Abstract
Purpose
Naturally occurring tumor suppressor microRNA-34a (miR-34a) downregulates the expression of >30 oncogenes across multiple oncogenic pathways, as well as genes involved in tumor immune evasion, but is lost or under-expressed in many malignancies. This first-inhuman, phase I study assessed the maximum tolerated dose (MTD), safety, pharmacokinetics, and clinical activity of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumors.
Patients and Methods
Adult patients with solid tumors refractory to standard treatment were enrolled in a standard 3+3 dose escalation trial. MRX34 was given intravenously twice weekly (BIW) for three weeks in 4-week cycles.
Results
Forty-seven patients with various solid tumors, including hepatocellular carcinoma (HCC; n=14), were enrolled. Median age was 60 years, median prior therapies was 4 (range, 1–12), and most were Caucasian (68%) and male (57%). Most common adverse events (AEs) included fever (all grade %/G3 %: 64/2), fatigue (57/13), back pain (57/11), nausea (49/2), diarrhea (40/11), anorexia (36/4), and vomiting (34/4). Laboratory abnormalities included lymphopenia (G3 %/G4 %: 23/9), neutropenia (13/11), thrombocytopenia (17/0), increased AST (19/4), hyperglycemia (13/2), and hyponatremia (19/2). Dexamethasone premedication was required to manage infusion-related AEs. The MTD for non-HCC patients was 110 mg/m2, with two patients experiencing dose-limiting toxicities of G3 hypoxia and enteritis at 124 mg/m2. The half-life was >24 h, and Cmax and AUC increased with increasing dose. One patient with HCC achieved a prolonged confirmed PR lasting 48 weeks, and four patients experienced SD lasting ≥4 cycles.
Conclusion
MRX34 treatment with dexamethasone premedication was associated with acceptable safety and showed evidence of antitumor activity in a subset of patients with refractory advanced solid tumors. The MTD for the BIW schedule was 110 mg/m2 for non-HCC and 93 mg/m2 for HCC patients. Additional dose schedules or MRX34 have been explored to improve tolerability.
Keywords: microRNA, miR-34a, experimental therapeutics, phase I trial, advanced solid tumors
Introduction
MicroRNAs (miRNAs) are naturally occurring, short (~17–23 nucleotides), non-coding RNAs that comprise a new class of post-transcriptional regulators of gene expression [1]. More than 2500 human distinct miRNAs have been identified, many highly conserved from plants to humans and others that may be lineage and tissue specific [2]. miRNAs play critical roles in key biological processes as “master regulators,” simultaneously modulating the expression of up to several hundred genes across multiple cellular pathways [3]. miRNAs are involved in nearly all developmental and pathological processes in animals, and their dysregulation is associated with many human diseases, including cancer [1, 3]. Altered (increased or reduced) expression of miRNAs relative to normal tissue is apparent in virtually all solid tumors and hematological malignancies [4– 5] Numerous studies using cultured cells and animal models have identified miRNAs that can function as conventional tumor suppressors or oncogenes, and have demonstrated that the introduction or repression of a single miRNA can effectively contribute to tumorigenesis, tumor progression, or regression [6–7]. This collective science provides a rationale for developing miRNA-based cancer therapies, particularly given the potential to simultaneously repress multiple oncogenic processes in the tumor microenvironment, including growth and proliferation, drug resistance, cancer stem cells, metastasis, and immune evasion [6– 9] One potential therapeutic strategy is to introduce a miRNA mimic to restore the functionality of a tumor suppressor miRNA that may be lost or expressed at reduced levels in the tumor [10–12].
MRX34, a potential first-in-class miRNA mimic therapy for cancer, is a liposomal formulation of the naturally occurring tumor suppressor miR-34a [13–14]. In patients with a broad range of cancer types, miR-34a is lost or expressed at reduced levels, frequently in association with loss of p53 function, which normally induces its transcription [8, 15–17]. Retrospective clinical studies in a similarly broad range of cancers have linked low miR-34 expression to worse survival [18–23]. miR-34a normally functions to down-regulate the expression of more than 30 different oncogenes across multiple oncogenic pathways (eg, MET, MEK1, MYC, PDGFR-α, CDK4/6, BCL2, WNT 1/3, NOTCH1, CD44), as well as genes involved in tumor immune evasion, (PD-L1, DGKζ) [8, 24 - 26]. Studies introducing miR-34a mimics into cultured cancer cell lines derived from both solid tumors (lung, liver, colon, pancreatic, brain, skin, prostate, bone, ovary) and hematological malignancies (lymphoma, multiple myeloma, leukemias) show significantly reduced cell proliferation, migration and invasion, inhibition of cancer stem cells, and synergistic effects in combination with other anticancer therapies, such as tyrosine kinase inhibitors, cytotoxic chemotherapy, and radiation therapy [8 – 9, 27–31]. In various animal models of cancer, treatment with miR-34a delivered via a variety of vehicles, including liposomal, inhibited the growth of primary tumors, blocked metastasis, and extended survival [8, 13, 29 – 33]. In orthotopic mouse models of hepatocellular carcinoma (HCC), MRX34 demonstrated significant growth inhibition, including tumor regression in more than a third of the treated animals [13].
Here we report results from the first-in-human, phase I clinical trial of miRNA cancer therapy, in which adult patients with refractory advanced solid tumors were treated with escalating twice-weekly (BIW) doses of MRX34.
Patients and Methods
MRX34
MRX34 is a 23-nucleotide long, double-stranded, synthetic version of miR-34a (a miR-34a “mimic”), encapsulated in a liposomal nanoparticle with a diameter of ~110 nm. The liposomal component contains amphoteric lipids that are cationic during liposome formation under acidic conditions to ensure efficient encapsulation of the negatively charged miR-34a mimic, and anionic in vivo at neutral pH to minimize particle aggregation and electrostatic adherence to the cellular membranes of endothelial cells [34]. Consistent with these biochemical attributes, MRX34 has a long circulation time in blood and delivers high numbers of miR-34a mimics to tumors, liver, and a variety of other tissues, including bone marrow, spleen, and lung, when administered intravenously to mice and nonhuman primates [14 – 13, 35].
Patients
Eligible patients were 18 years or older, and had refractory advanced malignancies for which no standard treatment existed, ECOG performance status 0–2, acceptable hepatic, renal, and hematologic function, and anticipated life expectancy of at least 3 months. Patients with non-HCC cancers were required to have hepatic metastases. For patients with HCC, only those with liver disease classified as Child-Pugh A were eligible. Patients with CNS metastases were allowed 4 weeks after completion of treatment provided they were stable with no attributable symptoms. The study followed the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Patients were enrolled with approval from the ethics committees and institutional review boards at participating institutions, and all patients signed a written informed consent prior to starting study-specific procedures.
Study Design
This multi-center, open-label, dose escalation phase I clinical trial was conducted in the United States and Republic of Korea. Standard 3+3 dose-escalation rules were followed. Due to concerns about underlying liver dysfunction, patients with HCC underwent dose escalation in cohorts separate from patients with non-HCC tumors. The starting dose was 10 mg/m2 based on a 10-fold safety reduction of the 9 mg/kg no-observed-adverse-effect level (NOAEL) from a repeat-dose toxicity study in non-human primates. A modified Fibonacci scheme was used to escalate the dose to 20, 33, 50, 70, 93, and 124 mg/m2. When the 124 mg/m2 dose was determined to be above the maximum tolerated dose (MTD), an intermediate dose level of 110 mg/m2 was added. MRX34 was given by intravenous infusion twice a week (BIW) for 3 weeks followed by 1 week of rest, with each 28-day cycle including 6 doses of MRX34. MRX34 infusions were given over 2 to 4 hours using a controlled infusion pump without a filter, and were required to be completed within 5 hours of drug preparation. No premedications were given in the initial two dose levels of 10 mg/m2 and 20 mg/m2. Following the observation of fever, chills, rigors, and back pain at these initial dose levels, routine premedication with a single dose of dexamethasone 10 mg IV was subsequently given to all patients at dose levels of 33 mg/m2 or higher just prior to each dose of MRX34 in cycles 1 and 2. In subsequent cycles, reduction or omission of the dexamethasone dosage was allowed at the investigators’ discretion. The primary objectives of the study were to determine the MTD and the recommended phase II dose. Secondary objectives included assessments of safety and tolerability, pharmacokinetics (PK), and clinical activity.
Study Evaluations
All patients who received treatment with MRX34 were considered evaluable for safety, with adverse events (AEs) graded by the NCI-CTCAE version 4.03. AEs and concomitant medications were evaluated and recorded at each patient visit, with laboratory abnormalities recorded separately. A Cohort Review Committee comprised of the investigators, site coordinators, and medical monitor met weekly via teleconference to review safety and manage the dose-escalation process. An AE was considered a dose-limiting toxicity (DLT) if it occurred during cycle 1, was clinically significant, grade 3 or 4, and related to study treatment. Grade 3 (G3) nausea, vomiting, diarrhea, or cytokine release syndrome (CRS) related to infusion reactions and associated with suboptimal prophylactic and other supportive treatment were not considered DLTs.
Patients were evaluated for antitumor activity by CT or MRI performed at screening, at the end of cycle 2, and then after every even cycle. If a response was noted (RECIST version 1.1), a follow-up radiographic assessment was required at ≥4 weeks (>28 days) for confirmation.
Pharmacokinetics
Blood samples for PK analysis of MRX34 were collected during cycle 1 pre-dose and at 24 and 48 hours post-infusion, as well as more frequently at multiple time points on days 1 and 18. Concentrations of miR-34a mimic extracted from the blood samples were measured by qRT- PCR.14 PK parameters were estimated from blood concentration versus time profiles using commercial software (Phoenix WinNonLin, Pharsight) and a non-compartmental model, with means and standard deviations for each parameter calculated for each dose group.
Statistical Analyses
All patients who received at least one dose of MRX34 were included in the safety analysis, and all patients, including those who had at least one post-treatment response assessment or discontinued before having a response assessment due to rapid clinical disease progression or death, were included in the analysis of response. Descriptive statistics were used for the evaluation of safety, PK, and response data.
Results
Patients and Drug Exposure
From April 2013 to September 2014, we treated 47 patients with Stage IV, advanced cancers non-responsive to standard-of-care treatments with at least one dose of MRX34. The median age was 60 years, and most were Caucasian (68%) and male (57%) (Table 1). Tumor types included HCC (n=14), pancreatic cancer (n=5), cholangiocarcinoma (n=4), and a broad range of other cancers. While most patients had good performance status (0, 30%; 1, 62%), this was a heavily pretreated population, with a median of 4 prior treatments (range 1–12) and 90% having received >3 prior therapies. The median number of cycles of MRX34 received by all patients was 2 (range, 1–16); half of the patients received a single cycle (up to 6 doses), another third received 2 cycles, and seven patients received ≥3 cycles. Thirty-one patients discontinued the study due to disease progression, two patients discontinued due to death secondary to disease progression, another eight withdrew consent, one was lost to follow-up, and five discontinued due to AEs (one with sepsis judged unrelated to study drug, and one each with acute renal injury, low back pain, fatigue, and altered mental status, all judged as possibly related to study drug). Five of the 8 patients that withdrew consent in Cycle 1 had infusion related events (chills, fever, pain) and one patient had 7 cycles then withdrew due to fatigue. Two patients had SAEs (hypoxia and enterocolitis) in Cycle 1 and withdrew consent prior to complete assessment of causality.
Table 1.
Patient Demographics, Disease Characteristics, and MRX34 Treatment Exposure
| Characteristic | Patients (N = 47) |
|---|---|
| Age, median (range) | 60 (29–86) |
| Sex: Male, n (%) | 26 (57) |
| Race: White/Black/Asian/other, % | 68/11/13/8 |
| ECOG Performance Score 0/1/2, % | 30/62/4a |
| Cancer type, n (%) | |
| Hepatocellular | 14 (30) |
| Pancreatic | 5 (11) |
| Cholangiocarcinoma | 4 (9) |
| Neuroendocrine tumor | 3 (6) |
| Colorectal | 3 (6) |
| Breast | 3 (6) |
| Cervical | 3 (6) |
| Bladder | 2 (4) |
| Esophageal | 2 (4) |
| Leiomyosarcoma | 2 (4) |
| Other (1 each) b | 6 (13) |
| Prior therapies, median (range) | 4 (1–12) |
| Prior therapy type, n (%) | |
| Surgery | 30 (64) |
| Chemotherapy | 47 (100) |
| Radiotherapy | 17 (36) |
| Immunotherapy | 2 (4) |
| Investigational | 8 (17) |
| MRX34 cycles delivered, median (range) | 2 (1–16) |
| No. MRX34 cycles delivered, n (%) | |
| 1 | 24 (51) |
| 2 | 16 (34) |
| 3–4 | 4 (9) |
| ≥5 | 3 (6) |
Missing for 2 patients.
Includes adenocarcinoma of unknown primary, appendiceal adenocarcinoma, ovarian, GIST, NSCLC, pheochromocytoma.
Safety
Most AEs and laboratory abnormalities were grade 1 or 2 (Tables 2 and 3). The most frequently observed non-laboratory AEs, regardless of grade and relationship to MRX34, were fever (64%), fatigue (57%), chills (57%), and back pain (57%). Treatment-related serious AEs (SAEs) did not show any notable pattern. Grade 3/4 laboratory abnormalities were recorded frequently but most were not associated with clinical symptoms. Compared to the group of patients with other cancers, the large group of patients with HCC had a higher frequency of laboratory abnormalities related to liver function, but otherwise the safety profiles of MRX34 in the two groups were similar (Table S1). AEs likely related to infusion reactions (ie, fever, chills, back pain) did not increase in frequency or severity with increasing doses of MRX34 administered with routine dexamethasone premedication subsequent to the first two dose cohorts.
Table 2.
Adverse Events (AEs) in Patients with Advanced Solid Tumors Treated with Biweekly MRX34 Monotherapy
| AEs in ≥20 of all patients, n (%) | Dosing Cohort, mg/m2 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (N=47) | ≤50 (n=21)a | 70 (n=11) | 93 (n=7) | 110 (n=6) | 124 (n=2) | |||||||||||||
| All | G3 | G4 | All | G3 | G4 | All | G3 | G4 | All | G3 | G4 | All | G3 | G4 | All | G3 | G4 | |
| All | 45 (96) | 19 (40) | - | 21 (100) | 8 (38) | - | 9 (82) | 4 (36) | - | 7 (100) | 3 (43) | - | 6 (100) | 4 (67) | - | 2 (100) | - | - |
| Fever | 30 (64) | 1 (2) | - | 15 (71) | - | - | 5 (46) | 1 (9) | - | 5 (71) | - | - | 3 (50) | - | - | 2 (100) | - | - |
| Fatigue | 27 (57) | 6 (13) | - | 13 (62) | 3 (14) | - | 5 (46) | 1 (9) | - | 5 (71) | 1 (14) | - | 3 (50) | 1 (16) | - | 1 (50) | - | - |
| Chills | 27 (57) | - | - | 15 (71) | - | - | 4 (36) | - | - | 5 (71) | - | - | 2 (33) | - | - | 1 (50) | - | - |
| Back Pain | 27 (57) | 5 (11) | - | 14 (66) | 4 (19) | 4 (36) | - | - | 4 (57) | - | - | 4 (67) | 1 (16) | 1 (50) | - | - | ||
| Nausea | 23 (49) | 1 (2) | - | 13 (62) | - | - | 4 (36) | - | - | 2 (29) | 1 (14) | - | 2 (33) | - | - | 2 (100) | - | - |
| Diarrhea | 19 (40) | 5 (11) | - | 10 (48) | 3 (14) | 4 (36) | 1 (9) | - | 1 (14) | - | - | 3 (50) | 1 (16) | - | 1 (50) | - | - | |
| Decreased appetite | 17 (36) | 2 (4) | - | 7 (15) | - | - | 3 (27) | - | - | 4 (57) | 1 (14) | - | 2 (33) | 1 (16) | - | 1 (50) | - | - |
| Vomiting | 16 (34) | 2 (4) | - | 7 (15) | - | - | 2 (18) | - | - | 3 (43) | 1 (14) | - | 2 (33) | 1 (16) | - | 2 (100) | - | - |
| Dehydration | 11(23) | 3 (6) | - | 4 (19) | - | - | 3 (27) | 1 (9) | - | 1 (14) | - | - | 2 (33) | 1 (16) | - | 1 (50) | - | - |
| Dyspnea | 12 (25) | - | - | 5 (24) | - | - | 4 (36) | - | - | 2 (29) | - | - | 1 (17) | - | - | - | - | - |
| Insomnia | 11 (23) | - | - | 3 (14) | - | 3 (27) | - | - | 3 (43) | - | - | 2 (33) | - | - | - | - | - | |
| Serious AEs (SAE) | 24 (51) | 14 (30) | 4 (9) | 8 (38) | 5 (24) | 2 (9) | 7 (64) | 2 (18) | 1 (9) | 2 (29) | 1 (14) | 1 (14) | 5 (83) | 4 (67) | - | 2 (100) | 2 (100) | - |
| Treatment- related SAEsb | 18 (38) | 8 (17) | 3 (14) | 5 (24) | 1 (5) | 2 (9) | 4 (36) | 2 (18) | 1 (9) | 2 (29) | 1 (14) | - | 5 (83) | 2 (33) | - | 2 (100) | 2 (100) | - |
| Dose-limiting AEsc | 4 (9) | 4 (9) | - | 1 (5) | 1 (5) | - | - | - | - | - | - | - | 1 (17) | 1 (17) | - | 2 (100) | 2 (100) | - |
| On-study deathsd | 5 (11) | 2 (10) | 2 (18) | - | 1 (17) | - | ||||||||||||
Includes dosing cohorts of 10 (n=3), 20 (n=6), 33 (n=3), and 50 mg/m2 (n=9)
Events included (n=1 patient for each): ≤50- fatigue, acute kidney injury, diarrhea, dehydration, increased transaminases; 70- atrial fibrillation, altered mental status, neutropenic fever, fever; 93-right groin hematoma, anemia; 110- chest pain, dehydration, fatigue, small bowel obstruction, vomiting; 124-hypoxia, enterocolitis.
Events included: 1 G3 acute kidney injury at 20, 1 G3 fatigue at 110, and 1 G3 hypoxia and 1 G3 enteritis at 124 mg/m2; dosing of patients with HCC ended at 93 mg/m2 due to persistent thrombocytopenia which ended treatment after 3 doses in cycle 1 in one patient and anemia lasting 2 weeks that prolonged the start of cycle 2 in another.
From initial dose to 30 days after last dose; 4 deaths due to disease progression and 1 due to cardiac arrest unrelated to study drug.
Table 3.
Laboratory Abnormalities in Patients with Advanced Solid Tumors Treated with Biweekly MRX34 Monotherapy
| Laboratory abnormalities, n (%) | Dosing Cohort, mg/m2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (N=47) | ≤50 (n=21)a | 70 (n=11) | 93 (n=7) | ≥110 (n=8)b | |||||||||||
| G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | |
| Lymphocytopenia | 12 (26) | 11 (23) | 4 (9) | 2 (10) | 6 (29) | - | 4 (36) | 3 (27) | 1 (9) | 2 (29) | 1 (14) | 2 (29) | 4 (50) | 1 (13) | 1 (13) |
| Thrombocytopenia | 10 (21) | 8 (17) | - | 2 (10) | 3 (14) | - | 3 (27) | 3 (27) | - | 2 (29) | 1 (14) | - | 3 (38) | 1 (13) | |
| Neutropenia | 7 (15) | 6 (13) | 5 (11) | 3 (14) | 2 (10) | 3 (14) | 1 (9) | 2 (18) | 1 (9) | 2 (29) | 1 (14) | - | 1 (13) | 1 (13) | 1 (13) |
| Decreased albumin | 20 (43) | 2 (4) | - | 9 (19) | - | - | 5 (45) | - | - | 3 (43) | - | - | 3 (38) | 2 (25) | - |
| Increased ALT | 2 (4) | 2 (4) | 1 (2) | - | 2 (10) | 1 (5) | 1 (9) | - | - | 1 (14) | 1 (14) | - | - | - | - |
| Increased AST | 6 (13) | 9 (19) | 2 (4) | 2 (10) | 4 (19) | 2 (10) | - | 4 (36) | - | 4 (57) | - | - | - | 1 (13) | - |
| Increased bilirubin | 4 (9) | 2 (4) | - | 2 (10) | 1 (5) | - | 1 (9) | 1 (9) | - | - | - | - | 1 (13) | - | - |
| Hyperglycemia | 5 (11) | 6 (13) | 1 (2) | 2 (10) | - | - | 2 (18) | - | - | - | 2 (29) | 1 (14) | 1 (13) | 4 (50) | - |
| Hyponatremia | - | 9 (19) | 1 (2) | - | 4 (19) | - | - | 3 (27) | - | - | 1 (14) | - | - | 1 (13) | 1 (13) |
Includes dosing cohorts of 10 (n=3), 20 (n=6), 33 (n=3), and 50 mg/m2 (n=9).
Includes dosing cohorts of 110 (n=6) and 124 mg/m2 (n=2).
Dose Escalation
The MTD for non-HCC patients was 110 mg/m2. DLTs in these patients included G3 acute kidney injury at 20 mg/m2, G3 fatigue at 110 mg/m2, and G3 hypoxia and G3 enteritis at 124 mg/m2. The G3 acute kidney injury at 20 mg/m2 occurred in a patient with non-small-cell lung cancer (NSCLC) with signs of renal toxicity on previous cisplatin therapy; this AE resolved with supportive care following development of nausea, vomiting, and elevated creatinine on day 2 of cycle 1. In the second patient, who had pancreatic cancer, G3 fatigue occurred on day 8 of cycle 1 at 110 mg/m2; this patient lost 12 lbs in 10 days and withdrew consent on day 15 of cycle 1. The G3 hypoxia at 124 mg/m2 occurred after the first dose of MRX34 in a patient with breast cancer and metastases to the lung and liver. Drug-related pneumonitis was suggested by the work-up, which included bronchoscopy; the patient met the criteria for systemic inflammatory response syndrome (SIRS) and was empirically started on antibiotics. The G3 enteritis at 124 mg/m2 occurred in a pancreatic cancer patient, who was admitted with abdominal pain and fever after one dose of MRX34. The work-up demonstrated small bowel inflammation, with CT evidence of new ascites and focal thickening of the small bowel and right colon. After treatment with supportive antibiotics and resolution of symptoms, the patient withdrew consent. For patients with HCC, the cohort review committee recommended to discontinue dose escalation in the BIW schedule and explore QDX5 dosing. Therefore the MTD for HCC patients at the BIW schedule, was determined to be the last dose level studied of 93 mg/m2 -.
Pharmacokinetics
Blood concentration versus time curves showed variability within and between the dose levels (Figure 1). Large standard deviations were observed for all parameters (Table S2), likely related, in part, to differences in the length of infusions and discontinuous (start, stop) dosing. In general, estimated Cmax and AUC increased with increasing dose, with similar values within each dose cohort between days 1 and 18. The increases were non-dose proportional, however; for example, as the dose increased 9-fold from 10 to 93 mg/m2, the estimated AUC increased ~30-fold. This effect suggests the potential for dose-related saturation of the presumed mononuclear phagocytic system clearance of the liposomes. The half-life in the cohorts from 20–100 mg/m2 was approximately 35 hours. There were no observed differences in the AUCs of HCC versus non-HCC patients.
Figure 1.
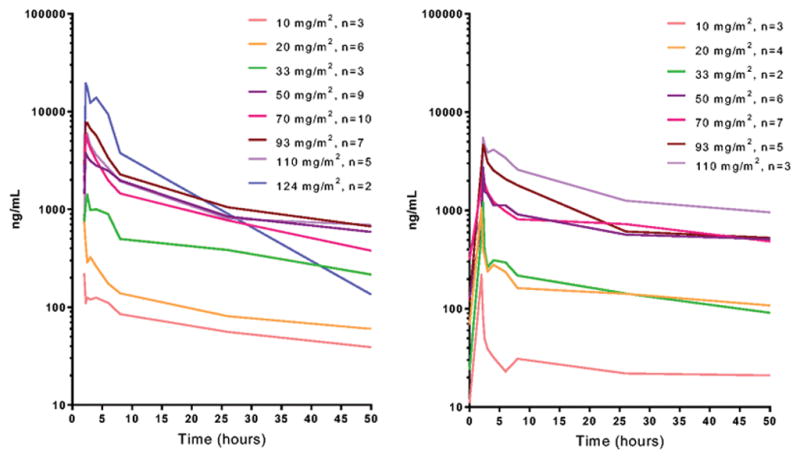
Blood concentration vs time profiles of miR-34a mimic showing mean values (ng/mL) at each dosing level for day 1 (left) and day 18 (right) in cycle 1. Blood samples were collected prior to infusion and at selected times post-infusion.
Efficacy
Of the 47 patients enrolled, 35 (75%) had at least one tumor reassessment on study. One prolonged confirmed PR lasting 48 weeks occurred after initial SD for 6 cycles on MRX34 in a 47-year-old Asian male patient with HBV-associated HCC that had been initially resected, but recurred in the lung after progression on sorafenib (Table 4, Figure 2). Unequivocal progression of a non-target lesion occurred after cycle 17, and both target and non-target lesions remained stable for an additional 2 cycles of MRX34 and 4 months off treatment (as of July 2016). Six patients had SD as their best overall response, including one extrahepatic cholangiocarcinoma for 3 cycles, one colorectal carcinoma for 4 cycles, two with HCC for 4 and 7 cycles, one leiomyosarcoma for 2 cycles, and one NSCLC for 8 cycles (Table S3). Eight patients who had received at least one dose of MRX34 were not evaluable for response, including three patients due to AEs (acute renal injury, back pain, and fatigue) and five who withdrew consent. The remaining 32 patients (68%) had PD as their best response.
Table 4.
Best Overall Response in Patients with Refractory Advanced Solid Tumors Treated with Twice Weekly MRX34 Monotherapy
| Best overall response, n (%) | Patients(N = 47) |
|---|---|
| CR | 0 |
| PRa | 1 (2) |
| SDb | 6 (13) |
| PD | 32 (68) |
| Not evaluablec | 8 (17) |
Advanced HBV-associated HCC patient with prolonged confirmed PR lasting 48 weeks following SD for the initial 6 MRX34 treatment cycles; patient had PD with progressing non-target lesion after cycle 17, and stable target and non-target lesions since for an additional 2 cycles and 4 months off treatment (as of July 2016).
Range, 2–8 cycles; 4 with SD lasting ≥4 cycles, includes 1 extrahepatic cholangiocarcinoma for 3 cycles, 1 colorectal carcinoma for 4 cycles, 2 HCC for 4 and 7 cycles, 1 leiomyosarcoma for 2 cycles, and 1 non-small-cell lung cancer for 8 cycles.
Includes 3 AE-related (acute renal injury, back pain, fatigue) and 5 withdrawing consent.
Figure 2. Prolonged Confirmed PR in Patient with Advanced HBV-associated HCC.
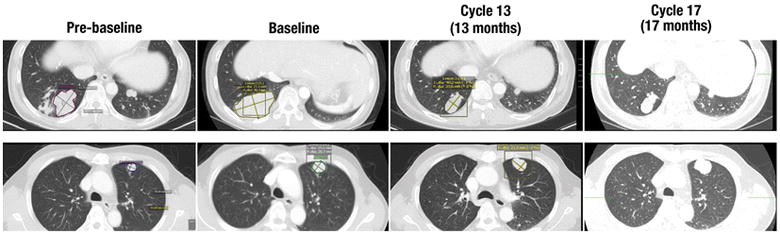
Note progressive disease (pre-baseline to baseline) in lung metastases target lesions prior to enrollment in study. MRX34 BIW 50 mg/m2 was initiated in August 2014, with stable disease for the initial 6 cycles and then prolonged confirmed PR lasting 48 weeks (34% maximum sum-of-diameters reduction for target lesions by RECIST 1.1). The patient remained in PR until cycle 17 when progression in a non-target lesion was noted. As of April 2016, the patient had received 2 additional cycles (19 total) of MRX34, with the size of both target and non-target lesions remaining stable.
Discussion
This first-in-human study of a miRNA therapy provides valuable insights on the potential use of a new class of oligonucleotide-based drugs in oncology. Although mostly grade 1 and 2, the AEs associated with MRX34, particularly those that appeared to be infusion-associated, made continuous administration of this drug on the BIW schedule difficult. While routine dexamethasone premedication moderated these AEs, it did not stop them from occurring. This, and difficulty in adhering to the schedule made compliance with therapy challenging, resulting in early discontinuation in many patients, which may have limited the ability to demonstrate treatment benefit. A second “QDx5” dosing regimen of MRX34 explores an alternate dosing schedule of dexamethasone 10 mg BID for 7 days and once-daily MRX34 infusions over 2 hours for 5 consecutive days in week 1, followed by 2 weeks off in 21-day cycles. This schedule is designed to improve tolerability and facilitate adherence, thereby increasing drug exposure; it also reduces the duration and total dose of steroid therapy per treatment cycle.
Our study does not allow direct attribution of AEs to the liposomal carrier versus the miR-34a mimic, but many of the AEs that appeared to be temporally related to the MRX34 infusions could potentially be attributed to the liposome carrier independent of the miR-34a mimic. Liposome-related toxicities have been well characterized and may include complement activation, pro-inflammatory effects, and thrombocytopenia secondary to activation of the macrophage phagocyte system and/or margination of platelets into liver and spleen [36]. Tolcher et al have reported phase I results for a BCL-2-targeted, single-stranded DNA oligonucleotide drug, PNT2258 (ProNAi Therapeutics, Inc., Plymouth, MI), that uses the same liposomal carrier as MRX34 [34]. In this study, 22 patients with refractory advanced solid tumors were given daily IV infusions of PNT2258 on days 1–5 in a 21-day cycle and completed a median 2 cycles of therapy. PNT2258 was well tolerated, with fatigue the most commonly reported treatment-related AE. While the two studies cannot be directly compared, the Tolcher et al results suggest that the liposomal carrier was not the cause of the more frequent and severe infusion-associated reactions we observed with MRX34. Furthermore, it is likely that effects related to liposome exposure would be effectively mitigated, as with other liposomal formulated drugs, by the dexamethasone premedication.
Another possibility that could account for the differences in AEs observed in the PNT2258 phase I study and ours is the different oligonucleotide types — single-stranded DNA in PNT2258 and double-stranded RNA (dsRNA) in MRX34. Non-specific inflammatory effects have been well characterized for dsRNA, whereby these molecules can act as triggers of innate immunity involved in the host response to viral infection [37–38] It is therefore plausible that the occasionally prolonged infusion reactions observed in our study could be associated with non-specific effects due to exposure to the dsRNA, miR-34a mimic component of MRX34. Interestingly, recent research results suggest that this non-specific mechanism might play a role in promoting effective anti-tumor immune responses [39–40].
Other MRX34-related AEs that tended to occur later post-infusion, such as diarrhea/enteritis, fatigue, altered mental status, and dyspnea/hypoxia, could potentially be immune-related toxicities similar to those that occur with the anti-CTLA-4 and anti-PD-1/PD-L1 immune checkpoint inhibitors [41]. This hypothesis is supported by the recent observation that miR-34a can repress immune-related genes such as PD-L1 [24 - 26]. Given the wide range of potential effects (liposomal-related, dsRNA-related non-specific inflammation, and miR-34a-related specific modulation of gene expression), it will be important in further development to clearly determine their relative contributions to MRX34 toxicity and anti-tumor activity, and studies focused on this question are underway. It will also be important to more fully understand the potential effects of the dexamethasone that appears to be necessary to tolerably administer MRX34.
In conclusion, our results show that miRNA therapy with MRX34 is feasible and tolerable under adequate dexamethasone premedication and provide preliminary evidence of antitumor activity. Additional clinical studies of MRX34 monotherapy with supportive translational research are underway in patients with refractory advanced tumors using the QDx5 regimen with required dexamethasone premedication.
Supplementary Material
Table S1. Adverse Events and Laboratory Abnormalities in Patients with HCC and non-HCC Solid Tumors Treated with Twice Weekly MRX34 Monotherapy
Table S2. Summary of Pharmacokinetic Parameters by Cohort
Table S3. Detailed Summary of Patients with Response and Stable Disease as Their Best Overall Response
Acknowledgments
Funded by Mirna Therapeutics, Inc.
We thank the patients and their families as well as the co-investigators and study teams for making this study possible. Assistance with medical writing and editing was provided by David E. Egerter, PhD, funded by Mirna Therapeutics.
Footnotes
Presented in part (preliminary results) at the 26th AACR-NCI-EORTC Symposium on Molecular Targets and Cancer Therapeutics, Barcelona, Spain, November 18–21, 2014, and the AACR-NCI-EORTC International Congress on Molecular Targets and Cancer Therapeutics, Boston, MA, November 5–9, 2015.
Clinical trial information: NCT01829971
Statement of Human Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Compliance with Ethical Standards
Conflicts of Interest
Muhammad S. Beg has consulting/advisory roles at Bayer, Celgene, and Ipsen, and has received research funding from Celgene, Mirna, and Precision Biologics, and travel expenses from Mirna and Precision Biologics. Andrew J. Brenner has consulting/advisory roles at NanoTX and Teleflex Medical, holds intellectual property with NanoTX, and has received research funding from Mirna and Threshold, and travel expenses from Vascular Biogenics. Jasgit Sachdev has a consulting/advisory role at Celgene and has received honoraria from Celgene. Mitesh Borad has no relationships to disclose. Yoon-Koo Kang has consulting/advisory roles at Lilly/ImClone, Novartis, Ono, Genentech, and Taiho, and has received research funding from Bayer, Novartis, and Genentech. Jay Stoudemire, Susan Smith, Andreas G. Bader, and Sinil Kim are, or were at the time of the study, employed by Mirna and own stock in Mirna; Dr Bader additionally is an inventor on patents and patent applications assigned to Mirna, and Dr Kim additionally owns stock in Pfizer. David S. Hong has received research funding from Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Genentech, Lilly, Merck, Mirati, Mirna, Novartis, and Pfizer, and travel expenses from Loxo and Mirna.
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Londin E, Loher P, Telonis AG, et al. Analysis of 13 cell types reveals evidence for the expression of numerous novel primate- and tissue-specific microRNAs. PNAS; Epub February. 2015;23:E1106–E1115. doi: 10.1073/pnas.1420955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 5.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 6.Kasinski AL, Slack FJ. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–64. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansson MD, Lund AH. MicroRNA and Cancer. Molecular Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bader AG. miR-34–a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3 doi: 10.3389/fgene.2012.00120. article 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortez MA, Ivan C, Valdecanas D, Wang X, et al. PDL1 regulation by p53 via miR-34. J Natl Cancer Inst. 2016;108:djv303. doi: 10.1093/jnci/djv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Res. 2010;70:7027–30. doi: 10.1158/0008-5472.CAN-10-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trang P, Wiggins JF, Daige DL, et al. Systemic Delivery of Tumor Suppressor microRNA Mimics using a Neutral Lipid Emulsion Inhibits Lung Tumors in Mice. Mol Ther. 2011;19:1116–22. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bader AG, Brown D, Stoudemire J, et al. Developing therapeutic microRNAs for cancer. Gene Ther. 2011;18:1121–6. doi: 10.1038/gt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daige CL, Wiggins JF, Priddy L, et al. Systemic delivery of a miR-34a mimic as a potential therapeutic for liver cancer. Mol Cancer Ther. 2014;13:2352–60. doi: 10.1158/1535-7163.MCT-14-0209. [DOI] [PubMed] [Google Scholar]
- 14.Kelnar K, Peltier HJ, Leatherbury N, et al. Quantification of therapeutic miRNA mimics in whole blood from non-human primates. Anal Chem. 2014;86:1534–42. doi: 10.1021/ac403044t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He L, He X, Lim LP, et al. A microRNA component of the p53 tumor suppressor network. Nature. 2007;447:1130–34. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–9. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Lammers P, Torrance CJ, et al. TP53-independent function of miR-34a via HDAC1 and p21(CIP1/WAF1) Mol Ther. 2013;21:678–86. doi: 10.1038/mt.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CH, Subramanian S, Beck AH, et al. MicroRNA profiling of BRCA1/2 mutation-carrying and non-mutation-carrying high-grade serous carcinomas of ovary. PloS One. 2009;4:e7314. doi: 10.1371/journal.pone.0007314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagman Z, Larne O, Edsjo A, et al. miR-34c is downregulated in prostate cancer and exerts tumor suppressive functions. Int J Cancer. 2010;127:2768–76. doi: 10.1002/ijc.25269. [DOI] [PubMed] [Google Scholar]
- 20.Nakatani F, Ferracin M, Manara MC, et al. miR-34a predicts survival of Ewing's sarcoma patients and directly influences cell chemo-sensitivity and malignancy. J Pathol. 2012;226:796–805. doi: 10.1002/path.3007. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson NB, Morran DC, Morton JP, et al. MicroRNA molecular profiles associated with diagnosis, clinicopathologic criteria, and overall survival in patients with resectable pancreatic ductal adenocarcinoma. Clin Cancer Res. 2012;18:534–45. doi: 10.1158/1078-0432.CCR-11-0679. [DOI] [PubMed] [Google Scholar]
- 22.Hiyoshi Y, Schetter AJ, Okayam H, et al. Increased microRNA-34b and -34c predominantly expressed in stromal tissues is associated with poor prognosis in human colon cancer. PloS One. 2015;10:e0124899. doi: 10.1371/journal.pone.0124899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Dan G, Zhao J, et al. The predictive effect of overexpressed miR-34a on good survival of cancer patients: a systematic review and meta-analysis. Onco Targets Ther. 2015;8:2709–19. doi: 10.2147/OTT.S84043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin J, Danli X, Zhong XP. MicroRNA-34a Enhances T Cell Activation by Targeting Diacylglycerol Kinase ζ. PLoS ONE. 2013;8:e77983. doi: 10.1371/journal.pone.0077983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortez MA, Valdecanas D, Niknam S, et al. In vivo delivery of miR-34a sensitizes lung tumors to radiation through RAD51 regulation. Molecular Therapy—Nucleic Acids. 2015;4:e270. doi: 10.1038/mtna.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Li J, Dong K, et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal. 2015;27(3):443–52. doi: 10.1016/j.cellsig.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li N, Fu H, Tie Y, et al. miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett. 2009;275:44–53. doi: 10.1016/j.canlet.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 29.Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–5. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Martino MT, Leone E, Amodio N, et al. Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: in vitro and in vivo evidence. Clin Cancer Res. 2012;18:6260–70. doi: 10.1158/1078-0432.CCR-12-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Kelnar K, Bader AG. In-depth analysis shows synergy between erlotinib and miR-34a. PLOS One Feb. 2014;14:e8910. doi: 10.1371/journal.pone.0089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiggins JF, Ruffino L, Kelnar K, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–30. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craig VJ, Tzankov A, Flori M, et al. Systemic microRNA-34a delivery induces apoptosis and abrogates growth of diffuse large B-cell lymphoma in vivo. Leukemia. 2012;26:2421–4. doi: 10.1038/leu.2012.110. [DOI] [PubMed] [Google Scholar]
- 34.Tolcher AW, Rodrigueza WV, Rasco DW, et al. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73:363–71. doi: 10.1007/s00280-013-2361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelnar K, Bader AB. A qRT-PCR method for determining the biodistribution profile of a miR-34a mimic. Chapter 8. In: Walther W, Stein U, editors. Gene Therapy of Solid Cancers: Methods and Protocols, Methods in Molecular Biology. Vol. 1317. 2015. pp. 125–33. [DOI] [PubMed] [Google Scholar]
- 36.Szebeni J, Muggia F, Gabizon A, et al. Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention. Adv Drug Del Rev. 2011;63:1020–30. doi: 10.1016/j.addr.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Robbins M, Judge A, Ambegia E, et al. Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation. Hum Gene Ther. 2008;19:991–9. doi: 10.1089/hum.2008.131. [DOI] [PubMed] [Google Scholar]
- 38.Chattopadhyay S, Sen GC. dsRNA-activation of TLR3 and RLR signaling: gene induction-dependent and independent effects. J Interfer Cytokine Res. 2014;34(6):427–436. doi: 10.1089/jir.2014.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiappinelli KB, Strissel PL, Desrichard A, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell. 2015;162:974–86. doi: 10.1016/j.cell.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dear AE. Epigenetic modulators and the new immunotherapies. N Engl J Med. 2016;374:684–6. doi: 10.1056/NEJMcibr1514673. [DOI] [PubMed] [Google Scholar]
- 41.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Adverse Events and Laboratory Abnormalities in Patients with HCC and non-HCC Solid Tumors Treated with Twice Weekly MRX34 Monotherapy
Table S2. Summary of Pharmacokinetic Parameters by Cohort
Table S3. Detailed Summary of Patients with Response and Stable Disease as Their Best Overall Response


