Abstract
The present study was aimed to evaluate adaptive mechanism in terms of seed characters of Phyllanthus amarus collected from ten different locations of Tamil Nadu, India. The adaptive variations among the collected populations were assessed based on the sink and float percentages of the seeds in water, the percentage of seed germination, total protein, carbohydrates and their seedling’s growth ability such as shoot and root lengths. From this, we observed that the population had a significantly higher germination percentage of sinking seeds that were attributed to its relatively higher carbohydrate and protein contents than the floating seeds. A comparison of the seed population by cluster analysis and principal coordinate analysis showed that the Chennai population constituted a single clade that was very distinct from the other nine populations, which were further grouped into two sub-clusters. They exhibited a trend consistent with their geographical proximity. Standardised Mantel’s t tests had revealed that the adaptive diversity of the P. amarus population was significantly affected by the geographic distance (r = 0.78, t = 2.68, P > 0.001), altitude (r = 0.35, t = 21.53, P > 0.05), minimum temperature (r = 0.43, t = 1.49, P > 0.01) and maximum temperature (r = 0.49, t = 1.67, P > 0.001). Seed’s characteristics and geographical conditions were correlated along with 19 bioclimatic variables. In dry season, the seedling’s rooting ability showed positive correlation, while its protein content exhibited a negative correlation. It is clearly evident from this study that the geographical variables significantly influence the adaptive ability of the P. amarus.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1236-9) contains supplementary material, which is available to authorized users.
Keywords: Phyllanthus amarus, Geographic variation, Mantel’s t test, PCoA analysis, Population diversity
Introduction
It is very important to understand the adaptive mechanism of species in the context of climate change and with a futuristic point of view. This will help in the conservation of species and their habitats (Doak et al. 2014; Hamilton 2004; Sutherland et al. 2016). Phyllanthus amarus Schum. & Thonn. is an annual herb that grows in sandy soil and is naturally distributed in the dry deciduous forests all over India, up to an altitude of 1000 m MSL. This plant has proven medicinal properties, including its effectiveness in curing many diseases such as hepatitis (Khare 2007). In the ayurvedic system of medicine, it is an important plant that is used to heal the problems of liver, spleen, stomach, kidney and genitourinary system. It is also used in treatments pertaining to ulcers, gastropathy, fevers, diarrhoea, dysentery, ophthalmopathy and scabies (Patel et al. 2011). The seeds of this plant are small and normally develop on the dorsal part of the leaf.
In general, seed development is the most critical stage in the life cycle of plants (Baskin and Baskin 1998), and it determines long-term survival of the species. During germination, the seed is highly sensitive to geographical conditions such as rainfall, temperature, wind speed, solar radiation, altitude, latitude, longitude and soil attributes (Tan et al. 2013). In general, germinability of the seed is determined by the quantity of reserve materials (carbohydrates and proteins), which varies in plants across different geographies (Shutov and Vaintraub 1987). It has been reported that carbohydrate content of the seeds plays a vital role in germinability. Seeds need high levels of soluble carbohydrate for germination, especially raffinose series oligosaccharides (RFOs) and sucrose. These soluble carbohydrates are reduced by the proteins in the seeds during the subsequent germination stages (Matheson and Saini 1977). Evidence to date suggests that soluble carbohydrates could act as cryoprotectants, antioxidants and osmotic regulators during drought conditions (Iskandar et al. 2011).
Most seeds are initially dormant, until they are hydrated. In general, high temperatures reinforce dormancy in them. On the other hand, low temperatures act as a stimulant (stratification response) in many species. In warm climates, the species distributed at higher elevations are expected to be at the greatest level of threat. Decrease in rainfall makes it more unfavourable for seed germination because it affects the allocation of biomass into the seeds, which is the main energy warehouse and it supports the early stages of growth (Engler et al. 2011). It has been observed that a strategy for survival during the very long dry season is to have a rapid growth of root rather than the shoot. This is because longer and bigger roots have the ability to absorb and conserve water more efficiently than the shorter and thinner roots (Saboya and Borghetti 2012). As a result of environmental adaptation, plants of the same species grown in different climatic regions differ in their composition of stored carbohydrates, proteins and secondary metabolites. The composition variations can be manifest within the species in different geographic regions (Kaushik et al. 2007; Ghosh and Singh 2011).
In India, only a few reports are available on climate change and its impact on distribution and richness of species; however, most of these reports are restricted to biodiversity hotspots (Gunnell 1997; Krishnan and Davidar 1996; Priti et al. 2016). To the best of our knowledge, there is no report exist on the climate change and its impact on the distribution and richness of P. amarus. So, 19 bioclimatic variables were used to project P. amarus’ future adaptability in their habitats. These bioclimatic variables were commonly used to project potential future changes in the geography to biodiversity conservation priorities of species.
Based on the above information, we have analysed and determined the germinability of seeds of P. amarus collected from ten different locations in the state of Tamil Nadu, India. Attempts have also been made to correlate germinability, protein and carbohydrate contents and the growth of seedlings along with their geographical variables and 19 bioclimatic variables.
Materials and methods
Study locations
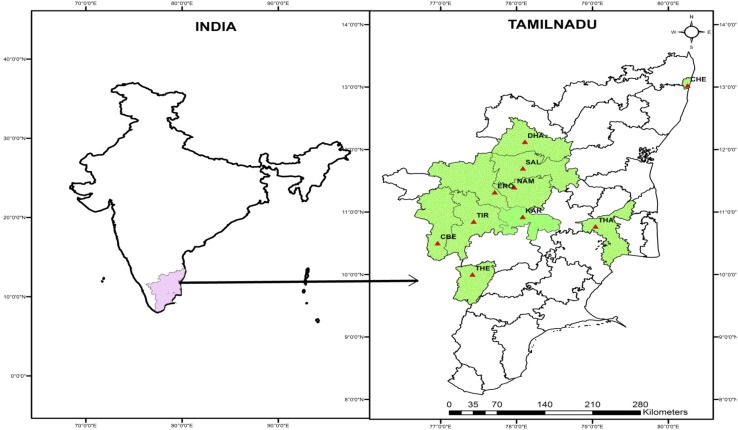
Seeds of P. amarus were collected from ten different areas in Tamil Nadu where the species grows naturally. This plant material was authenticated by taxonomist, Prof. N Mathivanan, and it was deposited in the Herbarium of Centre for Advanced Studies in Botany, University of Madras (Voucher No.: CASBAH1014). The criteria for choosing the sampling points for this study include agro-climatic zones and environmental pollution. The state of Tamil Nadu lies between 29°26′ and 31°28′N latitude and 77°49′ and 80°06′E longitude (Fig. 1). The state has three distinct rainfall seasons: (1) advancing monsoon seasons (June–September) with strong south-west winds; (2) northeast monsoon (October–December) with dominant northeast winds; and (3) dry season (January–May). The climate in Tamil Nadu ranges from dry sub-humid to semi-arid and is mostly dependent on monsoon rains and thereby is prone to droughts when the monsoons fail.
Fig. 1.
Sampling points. CHE Chennai, CBE Coimbatore, DHA Dharmapuri, ERO Erode, KAR Karur, NAM Namakkal, SAL Salem; THA Thanjavur, THE Theni, TIR Tiruppur
The sampling locations lie in the sea coast (CHE, urban area with very high pollution), near the Cauvery delta (THA, delta region with deposits and contaminated waters), industrial regions (TIR, DHA, KAR, NAM, ERO) and in the foothills of the Western Ghats (CBE, THE, SAL), away from industries and with low pollution. These ten sampling points lie at altitudes ranging from 11 to 470 m MSL and receive rainfall in the range of 517–1443 mm (Table 1).
Table 1.
Collection of P. amarus seeds from different locations of Tamil Nadu, India, with its geographical information
| S. no. | Locations | Locations code | Latitude/longitude | ALT (m) | Tmin (°C) | Tmax (°C) | RF (mm) |
|---|---|---|---|---|---|---|---|
| 1 | Adyar, Chennai | CHE | 13°0′16.7832″N 80°14′51.8856″E | 11 | 23.8 | 33.3 | 1211 |
| 2 | Aliyar Dam, Coimbatore District | CBE | 10°28′48.4752″N 76°59′9.0096″E | 318 | 22.3 | 32.1 | 517 |
| 3 | Nandhi Nagar, Dharmapuri District | DHA | 12°6′23.4972″N 78°8′10.1076″E | 340 | 22.0 | 31.0 | 1443 |
| 4 | Pallipalayam, Erode District | ERO | 11°20′57.6708″N 77°45′21.4272″E | 470 | 21.3 | 32.2 | 876 |
| 5 | Vellalapatty, Karur District | KAR | 10°3′32.1516″N 78°17′4.6680″E | 170 | 23.2 | 33.7 | 706 |
| 6 | Elaichipalayam, Namakkal District | NAM | 11°23′12.0300″N 78°0′52.0272″E | 148 | 23.5 | 33.7 | 584 |
| 7 | Periyar University, Salem District | SAL | 11°43′5.9988″N 78°4′34.3488″E | 194 | 23.1 | 33.7 | 790 |
| 8 | Thirumalaisamudram, Thanjavur District | THA | 10°43′39.3996″N 79°1′30.9720″E | 60 | 22.4 | 33.3 | 941 |
| 9 | Kodangipatti, Theni District | THE | 9°59′35.0484″N 77°25′40.6200″E | 345 | 24.2 | 33.3 | 840 |
| 10 | Kundadam, Tiruppur District | TIR | 10°51′9.8892″N 77°26′13.8372″E | 313 | 22.6 | 31.6 | 931 |
ALT altitude, Tmin minimum temperature, Tmax maximum temperature, RF rainfall, N North, E East
Seed collection
In this study, the seeds were collected from wildly growing plants from different regions. Though the plants grew alongside agriculture fields, the sampling targeted only the uncultivated lands since the additional/deficient water and nutrients from cultivated fields adversely influence the seeds’ nutritional composition, except in Chennai (CHE), where they were collected from open lands. Samples were randomly collected from uncultivated wastelands, where they grow wildly, away from the actions of any herbivorous animals and human activities. In Chennai, it was collected from an open private land with the consent of the land owner. This plant is not on the endangered list of IUCN, and none of the collection sites of this study was inside the national park or other restricted forest areas. Tamil Nadu mainly depends on the northeast monsoon, occurring from October to December. The plants were collected in their 3-month-old stage during the end of this monsoon, i.e. in December, 2011. Each location included three sampling points which are close together (within 100 m2). More than 50 plants were collected in a single sampling point; the number of seeds per plant varies from ~ 50 to 150. All the seeds collected from each sampling points were considered as a single population. To harvest mature seeds from the plants collected for the study and to remove the excess moisture in them, the shade drying was performed for 48 h. These harvested seeds were segregated into four parts and stored separately at 4 °C until they were used for the study. One-fourth of the stored voucher seed specimen from each location was frequently checked for germinability. It was found that the germinability of the seeds remained consistent for up to 6 months and the storage condition does not influence the germination percentage. The study was also completed within this time.
Determination of germinability and biochemical contents
The aim of this study was to determine the germinability and biochemical contents (protein and carbohydrate) of the seeds of P. amarus collected from ten different sampling points. A portion of the visibly healthy seeds was collected and separated into sink and float seeds by following a standard protocol (Edson et al. 2010). The percentage germinability of the seeds (50 seeds placed on wet filter paper with 5 mm distance between them) was measured by germinating them at room temperature. A seed was considered as germinated when there is an emergence of radicle, and then the germinability was recorded daily. Another portion of seeds was separated into sink and float seeds and analysed for their total carbohydrate and protein contents using the methods of Dubois et al. (1956) and Bradford (1976), respectively.
Ex situ field experiment
The aim of this experiment was to measure the length of the shoot and root of the seedlings grown in the experiment field from the P. amarus seeds collected from different regions. The ex situ field experimentation was conducted at the Field Research Laboratory, University of Madras, Maduravoyal Campus, Chennai, Tamil Nadu, in a completely randomised block design with three replications. The soil of the experiment site was luvisols, consisting of 16% sand, 29% silt and 55% clay fractions with pH 7.4 at 0–30 cm top soil. The third portion of sink seeds collected from all the locations was weighed into three 10 g lots. The seedlings were grown on field beds (5 × 4 m) between February and April 2012 and irrigated everyday by the surface irrigation method or when required. The field beds were prepared according to standard procedures. In brief, the soil was ploughed up to a depth of 30 cm and the top layer of the soil was mixed with 1 kg (per metre) of decomposed farmyard manure containing cow dung, cow urine, waste straw, fodder residues and other organic waste. They were watered twice in a day for 15 days; weeds were removed and 2% fungicide (Captan Gold® 80WDG, M/s. GNP Agrosciences Pvt. Ltd., India) solution was drenched into the soil two times at seven-day intervals to eradicate the fungal contaminants. After manual weeding, the sink seeds were sown in the field beds by mixing them with dry soil, which allowed a uniform distribution over the field beds. Later, a thin layer of top soil and farmyard manure mixture was spread to ~ 1 cm thickness to cover the field beds. Each plot was marked prominently with its respective sample code. On maturity of the seedlings (the 90th day), all the plants were harvested and 25 plants from each replication were measured using a ruler for their shoot and root lengths.
Data analysis
The aim of this study was to investigate the adaptive variation in the seed nutrient composition, the growth of seedlings and germination as a function of several environmental covariates such as rainfall and altitude. In general, the accumulation of biomass inside the seeds helps them to germinate and grow. The results obtained with a number of sinking and floating seeds, the percentage of seed germination, and carbohydrate and protein content were compared using analysis of variance (ANOVA), and the populations were compared with Tukey HSD with the documented germination percentage and seedling growth. The relationship of geographical attributes with the percentage of germination and biochemical content was established using Pearson rank correlation coefficient (r), since the data are normally distributed (according to Shapiro–Wilk test for normality). Bioclimatic variables were also used to correlate the variants which were obtained from the server (http://www.worldclim.org/bioclim; Table 2). Bioclimatic variables were used in this study to correlate further with its 19 meaningful variables along with seed’s protein and carbohydrate contents, and seedling establishment with existing geography from the collection site. Bioclimatic variables are derived from the monthly temperature and rainfall values to generate more biologically meaningful variables. These are often used in species distribution modelling and its related ecological modelling techniques. The multivariate techniques, cluster analysis (CA) and principal coordinate analysis (PCoA) are the most important strategies for classifying or analysing the geographical plasticity which was considered in this study as genetic distance and its relationships among the populations. The clustering was analysed using the unweighted pair group method with arithmetic averages (UPGMA; (Sneath and Sokal 1962). Principle coordinate analysis was performed to summarise variation between populations based on genetic distance by following Hu et al. (2010). The design of this method allows for visualising patterns of diversity across a surface in a three-dimensional space (x, y and z axes). The hypothesis that the adaptive variation between populations is due to distance or climatic conditions was tested according to Rousset (1997). To test for a correlation between dissimilarity and cophenetic matrices of the populations, Mantel’s t test was performed using NTSYSpc for Windows. Similarly, altitude, minimum and maximum temperature and rainfall matrices were subjected to Mantel’s t test of correlation to know the influence of environmental factors on the ten populations. Geographic distance (in km) was calculated as straight-line distances between pairs of sites using the latitude and longitude of each location. The rainfall data for 2007–2011 obtained from the Indian Meteorological Department (IMD) were used to calculate the mean annual rainfall of the study area. The ‘distance’ matrix based on altitude and rainfall was constructed from the absolute difference between pairs of populations. The 95% confidence interval around each r value was estimated by bootstrapping.
Table 2.
Bioclim details used in correlation studies
| BIO1 = annual mean temperature | BIO8 = mean temperature of wettest quarter | BIO15 = precipitation seasonality (coefficient of variation) |
| BIO2 = mean diurnal range (mean of monthly (max temp − min temp)) | BIO9 = mean temperature of driest quarter | BIO16 = precipitation of wettest quarter |
| BIO3 = isothermality (BIO2/BIO7) (× 100) | BIO10 = mean temperature of warmest quarter | BIO17 = precipitation of driest quarter |
| BIO4 = temperature seasonality (standard deviation × 100) | BIO11 = mean temperature of coldest quarter | BIO18 = precipitation of warmest quarter |
| BIO5 = max temperature of warmest month | BIO12 = annual precipitation | BIO19 = precipitation of coldest quarter |
| BIO6 = min temperature of coldest month | BIO13 = precipitation of wettest month | |
| BIO7 = temperature annual range (BIO5–BIO6) | BIO14 = precipitation of driest month |
Obtained from online source (http://www.worldclim.org/bioclim)
Results
Seed germination
The percentage of sink and float seeds was determined with seeds collected from all the ten different locations, and the data are presented in Table 3. The percentage of sink seeds was found to be between 51.7 and 61.9, while the percentage of float seeds was found to be between 48.3 and 38.1. The germination percentage of the sink seeds was found to be between 22.0 and 28.3, and the germination percentage of the float seeds was found to be in the range of 4.3–6.0. Based on this experiment, the sink seeds collected from Namakkal (NAM) recorded the highest germination, while the sink seeds collected from Thanjavur (THA) showed the least. Sink seeds of P. amarus are heavy and hence they sink in water. In general, heavier seeds have larger embryos and more nutrients in their endosperm, which contribute to the increased germination percentage. The accumulation of carbon reserve inside the seeds results in weight gain and renders them sink seeds. Increasing seed weight confers an advantage to developing seedlings for them to germinate (Eriksson 1999).
Table 3.
Determination of carbohydrate and protein contents of the sink and float seeds of P. amarus collected from different localities
| S. no. | Code | Percentage of | Germination % of | Total carbohydrates (%) | Total proteins (%) | Seedlings Length (cm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sinkers | Floaters | Sinkers | Floaters | Sinkers | Floaters | Sinkers | Floaters | Above | Below | ||
| 1 | CHE | 51.7 ± 0.6 | 48.3 ± 0.6 | 26.3 ± 2.3 | 5.0 ± 1.0 | 57 ± 0.34a | 41 ± 0.24a | 10 ± 0.01f | 6 ± 0.03de | 22.2 ± 0.8e | 6.0 ± 0.41g |
| 2 | CBE | 56.3 ± 0.6 | 43.7 ± 0.6 | 23.3 ± 0.6 | 4.3 ± 1.5 | 25 ± 0.15e | 18 ± 0.12de | 14 ± 0.07cf | 8 ± 0.03c | 37.2 ± 2.2cd | 6.9 ± 0.38fg |
| 3 | DHA | 58.3 ± 0.6 | 42.0 ± 1.0 | 25.0 ± 3.6 | 5.3 ± 0.6 | 47 ± 0.18bc | 26 ± 0.01c | 16 ± 0.09b | 5 ± 0.04ef | 38.6 ± 3.2bcd | 14.4 ± 0.86a |
| 4 | ERO | 59.3 ± 0.6 | 40.7 ± 0.6 | 23.0 ± 2.0 | 5.0 ± 1.0 | 31 ± 0.22e | 21 ± 0.09d | 13 ± 0.06de | 7 ± 0.02cd | 45.8 ± 3.2a | 7.9 ± 0.26ef |
| 5 | KAR | 59.0 ± 1.0 | 41.0 ± 1.0 | 22.7 ± 1.2 | 4.3 ± 0.6 | 45 ± 0.14cd | 35 ± 0.25b | 8 ± 0.02g | 4 ± 0.03f | 40.5 ± 1.9abcd | 8.2 ± 0.36def |
| 6 | NAM | 61.3 ± 0.6 | 38.7 ± 0.6 | 28.3 ± 1.2 | 6.0 ± 1.0 | 52 ± 0.36ab | 38 ± 0.13ab | 11 ± 0.07f | 6 ± 0.03de | 44.2 ± 1.7ab | 11.4 ± 0.44b |
| 7 | SAL | 59.0 ± 0.0 | 41.0 ± 0.0 | 23.7 ± 3.1 | 4.3 ± 1.5 | 48 ± 0.31bc | 29 ± 0.16c | 11 ± 0.06ef | 11 ± 0.08b | 33.8 ± 1.9d | 8.9 ± 0.13cde |
| 8 | THA | 60.3 ± 0.6 | 39.7 ± 0.6 | 22.0 ± 2.0 | 5.3 ± 0.6 | 38 ± 0.22d | 21 ± 0.14d | 15 ± 0.02bc | 12 ± 0.10a | 39.2 ± 3.4abcd | 7.1 ± 0.47fg |
| 9 | THE | 56.0 ± 0.0 | 44.0 ± 0.0 | 24.0 ± 3.6 | 5.7 ± 1.2 | 24 ± 0.16e | 16 ± 0.06e | 19 ± 0.11a | 7 ± 0.05cd | 36.4 ± 1.9d | 9.5 ± 0.50c |
| 10 | TIR | 56.7 ± 0.6 | 43.3 ± 0.6 | 26.0 ± 2.7 | 5.3 ± 0.6 | 26 ± 0.21e | 15 ± 0.09e | 17 ± 0.11ab | 11 ± 0.07ab | 43.5 ± 2.5abc | 9.5 ± 0.62cd |
Values followed by different lowercase letters in a column are significantly different (P < 0.05) among population’s location
Means with the same letter are not significantly different
CHE Chennai, CBE Coimbatore, DHA Dharmapuri, ERO Erode, KAR Karur, NAM Namakkal, SAL Salem, THA Thanjavur, THE Theni, TIR Tiruppur
Biochemical analysis
The percentage of total carbohydrate in sink seeds was relatively higher than the float seeds collected from all the different locations (Table 3). The ranges of carbohydrate in sink and float seeds were 24–57% and 15–41%, respectively (Table 3). Among the sink seeds, the highest carbohydrate (57%) was found in the seeds collected from Chennai (CHE), while the least percentage of carbohydrate (24%) was found in the seeds collected from Theni (THE). Among the float seeds, the seeds collected from CHE showed the highest percentage of carbohydrate (41%) and the seeds collected at Tiruppur (TIR) showed the least percentage of carbohydrate (15%) (Table 3). It has been reported that the cell wall-bound invertase, a crucial hydrolysing enzyme that breaks sucrose into glucose and fructose, contributes to establishing viability in young seeds, which results in germination (Weber et al. 1995).
Variations in the total protein content between the sink and float seeds were also recorded and the percentage was also determined, and they are presented in Table 3. A relatively higher protein content was recorded in the sink seeds than in the float seeds. The total protein content in the sink seeds ranged between 19 and 8%. The highest protein content (19%) was observed in the seeds collected from THE, while the least content of protein (8%) was recorded in the seeds collected from Karur (KAR). On the other hand, the percentage of protein content in the float seeds ranged between 11 and 4%. Among the float seeds, the highest protein content (11%) was recorded in seeds collected from SAL and TIR, and the lowest protein content (4%) was recorded in the seeds collected from KAR (Table 3). From earlier report, the seeds of P. amarus seeds contains the following protein − 11.33 ± 0.25% and carbohydrate − 68.70 ± 0.60%, respectively (Pius et al. 2015).
Experimental field observation
The shoot and root lengths of the plants were measured and recorded (Table 3). The highest shoot length was observed in the seedlings obtained from the seeds collected at NAM (44 cm), while the lowest shoot length was collected at CHE was 22.2 cm. The seedlings obtained from the seeds collected at Dharmapuri (DHA) showed the highest root length (14.4 cm), while the seeds collected at CHE showed the lowest root length (6.0 cm). The sink seeds had shown higher germinability, higher root and shoot lengths as well as higher carbohydrate and protein contents than float seeds. Seeds of larger size often have deeper root and shoot extension and greater sugar reserves, which provide a competitive advantage under stressful drought conditions (Eriksson 1999).
Multivariate analysis
The CA, PCoA and Mantel’s t tests revealed significant variations among the P. amarus populations in terms of seed germination and composition; these variations are determined by geographic proximity. This was in accordance with a report that showed that climatic variables contribute to the origin and maintenance of geographical gradients in seed mass (Murray et al. 2004). The dendrogram generated after UPGMA CA grouped the collected populations into two major clades in a tree scale length of 0.31 (Fig. 2). Major clade 1 consisted of the seeds collected from CHE alone, while major clade 2 had two subclades: subclade1 consisted of four populations (CBE, THE, SAL and DHA) and subclade 2 consisted of five populations (ERO, TIR, KAR, THA and NAM). A test for the possible effect of environment-specific natural selection on seed germination and CHO reserves was done. The results are consistent with three possible patterns expected under environment-specific selection. These patterns are: (a) in major clade 1, a distinct single population CHE, which is a characteristic coastal belt, is highly separated from the other populations; (b) subclade 1 of clade 2 includes populations from hills/foothills; and (c) subclade 2 of clade 2 includes river bank populations from the Cauvery delta zone, except NAM. The present study has demonstrated adaptation of P. amarus to environments that prevailed in a particular locality. A similar study conducted earlier with Jatropha curcas seeds showed variation in composition and germination ability between the samples collected at different locations which were mainly due to the populations that grown over a wide range of climatic conditions (Ginwal et al. 2005).
Fig. 2.
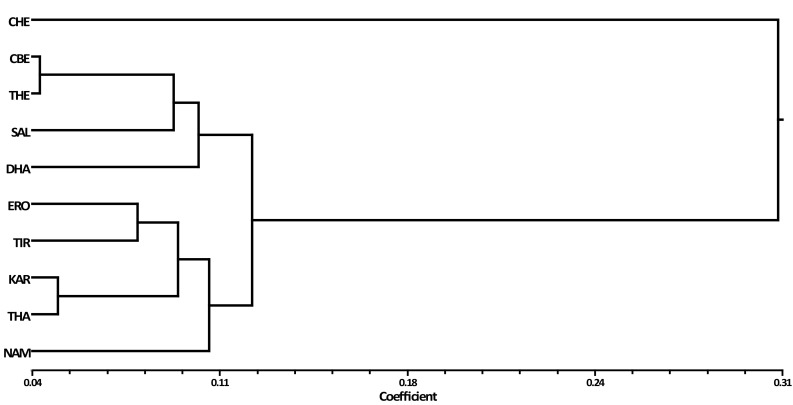
Cluster analyses of P. amarus based on genetic distance. CHE Chennai, CBE Coimbatore, DHA Dharmapuri, ERO Erode, KAR Karur, NAM Namakkal, SAL Salem, THA Thanjavur, THE Theni, TIR Tiruppur
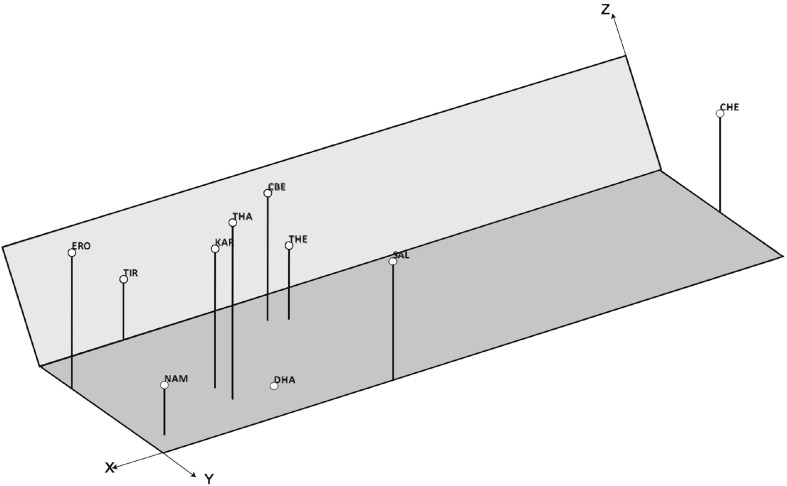
In PCoA, the first two axes explained 87.68% (78.45 and 9.23%) and axis three 7.23% of the variance. The x axis indicated the existence of strong differences between the genotypes. The y axis corresponded with intrapopulation variations between the populations. The z axis revealed the existence of differences between the regions. These data show clear-cut genetic structures (Fig. 3). Standardised Mantel’s t test (Table 4) revealed the seed’s diversity and structure of the P. amarus populations. These populations were significantly influenced by the geographic distance (), altitude (), minimum temperature () and maximum temperature (). However, rainfall showed a weak relationship with genetic distance of the populations (). Plants are sessile organisms, often characterised by limited dispersal. Seed dispersal is a critical stage of gene flow, often showing quite different modes and geographic distances and its variants (McCauley 1994, 1997; Heuertz et al. 2003; Garcia et al. 2007; Torimaru et al. 2012). Since altitude and temperature are the complex factors, which produce heterogeneous; environmental conditions were likely to affect the seed composition and growth variation markedly of a plant species (Sáenz-Romero and Tapia-Olivares 2003). Studies on seed weight of Glycine sp. have revealed that at low altitude, due to availability of more sunlight and photosynthesis, the seeds have increased the metabolic activity, which in turn leads to an increase in biomass and production of large seeds. This could also be due to that higher altitude experience low temperature, which reduces the more transpiration of water and they also receive good rainfall (Henry 1919; Gouvas et al. 2009). So, P. amarus also being a non-perennials plant showed variation between its populations collected from different localities.
Fig. 3.
PCoA analysis of P. amarus based on genetic distance (x axis latitude; y axes longitude; z axis genetic distances. CHE Chennai, CBE Coimbatore, DHA Dharmapuri, ERO Erode, KAR Karur, NAM Namakkal, SAL Salem, THA Thanjavur, THE Theni, TIR Tiruppur
Table 4.
Mantel’s t test of correlation analysis of P. amarus accessions based on genetic cophenetic matrix with geographic distance, altitude, temperature minimum, maximum and rainfall
| Mantel | r | Mantel t test | Significance | Significance |
|---|---|---|---|---|
| GD vs GED | 0.79513 | 2.6820 | 0.0752 | 0.0000** |
| GD vs ALT | 0.34501 | 1.5261 | 0.1849 | 0.0235* |
| GD vs Tmin | 0.43431 | 1.4869 | 0.1356 | 0.0036** |
| GD vs Tmax | 0.49218 | 1.6890 | 0.1332 | 0.0008** |
| GD vs RF | 0.21069 | 0.7558 | 0.1331 | 0.1750NS |
GD genetic distance, GED geographic distance, ALT altitude, Tmin temperature minimum, Tmax temperature maximum, RF rainfall
**P > 0.01, *P > 0.05, and NS not significant based on one tile probability with 999 permutation
Correlation analysis (Table 5) revealed that latitude has a negative correlation with the root lengths of P. amarus seedlings and they were also positively influenced by the minimum temperature of the coldest month (bio6), temperature annual range (bio7), precipitation in the driest month (bio14) and precipitation in the driest quarter (bio17), with significance. The temperature maximum in our collection location displayed negative correlation with the protein content in seed, with significance. The maximum temperature of the warmest month (bio5, highly significance) and the mean temperature of the driest month (bio9, significance) displayed a negative correlation, while the protein content displayed a positive correlation with the precipitation of coldest quarter (bio19). So, we observe there is a strong decrease in protein content in the seed with increase in temperature. During germination, seeds are highly sensitive to different environmental factors. Seeds often fail to germinate when their embryos imbibed at high-temperature environmental localities displaying low rate of protein synthesis. It was due to the lack of active mRNA in the seeds (Rilkey 1981).
Table 5.
Correlation between seed’s characteristics and climatic factors
| S | F | GS | GF | CHOS | CHOF | PROS | PROF | AG | BG | |
|---|---|---|---|---|---|---|---|---|---|---|
| S | 1 | |||||||||
| F | − 1.00** | 1 | ||||||||
| GS | − 0.292 | 0.292 | 1 | |||||||
| GF | 0.175 | − 0.175 | 0.492 | 1 | ||||||
| CHOS | 0.249 | − 0.249 | 0.406 | − 0.044 | 1 | |||||
| CHOF | 0.219 | − 0.219 | 0.261 | − 0.175 | 0.915** | 1 | ||||
| PROS | − 0.311 | 0.311 | 0.012 | 0.453 | − 0.693* | − 0.863** | 1 | |||
| PROF | 0.080 | − 0.080 | − 0.250 | 0.019 | − 0.409 | − 0.628 | 0.483 | 1 | ||
| AG | 0.681* | 0.681* | − 0.127 | 0.287 | − 0.164 | − 0.139 | − 0.055 | − 0.037 | 1 | |
| BG | 0.244 | − 0.244 | 0.419 | 0.588 | 0.018 | − 0.109 | 0.329 | − 0.242 | 0.304 | 1 |
| Lat | 0.219 | − 0.219 | − 0.297 | − 0.362 | 0.467 | 0.576 | − 0.62 | − 0.012 | − 0.127 | − 0.699* |
| Long | − 0.085 | 0.085 | 0.006 | 0.168 | 0.127 | 0.261 | − 0.188 | 0.024 | − 0.224 | − 0.45 |
| ALT | 0.267 | − 0.267 | − 0.382 | − 0.212 | − 0.358 | − 0.43 | 0.322 | 0.226 | 0.467 | 0.085 |
| Tmin | − 0.316 | 0.316 | 0.43 | 0.268 | 0.224 | 0.297 | − 0.207 | − 0.213 | − 0.418 | 0.049 |
| Tmax | 0.401 | − 0.401 | 0.055 | − 0.062 | 0.576 | 0.673* | − 0.729* | − 0.152 | − 0.091 | − 0.109 |
| Prec | − 0.207 | 0.207 | 0.127 | 0.262 | 0.212 | − 0.006 | 0.353 | 0.018 | − 0.164 | 0.043 |
| bio1 | − 0.122 | 0.122 | 0.261 | 0.243 | 0.188 | 0.333 | − 0.304 | − 0.183 | − 0.333 | − 0.049 |
| bio2 | 0.681* | 0.681* | − 0.612 | − 0.38 | 0.127 | 0.212 | − 0.492 | 0.287 | 0.358 | − 0.45 |
| bio3 | 0.116 | − 0.116 | 0.139 | − 0.069 | − 0.115 | − 0.321 | 0.255 | 0.177 | 0.224 | 0.48 |
| bio4 | − 0.128 | 0.128 | − 0.055 | 0.069 | 0.115 | 0.236 | − 0.17 | 0.079 | − 0.309 | − 0.486 |
| bio5 | 0.085 | − 0.085 | − 0.03 | − 0.268 | 0.636* | 0.782** | − 0.778** | − 0.323 | − 0.358 | − 0.383 |
| bio6 | − 0.129 | 0.129 | 0.485 | 0.498 | − 0.092 | − 0.08 | 0.203 | − 0.346 | − 0.006 | 0.763* |
| bio7 | 0.219 | − 0.219 | − 0.297 | − 0.362 | 0.467 | 0.576 | − 0.62 | − 0.012 | − 0.127 | − 0.699* |
| bio8 | − 0.073 | 0.073 | 0.164 | 0.168 | 0.345 | 0.273 | − 0.091 | 0.134 | − 0.552 | 0.036 |
| bio9 | 0.006 | − 0.006 | 0.188 | − 0.075 | 0.709* | 0.782** | − 0.675* | − 0.421 | − 0.418 | − 0.158 |
| bio10 | − 0.134 | 0.134 | 0.248 | 0.118 | 0.37 | 0.503 | − 0.419 | − 0.183 | − 0.418 | − 0.231 |
| bio11 | 0.277 | − 0.277 | 0.055 | 0.076 | 0.19 | 0.264 | − 0.271 | − 0.37 | − 0.006 | 0.554 |
| bio12 | − 0.207 | 0.207 | 0.127 | 0.262 | 0.212 | − 0.006 | 0.353 | 0.018 | − 0.164 | 0.043 |
| bio13 | − 0.186 | 0.186 | 0.176 | 0.131 | 0.304 | 0.055 | 0.25 | 0.009 | − 0.128 | 0.064 |
| bio14 | − 0.102 | 0.102 | 0.302 | 0.443 | − 0.419 | − 0.468 | 0.503 | − 0.152 | 0.314 | 0.815** |
| bio15 | − 0.073 | 0.073 | 0.236 | − 0.274 | 0.479 | 0.636* | − 0.602 | − 0.476 | − 0.115 | − 0.413 |
| bio16 | − 0.207 | 0.207 | 0.127 | 0.262 | 0.212 | − 0.006 | 0.353 | 0.018 | − 0.164 | 0.043 |
| bio17 | − 0.025 | 0.025 | 0.067 | 0.29 | − 0.313 | − 0.448 | 0.529 | − 0.025 | 0.202 | 0.769** |
| bio18 | 0.036 | − 0.036 | − 0.042 | 0.15 | 0.212 | − 0.055 | 0.304 | 0.14 | 0.018 | 0.097 |
| bio19 | − 0.632* | 0.632* | 0.261 | 0.299 | − 0.636* | − 0.612 | 0.687* | − 0.104 | 0.018 | 0.267 |
S sink seeds, F float seeds, GS germination percentage of sink seeds, GF germination percentage of float seeds, CHOS carbohydrate percentage of sink seeds, CHOF carbohydrate percentage of float seeds, PROS protein percentage of sink seeds, PROF protein percentage of float seeds, AG above ground growth, BG below ground growth, Lat latitude, Long longitude, ALT altitude, Tmin temperature minimum, Tmax temperature maximum, Prec rainfall (precipitation), bio1-19(different climatic modules)
*, **Significance of the Pearson correlation at P < 0.01, 0.05 respectively. The italic values are the possible correlation that were taken into consideration
Carbohydrate content in both sink and float seeds always displayed a negative correlation with the protein content in the seeds. It was significant in max temperature of warmest month (bio5) and mean temperature of driest quarter (bio9), while bio 19 (precipitation of coldest quarter) displayed high significance. Significant correlation is between bioclimatic modules (bio5, bio9 and bio19) and carbohydrate content in the seeds. Bio 5 and bio 9 displayed a positive correlation, while bio19 displayed a negative correlation. However, the well-adapted three plant species in the savannah regions showed longer roots due to well environmental adaptation from its long dry season (Saboya and Borghetti 2012). P. amarus’ root length was significantly high with positive correlation for both the Precipitation of Driest Month (bio14) and Precipitation of Driest Quarter (bio17). Based on the above correlation, it can be predicted that the P. amarus plant can disappear in a particular locality if the temperature raises beyond the regular temperature maximum limit, then their protein contents in their seeds drastically decrease. However, the existing P. amarus plant can withstand the high temperature as it positively correlates with the length of the root. This phenomenon will decrease the rate of germination of the plant. To ensure good germination in environments with a relatively high temperature that cause high seed mortality, seeds with good germinability should be introduced for better existence (Xu et al. 2014). This study is a plea to ensure the sustainability of P. amarus. It attempts to make a strong case for a concerted effort to conserve the species P. amarus owing to its less adaptability and its importance as an herb. In places where this species is naturally wild, especially in areas that have a week or unreliable monsoon, it is essential to save and preserve the seeds for the continuance of the species.
Conclusion
Species distribution models such as bioclimatic variables used in this study are useful tools to predict the adaptability of the species such as P. amarus. These findings will help in the conservation management of P. amarus under rapidly changing climate. This can provide wide information to the conversationalist about the degree of changes that can occur to this species in various climatic domains across different geographic distributions. This study showed that the climatic factors such as temperature and geographic distance altered the P. amarus’s seed composition and seed growth properties. Lower latitude seeds had shown positive rooting ability with high significance. Tmax had shown a positive correlation with carbohydrate content and negative correlation with protein content in the seeds. A negative correlation in protein content in their seeds can make them non-viable seeds. From the best of our knowledge, not many reports are present to conserve medicinal plants in their own habitat. This study can help to develop conservation strategies in a situation where unexpected changes in climatic variables mainly rise in temperature in the environment. Here, we demonstrate an overriding effect of confounding factors in experiments about local adaptation to climatic conditions, which calls for the careful experimental design of future studies in the survival of many plant species, such as P. amarus, in their natural habitat.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Our team would like to thank Mr. Sridhar, Mr. Lakshman and Mr. Ezkiel Raj for their help in the preparation of field bed to grow the Phyllanthus amarus seeds for ex situ studies. The first author is thankful to the land owners for giving details and permission to collect this plant, and we are also grateful to UGC-CPEPA, New Delhi, India, for providing financial support to carry out this work.
Author contributions
Conceived and designed the experiments: PP, SNK. Performed the experiments: SNK, MS. Analysed the data: EER, PR. Contributed reagents/materials/analysis tools: GVB, EER, MS. Wrote the paper: SNK, PP.
Compliance with ethical standards
Conflict of interest
The author declares that they have no conflict of interest in the publication.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1236-9) contains supplementary material, which is available to authorized users.
References
- Baskin CC, Baskin JM. Chapter 7—Germination ecology of seeds in the persistent seed bank. In: Baskin CC, Baskin JM, editors. Seeds. San Diego: Academic Press; 1998. pp. 133–179. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Doak DF, Bakker VJ, Goldstein BE, Hale B. What is the future of conservation? Trends Ecol Evol. 2014;29:77–81. doi: 10.1016/j.tree.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956 doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- Edson S, Adriana TN, Massanori T. The germination of seeds of Epiphyllum phyllanthus (L.) Haw. (Cactaceae) is controlled by phytochrome and by nonphytochrome related process. Biota Neotropica. 2010;10:115–119. [Google Scholar]
- Engler R, Randin CF, Thuiller W, Dullinger S, Zimmermann NE, Araojo MB, Pearman PB, Lay GL, Piedallu C, Albert CH, Choler P, Coldea G, Lamo XD, Dirnbock T, Gegout JC, Garcia DG, Grytnes JA, Heegaard E, Hoistad F, Bravo DN, Normand S, Puscar M, Sebastia MT, Stanisci A, Theurillant JP, Trivedi MR, Vittoz P, Guisan A. 21st century climate change threatens mountain flora unequally across Europe. Glob Chang Biol. 2011;17:2330–2341. doi: 10.1111/j.1365-2486.2010.02393.x. [DOI] [Google Scholar]
- Eriksson O. Seed size variation and its effect on germination and seedling performance in the clonal herb Convallaria majalis. Acta Oecologica. 1999;20:61–66. doi: 10.1016/S1146-609X(99)80016-2. [DOI] [Google Scholar]
- Garcia C, Jordano P, Godoy JA. Contemporary pollen and seed dispersal in a Prunus mahaleb population: patterns in distance and direction. Mol Ecol. 2007;16:1947–1955. doi: 10.1111/j.1365-294X.2006.03126.x. [DOI] [PubMed] [Google Scholar]
- Ghosh L, Singh L. Variation in seed and seedling characters of Jatropha curcas L. with varying zones and provenances. Trop Ecol. 2011;52:113–122. [Google Scholar]
- Ginwal HS, Phartyal SS, Rawat PS, Srivastava RL. Seed source variation in morphology, germination and seedling growth of Jatropha curcas Linn in Central India. Silvae Genet. 2005;54:76–80. [Google Scholar]
- Gouvas M, Sakellariou N, Xystrakis F. The relationship between altitude of meteorological stations and average monthly and annual precipitation. Stud Geophys Geod. 2009;53:557–570. doi: 10.1007/s11200-009-0039-1. [DOI] [Google Scholar]
- Gunnell Y. Relief and climate in south Asia: the influence of the Western Ghats on the current climate pattern of peninsular India. Int J Climatol. 1997;17:1169–1182. doi: 10.1002/(SICI)1097-0088(199709)17:11<1169::AID-JOC189>3.0.CO;2-W. [DOI] [Google Scholar]
- Hamilton AC. Medicinal plants, conservation and livelihoods. Biodivers Conserv. 2004;13:1477–1517. doi: 10.1023/B:BIOC.0000021333.23413.42. [DOI] [Google Scholar]
- Henry AJ. Increase of precipitation with altitude. Mon Weather Rev. 1919;47:33–41. doi: 10.1175/1520-0493(1919)47<33:IOPWA>2.0.CO;2. [DOI] [Google Scholar]
- Heuertz M, Vekemans X, Hausman JF, Palada M, Hardy OJ. Estimating seed vs. pollen dispersal from spatial genetic structure in the common ash. Mol Ecol. 2003;12:2483–2495. doi: 10.1046/j.1365-294X.2003.01923.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wang L, Xie X, Yang J, Li Y. Genetic diversity of wild populations of Rheum tanguticum endemic to China as revealed by ISSR analysis. Biochem Syst Ecol. 2010;38:264–274. doi: 10.1016/j.bse.2010.01.006. [DOI] [Google Scholar]
- Iskandar HM, Casu RE, Fletcher AT, et al. Identification of drought-response genes and a study of their expression during sucrose accumulation and water deficit in sugarcane culms. BMC Plant Biol. 2011;11:12. doi: 10.1186/1471-2229-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik N, Kumar K, Kumar S, Kaushik N, Roy S. Genetic variability and divergence studies in seed traits and oil content of Jatropha (Jatropha curcas L.) accessions. Biomass Bioenerg. 2007;31:497–502. doi: 10.1016/j.biombioe.2007.01.021. [DOI] [Google Scholar]
- Khare CP. Phyllanthus amarus Schum. & Thonn. Phyllanthus fraternus Webster. In: Khare CP, editor. Indian medicinal plants: an illustrated dictionary. New York: Springer; 2007. [Google Scholar]
- Krishnan RM, Davidar P. The shrubs of the Western Ghats (South India): floristics and status. J Biogeogr. 1996;23:783–789. doi: 10.1111/j.1365-2699.1996.tb00039.x. [DOI] [Google Scholar]
- Matheson NK, Saini HS. Polysaccharide and oligosaccharide changes in germinating lupin cotyledons. Phytochemistry. 1977;16:59–66. doi: 10.1016/0031-9422(77)83014-8. [DOI] [Google Scholar]
- McCauley DE. Contrasting the distribution of chloroplast DNA and allozyme polymorphism among local populations of Silene alba: implications for studies of gene flow in plants. Proc Natl Acad Sci USA. 1994;91:8127–8131. doi: 10.1073/pnas.91.17.8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley DE. The relative contributions of seed and pollen movement to the local genetic structure of Silene alba. J Hered. 1997;88:257–263. doi: 10.1093/oxfordjournals.jhered.a023103. [DOI] [Google Scholar]
- Murray BR, Brown AHD, Dickman CR, Crowther MS. Geographical gradients in seed mass in relation to climate. J Biogeogr. 2004;31:379–388. doi: 10.1046/j.0305-0270.2003.00993.x. [DOI] [Google Scholar]
- Patel JR, Tripathi P, Sharma V, Chauhana NS, Dixita VK. Phyllanthus amarus: ethnomedicinal uses, phytochemistry and pharmacology: a review. J Ethnopharmacol. 2011;138:286–313. doi: 10.1016/j.jep.2011.09.040. [DOI] [PubMed] [Google Scholar]
- Pius O, Babawale O, Sola EAA, Comfort O. A comparative study of nutritional and phytochemical composition of Phyllanthus amarus leaf and seed. Am J Toxicol Sci. 2015;7:321–327. [Google Scholar]
- Priti H, Aravind NA, Uma Shaanker R, Ravikanth G. Modeling impacts of future climate on the distribution of Myristicaceae species in the Western Ghats, India. Ecol Eng. 2016;89:14–23. doi: 10.1016/j.ecoleng.2016.01.006. [DOI] [Google Scholar]
- Rilkey GJP. Effects of high temperature on protein synthesis during germination of maize (Zea mays L.) Planta. 1981;151:75–80. doi: 10.1007/BF00384240. [DOI] [PubMed] [Google Scholar]
- Rousset F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics. 1997;145:1219–1228. doi: 10.1093/genetics/145.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboya P, Borghetti F. Germination, initial growth, and biomass allocation in three native Cerrado species. Braz J Bot. 2012;35(2):129–135. doi: 10.1590/S1806-99592012000200002. [DOI] [Google Scholar]
- Sáenz-Romero C, Tapia-Olivares BL. Pinus oocarpa isoenzymatic variation along an altitudinal gradient in Michoacán, México. Silvae Genet. 2003;52:237–240. [Google Scholar]
- Shutov AD, Vaintraub IA. Degradation of storage proteins in germinating seeds. Phytochemistry. 1987;26:1557–1566. doi: 10.1016/S0031-9422(00)82245-1. [DOI] [Google Scholar]
- Sneath PHA, Sokal R. Numerical taxonomy. Nature. 1962;193:855–860. doi: 10.1038/193855a0. [DOI] [PubMed] [Google Scholar]
- Sutherland WJ, Broad S, Caine J, Clout M, Dicks LV, Doran H, Entwistle AC, Fleishman E, Gibbons DW, Keim B, LeAnstey B, Lickorish FA, Markillie P, Monk AK, Mortimer D, Ockendon N, Pearce-Higgins PW, Peck LS, Pretty J, Rockström J, Spalding MD, Tonneijck FH, Wintle BC. A horizon scan of global conservation issues for 2016. Trends Ecol Evol. 2016;31:44–53. doi: 10.1016/j.tree.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Tan L, Chen S, Wang T, Dai S. Proteomic insights into seed germination in response to environmental factors. Proteomics. 2013;13:1850–1870. doi: 10.1002/pmic.201200394. [DOI] [PubMed] [Google Scholar]
- Torimaru T, Wennström U, Lindgren D, Wang XR. Effects of male fecundity, interindividual distance and anisotropic pollen dispersal on mating success in a Scots pine (Pinus sylvestris) seed orchard. Heredity (Edinb) 2012;108:312–321. doi: 10.1038/hdy.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Heim U, Buchner P, Wobus U. Seed coat-associated invertases of fava bean control both unloading and storage functions: cloning of cDNAs and cell type-specific expression. Plant Cell. 1995;7:1835–1846. doi: 10.1105/tpc.7.11.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li W, Zhang C, Liu W, Du G. Variation in seed germination of 134 common species on the eastern Tibetan Plateau: phylogenetic, life history and environmental correlates. PLoS ONE. 2014;9:e98601. doi: 10.1371/journal.pone.0098601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.