with individualized goal setting and both paper‐based and internet‐based tracking tools. Nutrition guidance was based on the Dietary Approaches to Stop Hypertension (DASH) diet 21, with recommendations to reduce calories by approximately 500 calories per day while focusing primarily on whole foods. Participants were encouraged to increase their exercise, primarily through walking, which was tracked using wireless activity monitors. Participants received up to 11 structured telephone calls, each lasting approximately 15 min, from a coach/dietician who was not otherwise affiliated with the trial during the first 26 weeks. Telephone calls were based on a pre‐set curriculum that was modelled after a Diabetes Prevention Program and Look AHEAD lessons 22, 23 and focused on topics such as reducing calories and healthy eating, increasing physical activity, managing stress, navigating difficult situations and rebounding from lapses. UC was a site‐based lifestyle intervention program intended to mimic the weight loss support that might typically be provided to patients in a primary care setting. UC participants were instructed at baseline and at week 10 to follow an exercise prescription that primarily consisted of walking and a hypocaloric diet, with a target daily caloric deficit of 500 kcal. UC participants were also given support tools such as a nutrition tracker, pedometer and healthy weight literature 24.
Treatment period (week 1 through week 26)
Following a screening period, participants were randomized in a 1.75:1 ratio to receive either open‐label active study medication (NB) along with CLI (NB + CLI) or UC. In accordance with prescribing information, participants randomized to NB + CLI initiated treatment with NB at a daily dose of 8 mg naltrexone/90 mg bupropion and escalated their dose over the subsequent 3 weeks. In addition, an evaluation of weight loss was performed in the NB + CLI group at week 16, with participants discontinued from NB if they had not lost at least 5% of their baseline body weight. Based on analysis of the phase 3 data, discontinuation of treatment at week 16 in the absence of at least a 5% reduction in body weight is part of the product labelling for NB 25. Additionally, participants were to discontinue NB if they experienced increases above baseline in systolic or diastolic blood pressure (BP) of ≥10 mm Hg at both week 10 and week 16.
Sample size determination
Based on the predicted difference in discontinuation rates and effect of a week 16 weight assessment, a randomization ratio of 1.75:1 was adopted. A total of 242 participants was sufficient to provide ≥90% power to detect a significant difference (α = 0.05) between the treatment groups at week 26 for the per protocol (PP) population using a two‐sample t‐test assuming a true mean difference of 0.6 common standard deviation. The assumptions are based on data from the NB phase 3 trials and publications pertaining to US for obesity 26, 27.
Study endpoints
Patient‐reported outcomes included the IWQOL‐Lite, BES and ASEX. The BES is not a diagnostic tool for binge eating disorder, as studies have shown poor concurrence between BES scores, other scales and various diagnostic criteria 28, 29, 30. Instead, it is a measure of behavioural manifestations and feelings and cognitions associated with binge eating, with higher scores indicating greater levels of binge eating behaviour 31. Total BES scores can range from 0 to 46 and are summarized categorically where scores less than or equal to 17 suggest that there is no significant binge eating, scores from 18 to 26 suggest moderate levels of binge eating and scores greater than or equal to 27 suggest severe levels of binge eating.
Arizona Sexual Experiences Scale is a measure of self‐assessed sexual function, with higher scores indicating greater dysfunction 32. This questionnaire was chosen because of its brevity and ease of use. It consists of five questions that quantify sex drive, arousal, vaginal lubrication/penile erection, ability to reach orgasm and satisfaction from orgasm. Except for one anatomically specific question, the questionnaire is identical for men and women. With a minimum value of 1 and a maximum value of 6 for each question, the total score for the questionnaire ranges from 5 to 30. The questionnaire, because of the limited number of questions, is easily completed in a timely manner. ASEX scores are correlated with clinician‐diagnosed dysfunction if any of the following criteria are met: (1) a total score of greater than or equal to 19, (2) any single section with a score of greater than or equal to 5 or (3) any three sections with individual scores greater than or equal to 4.
Impact of Weight on Quality of Life‐Lite consists of a total score, which measures overall weight‐related quality of life. The total score is composed of five domain scores: physical function, self‐esteem, sexual life, public distress and work 33. The total score and the subscale scores are transformed into a 0–100 scale where 100 represents the best quality of life. Clinically meaningful improvement in IWQOL‐Lite total score was defined according to a published algorithm as +7.7 to +12 points per participant, depending upon baseline severity of impairment 34. It is worth noting that the IWQOL‐Lite's ‘sexual life’ domain addresses enjoyment, avoidance and desire related to sexual activity as it relates to an individual's weight. The ASEX scale address specific aspects of sexual performance, outside of the context of an individual's weight, providing justification for the collection of the ASEX questionnaire in addition to collection of the IWQOL‐Lite questionnaire. PROs were measured at baseline (prior to receiving treatment) and while receiving treatment at week 16 and week 26. Additional outcomes collected in this trial, which have been summarized in a prior publication, included percent change in body weight, vital signs and obesity‐related risk factors 20.
Statistical analysis
The analysis of continuous variables was based on the adjusted least‐squares (LS) means estimated using analysis of covariance (ancova), using the week 26 PP population (defined as modified intent‐to‐treat participants in compliance with the protocol in the controlled treatment period), with no imputation of missing data. Categorical analyses for body weight were assessed using a logistic regression model with treatment group and baseline BMI category as factors and with baseline body weight as a covariate. Categorical analyses of BES and ASEX scores were performed using shift analysis and the Cochran–Mantel–Haenszel test comparing groups with baseline BMI category (<35, ≥35 to <40, ≥40 kg m−2) as a stratification factor. For treatment comparisons, LS mean with standard error of mean (SEM), LS mean difference and P value from ancova and odds ratio with 95% confidence interval from logistic regression are reported. Adverse events were summarized in the intent‐to‐treat (ITT) population.
Analyses were performed on observed data only. Because the analyses were performed in a PP analysis population, missing data were essentially non‐existent. All statistical tests were performed based on a two‐sided hypothesis test at the 0.05 level of significance. All statistical analyses were performed using SAS® software (Version 9.3, SAS Institute Inc., Cary, NC).
Results
Baseline characteristics and participant disposition
A total of 242 participants (ITT) were randomized and treated: 153 participants to the NB + CLI group and 89 participants to the UC group (Figure 1). Of the 242 randomized participants, the week 26 PP population included 153 participants (71 in the NB + CLI group and 82 in the UC group) who completed the 26‐week controlled treatment period, and were administered PROs. Participant demographics are summarized in Table 1. Within the ITT population, the most common reason for discontinuation from the NB + CLI group within the first 26 weeks was due to adverse events (n = 35 of 153, 22.9%), followed by discontinuation due to insufficient weight loss and/or elevated BP at week 16 [n = 32 of 153; 20.9% (n = 29 not satisfying the weight criteria alone, n = 1 had BP elevations that met the criteria for discontinuation alone and n = 2 were discontinued due to both weight and BP criteria)]. The most common reason for discontinuation from the UC group was lost to follow‐up (n = 5 of 89, 5.6%). It is important to note that unless otherwise specified, reported results for NB + CLI participants at week 26 exclude participants who did not lose at least 5% of their baseline body weight at week 16.
Figure 1.
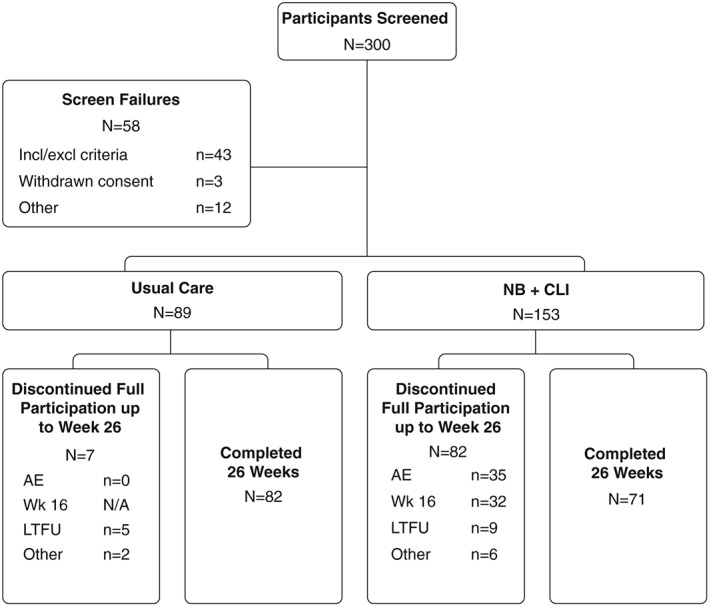
Participants and group assignments. A total of 242 intent‐to‐treat participants were randomly assigned 1.75:1 to NB + CLI and UC groups. AE, adverse event; LTFU, lost to follow‐up; Other, protocol deviation or withdrawal of consent; N/A, not applicable; CLI, comprehensive lifestyle intervention; NB, sustained release combination of naltrexone and bupropion; Wk 16, evaluation to continue treatment at week 16 visit.
Table 1.
Participant demographic and baseline characteristics
| Parameter | Usual care (N = 82) | NB + CLI (N = 71) |
|---|---|---|
| Age (years), mean (SD) | 48 (10) | 48 (9) |
| Sex (% female) | 87 | 80 |
| Race (%) | ||
| White | 74 | 86 |
| Black or African American | 24 | 14 |
| Other | 1 | 0 |
| Weight (kg), mean (SD) | 100 (17) | 102 (15) |
| IWQOL‐Lite total score, mean (SD) | 63.8 (16.5) | 67.1 (17.5) |
| BES total score, mean (SD) | 15.8 (8.6) | 15.0 (8.6) |
| % With moderate/severe problem | 41 | 32 |
| ASEX total score, mean (SD) | 16.3 (4.5) | 15.8 (4.1) |
| % With dysfunctional status | 45 | 35 |
Data are mean (SD) or percentage of participants.
ASEX, Arizona Sexual Experiences Scale; BES, Binge Eating Scale; CLI, comprehensive lifestyle intervention; IWQOL, Impact of Weight on Quality of Life; NB, sustained release combination of naltrexone and bupropion.
Summary of previously published results from this trial
As previously published, participants in the PP population treated with NB + CLI demonstrated a significant decrease in body weight compared with those in the UC group at week 26 (9.46% reduction in the NB + CLI group; 0.94% reduction in the UC group; 8.52% difference, P < 0.0001). Consistent with the greater percentage body weight reduction observed in the NB + CLI group, significantly more NB + CLI participants achieved ≥5% weight loss than UC participants at week 26 [84.5% NB + CLI vs. 12.2% UC (P < 0.0001)]. Similarly, significantly more NB + CLI participants achieved ≥10% weight loss than UC participants at week 26 [42.3% NB + CLI vs. 3.7% UC (P < 0.0001)]. In comparison with participants in the UC group, participants in the NB + CLI group also had significantly greater improvements in obesity‐related risk factors including waist circumference, triglycerides, glucose, insulin, a measure of insulin resistance (HOMA‐IR) and high‐density lipoprotein cholesterol 20.
Treatment with NB + CLI led to greater improvements in Impact of Weight on Quality of Life‐Lite
At both week 16 and 26, compared with participants treated with UC, participants in the NB + CLI group had a significantly greater increase in IWQOL‐Lite total score (LS mean difference at week 16: 14.6, P < 0.0001; week 26: 17.4, P < 0.0001) (Figure 2a). By week 26, 67% of NB + CLI‐treated participants achieved a clinically meaningful improvement in IWQOL‐Lite total score, vs. 20% of UC participants (odds ratio 8.17, P < 0.0001). A significant increase in score was also observed across all five IWQOL‐Lite domains, with the largest increases in the physical function, self‐esteem and sexual life subscales (P < 0.0001) (Figure 2b). In general, improvement in IWQOL‐Lite total score was greater with greater weight loss in the NB + CLI group (Figure 2c).
Figure 2.
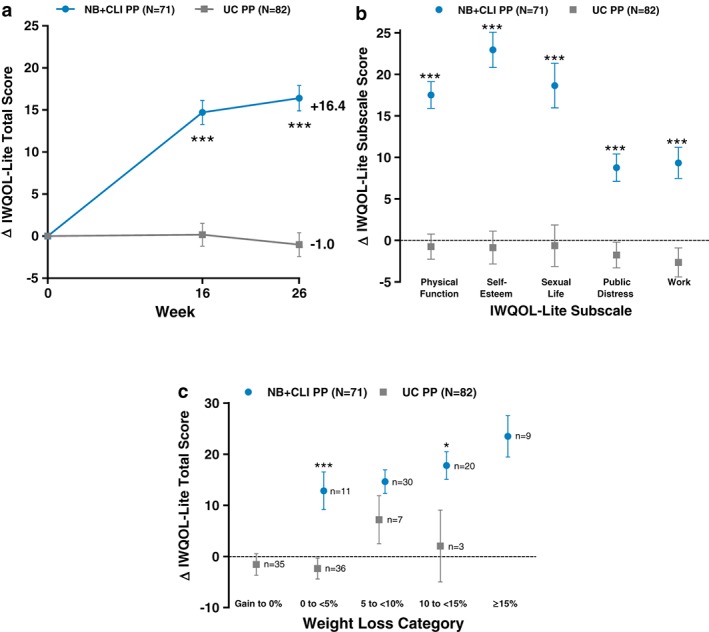
Change in Impact of Weight on Quality of Life‐Lite (IWQOL‐Lite). (a) Change in total score from baseline at weeks 16 and 26. (b) Change in IWQOL‐Lite subscale scores from baseline to week 26. Change in score was greater in NB + CLI than UC for all subscales. (c) Change in total score from baseline to week 26 by weight loss category. Change in IWQOL‐Lite total score was significantly greater in NB + CLI than UC for weight loss categories of 0 to 5% (P = 0.0004) and 10 to <15% (P = 0.0384). For all panels, points represent least square means and error bars are standard error, *P < 0.05, ***P < 0.001 (NB + CLI vs. UC).
Treatment with NB + CLI led to greater reductions in binge eating
The BES was used to assess patient‐reported control over eating behaviour. Mean (SD) score at baseline of 15.0 (8.6) in NB + CLI and 15.8 (8.6) in UC participants indicated that on average, the group did not have a significant problem with binge eating behaviour; 11% of NB + CLI and 10% of UC participants were rated as having a severe binge eating problem at baseline. Participants in the NB + CLI group had a decrease in BES total score compared with a slight increase in the UC group (LS mean difference at week 16: −7.8, P < 0.0001; week 26: −7.9, P < 0.0001) (Figure 3a). Shifts from baseline in BES severity category at week 26 are summarized in Table 2. At week 26, a significantly higher proportion of participants in the NB + CLI group had reduction in BES severity (i.e. shift from a more severe to less severe category) compared with participants in the UC group (P < 0.0001) (Table 2). In participants with moderate or severe BES scores at baseline, categorical improvement was observed in 91% of NB + CLI participants vs. 18% in UC. Among participants with moderate or severe (i.e. total BES score 18 or greater) binge eating at baseline [n = 23 (32%) in NB + CLI, n = 34 (41%) in UC participants], participants in the NB + CLI group experienced a greater reduction in BES at week 26 compared with the UC group [LS mean (SEM): NB + CLI, 12.0 (1.3); UC, 1.2 (1.1), P < 0.0001] (Figure 3b).
Figure 3.
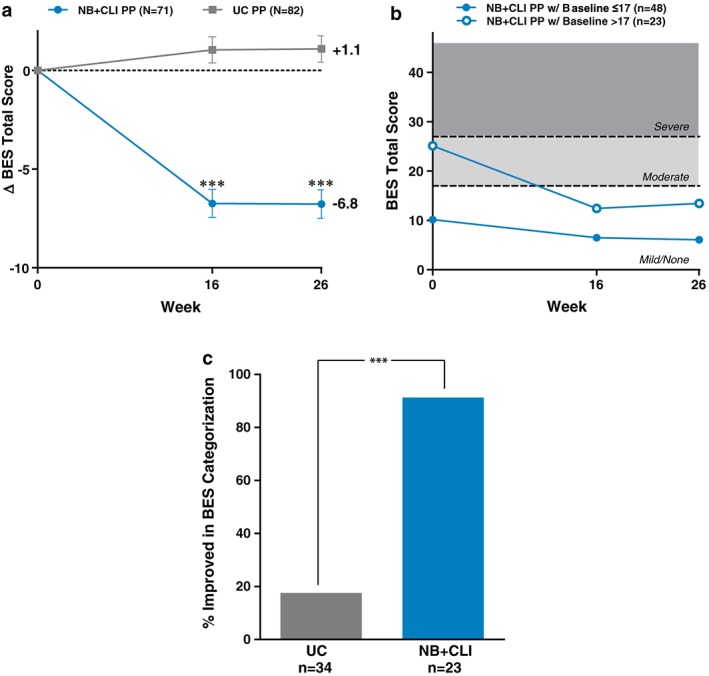
Binge Eating Scale. (a) Change in total BES from baseline. (b) Total score over time for NB participants with and without significant binge eating problem at baseline (dashed lines provide reference to cut‐off thresholds for binge eating). Total score ≤17 indicates no significant binge eating problem, scores from 18 to 26 indicate moderate binge eating and scores ≥27 indicate severe binge eating. (c) Participants with moderate or severe binge eating at baseline (i.e. BES score >17). Proportion of participants with improvement in Binge Eating Scale categorization from baseline to week 26. A greater proportion of NB + CLI (91.3%) than UC (17.6%) participants improved in BES categorization from baseline to week 26 (P < 0.001). For all panels, points represent least square means and error bars are standard error, ***P < 0.001 when UC is compared with NB + CLI for the same time point.
Table 2.
Shift from baseline in Binge Eating Scale total score severity category at week 26
| Parameter | Usual Care (N = 82) n (%) | NB + CLI (N = 71) n (%) |
|---|---|---|
| n a | 82 | 71 |
| Improved | 21 (29.6) | |
| From moderate to none | 5 (6.1) | 14 (19.7) |
| From severe to none | 1 (1.2) | 2 (2.8) |
| From severe to moderate | 0 (0.0) | 5 (7.0) |
| No change | 61 (74.4) | 48 (67.6) |
| From none to none | 37 (45.1) | 46 (64.8) |
| From moderate to moderate | 17 (20.7) | 1 (1.4) |
| From severe to severe | 7 (8.5) | 1 (1.4) |
| Worsened | 15 (18.3) | 2 (2.8) |
| From none to moderate | 9 (11.0) | 2 (2.8) |
| From none to severe | 2 (2.4) | 0 (0.0) |
| From moderate to severe | 4 (4.9) | 0 (0.0) |
| P valueb | <0.0001 |
BES scores were categorized as follows: none = scores ≤17 indicated no significant binge eating, moderate = scores from 18 to 26 (inclusive), severe = scores ≥27 indicated severe levels of binge eating.
BES, Binge Eating Scale; CLI, comprehensive lifestyle intervention; NB, sustained release combination of naltrexone and bupropion.
The number of participants with a score at both baseline and the week 26 visit. The percentages are calculated based on this n.
From a Cochran–Mantel–Haenszel test comparing shift frequencies for participants assigned to NB + CLI to those assigned to usual care, stratified by baseline BMI category.
Treatment with NB + CLI led to greater improvements in Arizona Sexual Experiences Scale
At baseline, 25 (35%) of NB + CLI participants and 37 (45%) of UC participants met the ASEX criteria for sexual dysfunction (Table 1). At weeks 16 and 26, participants in the NB + CLI group had a significantly greater decrease in ASEX total score compared with the UC group (LS mean difference at week 16: −1.7, P = 0.0005; week 26: −2.1, P < 0.0001). At week 26, participants in the NB + CLI group had a favourable pattern of categorical shifts in ASEX dysfunction compared with participants in the UC group (Table 3). Among participants with ASEX scores indicative of dysfunction at baseline, participants in the NB + CLI group experienced a greater improvement in ASEX at week 26 compared with the UC group [LS mean (SEM): NB + CLI, −3.6 (0.8); UC, −0.3 (0.7), P = 0.0011] (Figure 4a). Over half (58%) of NB + CLI participants who met the ASEX criteria for sexual dysfunction no longer met such criteria, compared with 19% of UC participants (Figure 4b).
Table 3.
Shifts from baseline in patient‐reported Arizona Sexual Experiences Scale total score dysfunction categories at week 26
| Parameter | Usual care (N = 82) n (%) | NB + CLI (N = 71) n (%) |
|---|---|---|
| n a | 79 | 70 |
| Improved | 7 (8.9) | 14 (20.0) |
| From dysfunctional to not dysfunctional | 7 (8.9%) | 14 (20.0) |
| No change | 69 (87.3) | 50 (71.4) |
| Not dysfunctional at baseline and week 26 | 40 (50.6) | 40 (57.1) |
| Dysfunctional at baseline and week 26 | 29 (36.7) | 10 (14.3) |
| Worsened | 3 (3.8) | 6 (8.6) |
| From not dysfunctional to dysfunctional | 3 (3.8) | 6 (8.6) |
| P valueb | 0.0040 |
ASEX results were correlated with the presence of clinician‐diagnosed sexual dysfunction if one or more of the following criteria were met: (1) total score ≥19, (2) any individual score of ≥5 and (3) any three items with an individual score of ≥4. An assessment meeting one or more of these criteria was classified as ‘dysfunctional’.
ASEX, Arizona Sexual Experiences Scale; CLI, comprehensive lifestyle intervention; NB, sustained release combination of naltrexone and bupropion.
The number of participants with a score at both baseline and the week 26 visit. The percentages are calculated based on this n.
From a Cochran–Mantel–Haenszel test comparing shift frequencies for participants assigned to NB + CLI to those assigned to usual care, stratified by baseline BMI category.
Figure 4.
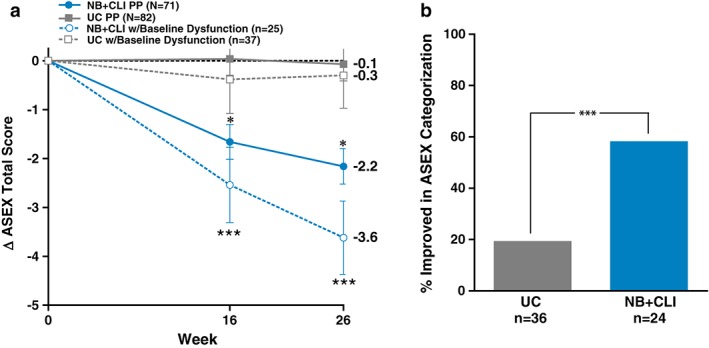
Arizona Sexual Experiences Scale. (a) Change in total ASEX from baseline. Results are displayed for the entire PP population (groups ‘NB + CLI PP’ and ‘UC PP’) as well as those participants who were classified as having sexual dysfunction at baseline (groups ‘NB + CLI dysfunction’ and ‘UC dysfunction’). (b) Proportion of participants with improvement in ASEX categorization from baseline to week 26 in the population of participants with baseline scores indicative of dysfunction. A greater proportion of NB + CLI (58.3%) than UC (19.4%) participants improved in ASEX categorization from baseline to week 26 (P < 0.002). 2 participants did not have data at week 26 so were excluded. For all panels, points represent least square means and error bars are standard error, *P < 0.05 when UC PP is compared with NB PP for the same timepoint, ***P < 0.001 when UC dysfunction is compared with NB + CLI dysfunction for the same time point.
The change in ASEX total score and the change in IWQOL‐Lite sexual domain score are correlated in a concordant manner in the NB‐treated participants, meaning that improvement (i.e. a decrease) in ASEX score also tended to pair with improvement (i.e. an increase) in IWQOL‐Lite sexual domain score, even though the objectives of the two questionnaires are somewhat different. The magnitude of the correlation was generally in the range of −0.3 to −0.2 and was similar between week 16 and week 26 in participants receiving NB.
Week 16 results (modified intent‐to‐treat population)
As a sensitivity analysis, PRO data at week 16 were also reported for the modified ITT (mITT) population, defined as participants who had a baseline body weight measurement, had at least one postbaseline body weight measurement and completed the week 2 study visit while on study medication. Of the 242 randomized participants, the week 16 mITT population included 82 UC participants and 108 NB + CLI participants. Treatment differences at week 16 between UC and NB + CLI tended to be blunted in the mITT population relative to those in the PP population, but these differences were still significant. LS mean treatment differences were IWQOL‐Lite total score (12.4, P < 0.0001), BES (−5.4, P < 0.0001) and ASEX (−1.2, P = 0.0093).
Safety
Safety and tolerability of NB were thoroughly studied in the phase 3 trials; therefore, only adverse events (AEs) leading to discontinuation of study medication and serious AEs were collected in this trial. AEs leading to discontinuation of full participation before week 26 in the NB + CLI group included nausea (10.5%); anxiety (3.3%); headache, hypertension, insomnia and palpitations (1.3% each); and diarrhea, dizziness, gastroesophageal reflux disease, irritability, pruritus and vomiting (0.7% each). AEs leading to discontinuation of full participation before week 26 in the UC group included one incidence of sphincter of oddi dysfunction (1.1%). Serious AEs included one incidence of breast cancer in an NB + CLI participant, diagnosed 3 months into NB treatment. The investigator deemed this event not related to NB.
Discussion
In this randomized open‐label trial designed to approximate the real‐world setting, treatment with 32 mg naltrexone SR/360 mg bupropion SR (NB + CLI), in a manner consistent with its prescribing information, was shown to result in significantly greater improvements in weight‐related quality of life, control over eating behaviour and sexual function scores compared with treatment with UC, a diet and exercise advice program designed to reflect what a patient might receive in a primary care setting.
Prior studies have shown that following weight loss interventions, subjects experience significant improvements in weight‐related quality of life with improvements often occurring early in the weight loss experience when weight loss is rapid 4. IWQOL‐Lite was previously measured in four randomized double‐blind NB phase 3 trials for 56 weeks 19. Pooled results across these four trials showed significant improvement in NB participants compared with placebo in change in IWQOL‐Lite total score from baseline as early as week 8. The majority of NB score improvements occurred by week 28 and were maintained at week 56; improvements in weight‐related quality of life were associated with reductions in weight. Approximately half of pooled NB patients compared with 32% of pooled placebo patients achieved clinically meaningful improvements in IWQOL‐Lite total score. Similarly, in this trial, significantly greater improvements in IWQOL‐Lite were observed in NB + CLI compared with UC as early as week 16 (the earliest timepoint measured) and maintained at week 26, with a greater proportion of NB + CLI patients achieving clinically meaningful improvements in IWQOL‐Lite (67 vs. 20%) at week 26. Collectively, these results reaffirm prior reports of improved weight‐related quality of life for patients with obesity in the presence of weight loss 35.
Many medications under investigation for or approved for long‐term weight management have demonstrated improvements in BES scores 36, 37, 38. Although NB is not indicated for the treatment of BED, this trial aimed to evaluate changes in BES results at 26 weeks in participants with 5% weight loss after 16 weeks of NB + CLI, compared with participants who were not treated with NB. BES scores have also been used in the evaluation of participants undergoing pharmaceutical treatment for obesity, both with (rimonabant, sibutrimine) 36, 37 and without (pramlitide) 38 BED. These studies showed reductions in baseline BES scores ranging from 25 to 51% across all three evaluations. Previously, BES scores were assessed in an open‐label, single‐arm study of participants with obesity and major depressive disorder (MDD) who were treated with NB and dietary and behaviour counselling for 24 weeks 39. The BES results from this current study are the first reported in a more typical real‐world NB population. Mean baseline BES scores were notably higher in participants with MDD and obesity compared with the NB + CLI participants in this trial (28.4 vs. 15.0). However, both cohorts demonstrated significant reductions in BES scores with NB treatment over the study period (57 and 45% reduction in participants with MDD and the current study population, respectively), supporting the notion that NB may help participants across a range of baseline BES scores control their eating behaviour. It should be noted, however, that participants in the current study with baseline BES scores of 17 or less (i.e. those defined by the scale as having little to no binge eating problem) had a smaller reduction in BES score over time, likely reflecting a floor effect of the measure. Although eating behaviour/binge eating was not directly assessed in the placebo‐controlled, double‐blind, phase 3 studies, increased control of eating was demonstrated by the results of question 19 of the Control of Eating Questionnaire (“Generally, how difficult has it been to control your eating?”) 15, 16, 18.
Arizona Sexual Experiences Scale was developed in response to the need for developing a more pertinent, expedient and less intrusive method for evaluating psychotropic drug‐induced sexual dysfunction and changes in sexual function 32. It has not been widely used in patients undergoing weight loss interventions; however, it has previously been used to successfully detect changes in sexual function in populations outside of psychotropic drug use 40, 41, 42. Prior studies of weight loss interventions for populations with obesity have reported improvements in the Female Sexual Functioning Index as a result of weight loss 5, 10, 43. In this study, participants in the NB + CLI group had greater improvements in ASEX scores compared with participants in the UC group (LS mean difference at week 26: −2.1, P < 0.0001). Although this is the first trial to report ASEX results in an NB population, topics of sexual life and sexual function in patients with obesity have been previously studied. In a study of participants seeking various obesity intervention methods, higher BMI was associated with greater impairments in sexual quality of life as assessed by the sexual life subscale of the IWQOL‐Lite scale including lack of enjoyment of sexual activity, lack of sexual desire, difficulties with sexual performance and avoidance of sexual encounters due to weight 44. In NB phase 3 studies, the IWQOL‐Lite subscore for sexual life was significantly improved at week 56 in NB participants compared with placebo. Sexual function results in NB + CLI participants from the current trial include significant reduction from baseline in percentage of patients with sexual dysfunction and greater improvement in ASEX scores when compared with participants treated with UC. These results are supportive of previous results describing improvements in sexual function observed in patients taking bupropion, a component of NB, for major depressive disease 12.
A caveat of this trial is inherent to its design. To test NB effects in a manner consistent with the approved prescribing information, continued participation in the NB treatment group was limited to those who responded to the drug by demonstrating 5% weight loss at week 16, whereas no such limitation applied to the control group. In pooled phase 3 studies, approximately 51% of NB‐treated participants achieved weight loss of at least 5% at week 16 25. In the current trial, up to an additional 32 participants (approximately 45% more participants) may have been included in the NB + CLI analysis without the use of these criteria. The authors acknowledge that the use of a selection variable (weight loss) that has a known relationship with the study outcomes (IWQOL‐Lite, ASEX and BES) may inflate the observed effect size of the treatment group. Although the differences between NB + CLI and UC were greater in the PP population than the mITT population, significantly greater improvements in outcomes for NB + CLI compared with UC were observed in both populations. Other limitations of the present trial include the high number of dropouts, and its open‐label nature, which can have a meaningful impact on patient‐reported data. The NB phase 3 trials were specifically designed to test the effects of NB, and thus utilized a common lifestyle intervention program in both NB and placebo groups. The study design in the current trial compared lifestyle intervention that is common in clinical care for patients initiating treatment for weight loss (i.e. encouragement to lose weight, educational materials and tools designed to enable weight loss, but without regular, consistent reinforcement of the behaviour change), against a more supportive lifestyle intervention combined with NB treatment with the specific goal of generating results that may be reflective of those observed when NB is used as intended in a clinical setting. The lack of a CLI group without study medication could be viewed as a limitation, as one cannot estimate the contribution from NB and CLI separately to the observed weight loss, or improvements in patient‐reported sexual function, binge eating scale scores and quality of life observed in this study. Further, participants in the NB + CLI group received coaching on behaviour modifications that may have impacted BES scores; given that there was no CLI only group, it is not possible to tell whether the improvements in the BES scores in NB + CLI vs. UC are reflective of NB, CLI or a combination of the two.
This trial, conducted to approximate the real‐world experience, is consistent with and builds on the results of the previous NB phase 3 clinical trials, all of which demonstrated that NB used as an adjunct to lifestyle modification is associated with weight‐related quality of life improvements greater than those seen with placebo participants receiving the same lifestyle intervention in participants with overweight or obesity 15, 16, 18. This trial assessed the effects of NB in the population of participants who responded to the medication by losing at least 5% of their initial body weight by week 16; prior studies did not include this efficacy assessment. These results strengthen the body of evidence suggesting combination therapy of NB along with lifestyle to promote weight loss is a promising approach to lowering the prevalence of obesity while simultaneously improving health‐related quality of life and sexual function while increasing control over eating behaviour.
Conclusion
Greater improvements in weight‐related quality of life, control over eating behaviour and sexual function were observed in participants treated with NB + CLI compared with participants treated with UC.
Conflict of Interest Statement
No conflict of interest was declared.
Disclosure
AH, KS, KG, MM and LA are employees and stockholders of Orexigen Therapeutics, Inc. KF is a consultant for Orexigen Therapeutics, Inc.
Clinical Trial Registration
Funding
Orexigen Therapeutics, Inc.
Acknowledgements
We thank the participants, study team and the study sites for their participation in this clinical trial. We also thank Dr. Thomas A. Wadden and Dr. Ronette Kolotkin for their thoughtful input into the design of this study.
Halseth, A. , Shan, K. , Gilder, K. , Malone, M. , Acevedo, L. , and Fujioka, K. (2018) Quality of life, binge eating and sexual function in participants treated for obesity with sustained release naltrexone/bupropion. Obesity Science & Practice, 4: 141–152. doi: 10.1002/osp4.156.
References
- 1. Centers for Disease Control and Prevention . Obesity and overweight: CDC; 2016. [cited 2017 12/20/2017]. Available from: https://www.cdc.gov/nchs/fastats/obesity-overweight.htm.
- 2. World Health Organization . Obesity data and statistics [web site]. 2017. [cited 2017 5/4/2017]. Available from: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/obesity/data-and-statistics.
- 3. Kolotkin RL, Head S, Hamilton M, Tse CK. Assessing impact of weight on quality of life. Obes Res 1995; 3: 49–56. [DOI] [PubMed] [Google Scholar]
- 4. Sarwer DB, Steffen KJ. Quality of life, body image and sexual functioning in bariatric surgery patients. Eur Eat Disord Rev 2015; 23: 504–508. [DOI] [PubMed] [Google Scholar]
- 5. Moore RH, Sarwer DB, Lavenberg JA, et al. Relationship between sexual function and quality of life in obese persons seeking weight reduction. Obesity (Silver Spring) 2013; 21: 1966–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guerdjikova AI, Mori N, Casuto LS, McElroy SL. Binge eating disorder. Psychiatr Clin North Am 2017; 40: 255–266. [DOI] [PubMed] [Google Scholar]
- 7. de Zwaan M. Binge eating disorder and obesity. Int J Obes Relat Metab Disord 2001; 25: S51–S55. [DOI] [PubMed] [Google Scholar]
- 8. Guerdjikova AI, Walsh B, Shan K, Halseth AE, Dunayevich E, McElroy SL. Concurrent improvement in both binge eating and depressive symptoms with naltrexone/bupropion therapy in overweight or obese subjects with major depressive disorder in an open‐label, uncontrolled study. Adv Ther 2017; 34: 2307–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steffen KJ, King WC, White GE, et al. Sexual functioning of men and women with severe obesity before bariatric surgery. Surg Obes Relat Dis 2017; 13: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarwer DB, Spitzer JC, Wadden TA, et al. Changes in sexual functioning and sex hormone levels in women following bariatric surgery. JAMA Surg 2014; 149: 26–33. [DOI] [PubMed] [Google Scholar]
- 11. Sarwer DB, Lavery M, Spitzer JC. A review of the relationships between extreme obesity, quality of life, and sexual function. Obes Surg 2012; 22: 668–676. [DOI] [PubMed] [Google Scholar]
- 12. Patel K, Allen S, Haque MN, Angelescu I, Baumeister D, Tracy DK. Bupropion: a systematic review and meta‐analysis of effectiveness as an antidepressant. Ther Adv Psychopharmacol 2016; 6: 99–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond) 2015; 39: 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev 2013; 14: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity‐related risk factors (COR‐II). Obesity (Silver Spring) 2013; 21: 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR‐I): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2010; 376: 595–605. [DOI] [PubMed] [Google Scholar]
- 17. Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained‐release/bupropion sustained‐release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013; 36: 4022–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: The COR‐BMOD Trial. Obesity (Silver Spring) 2011; 19: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolotkin RL, Chen S, Klassen P, Gilder K, Greenway FL. Patient‐reported quality of life in a randomized placebo‐controlled trial of naltrexone/bupropion for obesity. Clin Obes 2015; 5: 237–244. [DOI] [PubMed] [Google Scholar]
- 20. Halseth A, Shan K, Walsh B, Gilder K, Fujioka K. Method‐of‐use study of naltrexone sustained release (SR)/bupropion SR on body weight in individuals with obesity. Obesity (Silver Spring) 2017; 25: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heller M. The Dash Diet Weight Loss Solution: 2 Weeks to Drop Pounds, Boost Metabolism, and Get Healthy (A DASH Diet Book). Grand Central Life & Style: New York, 2012. [Google Scholar]
- 22. Look Ahead Research Group , Gregg EW, Jakicic JM, et al. Association of the magnitude of weight loss and changes in physical fitness with long‐term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post‐hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016; 4: 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997; 20: 537–544. [DOI] [PubMed] [Google Scholar]
- 24. National Heart, Lung, and Blood Institute (ed.). Aim for a Healthy Weight (Publication No. 06‐5831). National Institutes of Health: Bethesda, MD, pp. 1–2. [Google Scholar]
- 25. Fujioka K, Plodkowski R, O'Neil PM, Gilder K, Walsh B, Greenway FL. The relationship between early weight loss and weight loss at 1 year with naltrexone ER/bupropion ER combination therapy. Int J Obes (Lond) 2016; 40: 1369–1375. [DOI] [PubMed] [Google Scholar]
- 26. National Heart, Lung, and Blood Institute . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. National Institute of Health: Bethesda, MD, 1998. [PubMed] [Google Scholar]
- 27. Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet 2007; 369: 71–77. [DOI] [PubMed] [Google Scholar]
- 28. Celio AA, Wilfley DE, Crow SJ, Mitchell J, Walsh BT. A comparison of the binge eating scale, questionnaire for eating and weight patterns‐revised, and eating disorder examination questionnaire with instructions with the eating disorder examination in the assessment of binge eating disorder and its symptoms. Int J Eat Disord 2004; 36: 434–444. [DOI] [PubMed] [Google Scholar]
- 29. Gladis MM, Wadden TA, Foster GD, Vogt RA, Wingate BJ. A comparison of two approaches to the assessment of binge eating in obesity. Int J Eat Disord 1998; 23: 17–26. [DOI] [PubMed] [Google Scholar]
- 30. Greeno CG, Marcus MD, Wing RR. Diagnosis of binge eating disorder: discrepancies between a questionnaire and clinical interview. Int J Eat Disord 1995; 17: 153–160. [DOI] [PubMed] [Google Scholar]
- 31. Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav 1982; 7: 47–55. [DOI] [PubMed] [Google Scholar]
- 32. McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther 2000; 26: 25–40. [DOI] [PubMed] [Google Scholar]
- 33. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001; 9: 102–111. [DOI] [PubMed] [Google Scholar]
- 34. Crosby RD, Kolotkin RL, Williams GR. An integrated method to determine meaningful changes in health‐related quality of life. J Clin Epidemiol 2004; 57: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 35. Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health‐related quality of life and weight loss. Obes Res 2001; 9: 564–571. [DOI] [PubMed] [Google Scholar]
- 36. Appolinario JC, Godoy‐Matos A, Fontenelle LF, et al. An open‐label trial of sibutramine in obese patients with binge‐eating disorder. J Clin Psychiatry 2002; 63: 28–30. [DOI] [PubMed] [Google Scholar]
- 37. Pataky Z, Gasteyger C, Ziegler O, Rissanen A, Hanotin C, Golay A. Efficacy of rimonabant in obese patients with binge eating disorder. Exp Clin Endocrinol Diabetes 2013; 121: 20–26. [DOI] [PubMed] [Google Scholar]
- 38. Smith SR, Blundell JE, Burns C, et al. Pramlintide treatment reduces 24‐h caloric intake and meal sizes and improves control of eating in obese subjects: a 6‐wk translational research study. Am J Physiol Endocrinol Metab 2007; 293: E620–E627. [DOI] [PubMed] [Google Scholar]
- 39. McElroy SL, Guerdjikova AI, Kim DD, et al. Naltrexone/Bupropion combination therapy in overweight or obese patients with major depressive disorder: results of a pilot study. Prim Care Companion CNS Disord 2013; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ozcan T, Benli E, Ozer F, Demir EY, Kaya Y, Ayyildiz A. The association between symptoms of sexual dysfunction and age at onset in Parkinson's disease. Clin Auton Res 2016; 26: 205–209. [DOI] [PubMed] [Google Scholar]
- 41. Kurek Eken M, Ilhan G, Temizkan O, Celik EE, Herkiloglu D, Karateke A. The impact of abdominal and laparoscopic hysterectomies on women's sexuality and psychological condition. Turk J Obstet Gynecol 2016; 13: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hekmat R, Maghsudloo F, Mohebi M, Rezaee SA, Vakili R, Panah HR. A study of the main determinants of sexual dysfunction in women aged 15–45 years on chronic hemodialysis. Saudi J Kidney Dis Transpl 2016; 27: 916–920. [DOI] [PubMed] [Google Scholar]
- 43. Bond DS, Wing RR, Vithiananthan S, et al. Significant resolution of female sexual dysfunction after bariatric surgery. Surg Obes Relat Dis 2011; 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kolotkin RL, Binks M, Crosby RD, Ostbye T, Gress RE, Adams TD. Obesity and sexual quality of life. Obesity (Silver Spring) 2006; 14: 472–479. [DOI] [PubMed] [Google Scholar]


