Abstract
Introduction
Linac-based stereotactic radiosurgery (SRS) for brain metastases may be influenced by the time interval between treatment preparation and delivery, related to risk of anatomical changes. We studied tumor position shifts and its relations to peritumoral volume edema changes over time, as seen on MRI.
Methods
Twenty-six patients who underwent SRS for brain metastases in our institution were included. We evaluated the occurrence of a tumor shift between the diagnostic MRI and radiotherapy planning MRI. For 42 brain metastases the tumor and peritumoral edema were delineated on the contrast enhanced T1weighted and FLAIR images of both the diagnostic MRI and planning MRI examinations. Centre of Mass (CoM) shifts and tumor borders were evaluated. We evaluated the influence of steroids on peritumoral edema and tumor volume and the correlation with CoM and tumor border changes.
Results
The median values of the CoM shifts and of the maximum distances between the tumor borders obtained from the diagnostic MRI and radiotherapy planning MRI were 1.3 mm (maximum shift of 5.0 mm) and 1.9 mm (maximum distance of 7.4 mm), respectively. We found significant correlations between the absolute change in edema volume and the tumor shift of the CoM (p < 0.001) and tumor border (p = 0.040). Patients who received steroids did not only had a decrease in peritumoral edema, but also had a median decrease in tumor volume of 0.02 cc while patients who did not receive steroids had a median increase of 0.06 cc in tumor volume (p = 0.035).
Conclusion
Our results show that large tumor shifts of brain metastases can occur over time. Because shifts may have a significant impact on the local dose coverage, we recommend minimizing the time between treatment preparation and delivery for Linac based SRS.
Keywords: SRS, Brain metastasis, Edema, Steroids, Tumor shifts
Introduction
Brain metastases occur in approximately 10–30% of all cancer patients with solid tumors [1]. Different treatment modalities are available, including surgery, whole brain radiation therapy (WBRT), stereotactic radiosurgery (SRS) and best supportive care. Choice of treatment is based on patient related factors and tumor characteristics and is to be determined in multidisciplinary teams [2]. SRS is often the treatment of choice for patients with smaller tumors, limited number of lesions, and for patients with unresectable tumors or who are medically inoperable. The maximum tumor volume and the number of lesions that can safely be treated simultaneously with SRS is a subject of investigation [3], [4]. Different systems are used for SRS, such as the Gammaknife®, Cyberknife® and Linac based systems [5]. Many institutes apply Linac-based SRS, which requires a robust positioning of the skull in SRS frames or image guidance based on CBCT with skull focused registration [6]. However, the variation of tumor location in time and the possible influence of steroids hereon are unknown. For Linac-based SRS the time between the pretreatment MRI and the actual treatment delivery may take several days in which tumor shifts can occur resulting in suboptimal target coverage.
Patients with brain metastasis often experience neurologic symptoms triggered by the tumor mass, and often by the surrounding edema. For patients with significant or symptomatic peritumoral edema, steroids (i.e. dexamethasone) are commonly prescribed. Although the mechanism is not entirely clear, studies show a decrease in radiographic edema after the administration of dexamethasone [7]. We hypothesize that a change (increase or decrease) of edema may have an impact on the tumor position.
To our knowledge, there is currently no literature available about the extent of tumor shifts in patients with brain metastases. In this work, we used the time between the diagnostic MRI and the radiotherapy planning MRI to study the changes in tumor volume, spatial location and edema volume as function of time.
Materials and methods
Patients
For this study we included 26 patients receiving a single fraction of SRS treatment for brain metastasis between October 2015 and February 2016 at the Netherlands Cancer Institute (NKI). Patients were excluded when the patient had previous WBRT, when the SRS was given to surgical cavity (i.e. post-resection), when the tumor location was not in the brain parenchyma or if one of the MRI sequences was not available. Information about steroid use and systemic cytotoxic treatment was retrospectively retrieved from the electronic patient file and binary scored (i.e., yes/no).
Imaging
The MR examination included a Fluid-attenuated inversion recovery (FLAIR) sequence and a T1 weighted sequence with contrast (T1w + c) for both the diagnostic MRI (MRD) protocol as for the radiation treatment planning MRI (MRRT) protocol. For 14 patients the MRD was performed at the NKI and 12 patients were referred from another hospital (with a MRD executed at the referring hospital). There were 8 different referring hospitals. Slice thickness of the MRD images varied from 0.9 to 6 mm for the T1w sequence and 4.4 to 6 mm for the FLAIR sequence. For the MRRT the slice thickness was 1 mm and 3.3 mm for the T1w + c and FLAIR sequence, respectively. The contrast agent (Dotarem, Guerbet, France, 15 ml) was injected using an automated injection pump (Spectris Solaris, Medrad Inc.). Details about chemical shift artifacts, deviations in localization due to gradient non-linearity and slice thickness are given in the Supplementary data (Table S1).
Registrations and delineations
In house developed software was used for registration and volume determination. Both the MRD and MRRT images were skull based rigidly registered with the planning CT scan (CTRT). Both the gross tumor volume (GTV) and edema were delineated by a single observer (EH) and reviewed by an experienced CNS radiation oncologist (GB).
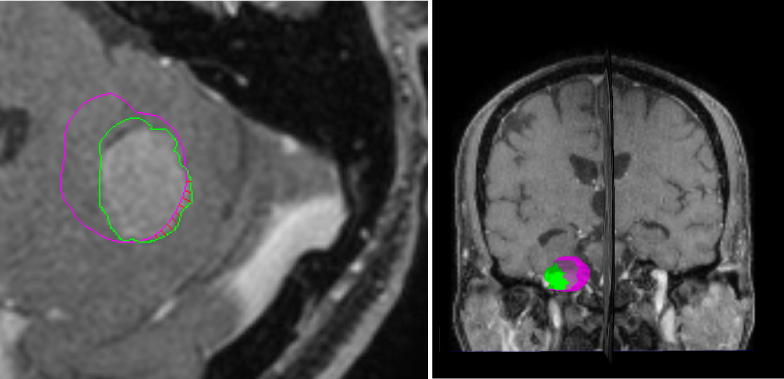
The tumor was contoured on the T1w + c, and the peritumoral edema was contoured on the FLAIR sequence (Fig. 1).
Fig. 1.
Example of patient who received the day before the MRD one gift of dexamethasone and continued dexamethasone intake hereafter (2dd4mg). The MRRT was made 8 days later and the tumor contours overlaid on sagittal (left) and coronal (right) view of the MRRT T1w + c image. The pink contour is the reference contour from the MRD examination, whereas the green contour is delineated on the data from the MRRT examination. The red lines in the left image represent the positive distances between the two tumor surfaces. The right image shows a 3-dimentional depiction of these tumor contours. Here, the MRRT tumor volume which is shifted outside the MRD tumor volume is indicated in green. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For all delineations, the volume and volume change between MRD and MRRT were determined. To calculate the edema volume, the tumor volume was excluded by subtracting the intersection between the edema and the tumor.
Tumor shift
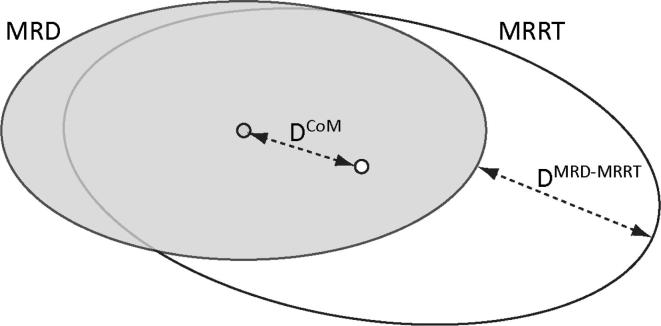
The magnitude of the tumor shifts was evaluated by two parameters: the displacement of the Centre of Mass (DCoM) of the tumor on the MRD and MRRT (with CoM representing the central point of the tumor within its contour) and by the maximal perpendicular distance between the two tumor delineations on the MRD and MRRT (DMRD-MRRT; Fig. 2).
Fig. 2.
Schematic 2D view of the distances DCoM and DMRD-MRRT. DCoM represents the shift of the Centre of Mass of the tumor on the MRD (in grey) and MRRT (in white), whereas DMRD-MRRT is the maximal perpendicular distance between the two tumor delineations on the MRD and MRRT. We did correct for possible tumor volume changes as explained in the material and methods (but this is not schematically represented in this graph).
For the DMRD-MRRT, the delineation on MRD was used as the reference delineation and the delineation on MRRT as the target delineation. The delineations were automatically triangulated and resampled to 1 mm point spacing on the reference delineation. The distance perpendicular on the resample point of the reference scan towards the target scan was then automatically measured (Fig. 1).
DMRD-MRRT was corrected for tumor growth by subtracting the difference in radius between the two tumor contours from the maximum distance (assuming a spherical tumor with radius r = (V/(4/3π))⅓ and isotropic growth).
To determine the influence/dependence of the tumor location on tumor shifts, the shortest distance of the tumor surface to the internal bone surface of the skull was measured on the transversal T1w + c MRI images.
Statistics
We constructed summary statistics (sum, mean, median, standard deviation (SD), median and ranges) for the baseline variables. Graphics were made in Microsoft Excel software version 2010 and all statistical analyses were performed using IBM SPSS Statistics software version 22.
We used the nonparametric Mann–Whitney U test for variables without normal distribution, Spearman's rank correlation coefficient (rs) to evaluate correlations and p values smaller than smaller than 0.05 was considered as statistically significant.
For patients with multiple metastases we executed the correlation analysis for all these metastasis independently, but repeated the analysis including only the metastasis with the largest shift if located in the same edema region. This was to avoid biases caused by the effect of the same edema region on multiple metastases.
Results
Patients and MRI scans
A total of 26 consecutive treated patients were included in this study with a total number of 62 brain metastases on MRD and 65 on MRRT. Twenty-three lesions were excluded because they were not located in the brain parenchyma (e.g. brainstem or dural layer), were already treated with SRS in the past or were not visible on MRD. Final analyses included 42 lesions. The median time between the two MRI scans was 22 days (range 6–43 days) and between MRRT and SRS delivery 8 days (range 4–13 days).
The median tumor volume on MRD and MRRT was 1.05 cc (range 0.05–20.38 cc), and 0.83 cc (range 0.01–21.47 cc), respectively. The median volume of edema on MRD was 7.96 cc (range 0–197.92 cc), on MRRT 8.39 cc (range 0–129.63 cc).
Forty-six percent of the patients (n = 12) received steroids because of neurological symptoms. Symptoms included headache, seizures, neurologic deficit, aphasia and cognitive dysfunction. One patient was using steroids due to symptoms secondary to a lobectomy of the primary lung carcinoma.
Peritumoral edema and the effect of steroids
Forty percent of the tumors (n = 17) had a decrease in peritumoral edema (median 20.59 cc, range 0.004–95.14) and 50% of the tumors (n = 21) had an increase in edema (median 1.67 cc, range 0.03–77.38 cc). Only 10% (n = 4) of the tumors did not have any peritumoral edema on both MRD and MRRT.
Patients receiving steroids had a larger volume of peritumoral edema on MRD than patients who did not receive steroids (p = 0.004). The median decrease in the volume of the edema for the patients using steroids was −16.68 cc (range of 95.14 cc decrease to 9.23 cc increase), which was significantly different from the 0.71 cc median increase (range of 2.45 cc decrease to 77.38 cc increase) for the patients without steroids (p < 0.001).
Edema changes, DCoM and DMRD-MRRT
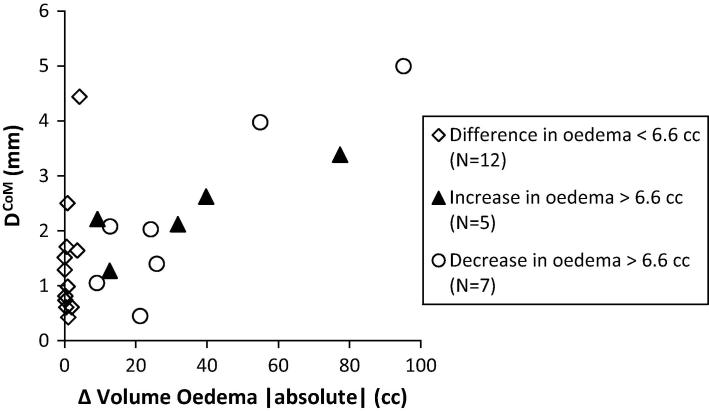
The median DCoM was 1.3 mm (range 0.2–5.0 mm) for the whole group. We found a significant correlation between the absolute change in edema volume and DCoM (rs = 0.459, p = 0.002), showing that larger volume changes of edema result in larger shifts of the DCoM of the tumor. If we select in patients with multiple metastases the tumor with largest DCoM, this correlation is increased (rs = 0.640, p < 0.001, Fig. 3). The median absolute change in edema volume was 3 cc (increase or decrease). For tumors with a change of edema more than 3 cc there was a significant larger DCoM compared to DCoM of tumors with an edema volume change less than 3 cc (Table 1).
Fig. 3.
DCoM as function of absolute volume change of oedema. The group ‘difference in oedema >6.6 cc’ is split up in patients with a decrease and increase of oedema. For the whole group a significant Spearman correlation of r2 = 0.640 (p < 0.001) is found. For 2 patients no oedema is seen on MRD. For patients with multiple metastases, only the tumor with largest DCoM is included resulting in a cut off of 6.6 cc.
Table 1.
Median DCoM and DMRD-MRRT for absolute oedema volume change larger or smaller than 3 cc.
| |Δ Volume oedema| | DCoM (mm) | Range | p* |
| Median (25, 75) | |||
| <3.0 cc (n = 21) | 0.89 (0.56, 1.44) | 0.22–2.50 | p = 0.005 |
| >3.0 cc (n = 21) | 1.40 (1.06, 2.42) | 0.32–5.00 | |
| DMRD-MRRT (mm) | |||
| |Δ Volume oedema| | Median (25, 75) | Range | p* |
| <3.0 cc (n = 21) | 1.7 (1.11, 2.16) | 0.43–3.78 | p = 0.032 |
| >3.0 cc (n = 21) | 2.63 (1.23, 4.65) | 0.15–7.42 | |
Mann–Whitney U test.
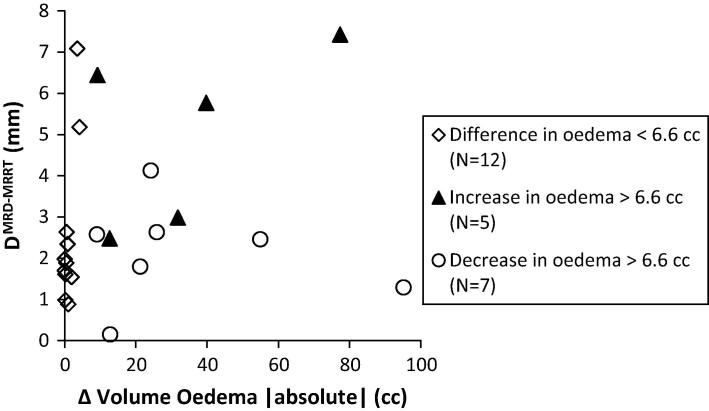
There was no significant correlation between the change in edema volume and DMRD-MRRT (rs = 0.206, p = 0.191) with a median DMRD-MRRT of 1.9 mm (range 0.2–7.4 mm) for the whole group although a significant correlation was found selecting the tumors with largest DMRD-MRRT (rs = 0.405, p = 0.040, Fig. 4). We observed significant larger DMRD-MRRT values for tumors with an edema volume change larger than 3.0 cc as opposed to tumors with an edema volume change smaller than 3.0 cc (p = 0.032; Table 1).
Fig. 4.
DMRD-MRRT as function of absolute volume change of oedema. The group ‘difference in oedema >6.6 cc’ is split up in patients with a decrease and increase of oedema. For the whole group no significant Spearman correlation is found (r2 = 0.405 (p = 0.040)). For 2 patients no oedema is seen on MRD. For patients with multiple metastases, only the tumor with largest DCoM is included resulting in a cut off of 6.6 cc.
A significant correlation between tumor volume and DCoM shifts, with larger shifts for larger tumors (rs = 0.322, p = 0.038) was observed. Additionally, there was a significant correlation between tumor volume and DMRD-MRRT, with a larger DMRD-MRRT for larger tumors (rs = 0.443, p = 0.003). This may be influenced by the observations that larger tumors are more likely to have peritumoral edema (rs = 0.735, p < 0.001). For almost half of the tumors (n = 19) the DMRD-MRRT was larger than 2 mm.
We also evaluated the time dependence of DCoM and DMRD-MRRT, but no significant correlation was found (rs = 0.145, p = 0.358 for DCoM and rs = 0.174, p = 0.270 for DMRD-MRRT).
Changes in tumor volume
The median tumor volume change for the whole group was 0.04 cc (range −10.4 to 10.07 cc) between the MRD and MRRT. Tumors in patients who received steroids (n = 18) had a median decrease in tumor volume of 0.02 cc (−10.4 to 2.83 cc), while tumors in patients who did not receive steroids (n = 24) had a median increase of 0.06 cc (range −0.25 to 10.07 cc) (p = 0.035, Table 2). The tumor volume changes of these two groups did not correlate with time between the two scans (rs = 0.386, p = 0.062 for patients with steroids and rs = 0.193, p = 0.443 for patients without steroids).
Table 2.
Median changes in tumor volume.
| Steroids | ΔVolume Tumor (cc) | Range | p* |
| Median (25, 75) | |||
| No steroids (n = 24) | 0.06 (0.01, 0.81) | −0.25 to 10.07 | p = 0.035 |
| Steroids (n = 18) | −0.02 (−1.37, 0.41) | −10.4 to 2.83 | |
| Systemic treatment (ST) | |||
| No ST (n = 17) | 0.04 (−0.01, 1.14) | −0.25 to 10.07 | p = 0.276 |
| Received ST (n = 25) | 0.02 (−0.23, 0.74) | −10.40 to 4.69 | |
Mann–Whitney U test.
Since systemic treatment may have an influence on tumor growth we evaluated this effect. For patients who received systemic treatment at any point in their treatment of the primary tumor we did not observe a significant difference in tumor volume changes compared to patients who did not receive any systemic treatment (p = 0.276, Table 2). Of the patients who received systemic treatment only one received immunotherapy (Table 3).
Table 3.
Characteristics of patients with tumor shrinkage.
| Patient | No. Tumors with shrinkage (No. tumors delineated) | Primary tumor | Steroids | Systemic treatment | Last gift ST (days prior to MRRT) | Days between MRD and MRRT | Tumor volume on MRD (cc) |
|---|---|---|---|---|---|---|---|
| 1 | 1 (1) | Lung | – | – | – | 28 | 1.14 |
| 2 | 1 (3) | Lung | – | Nivolumab | 11 | 19 | 0.19 |
| 7 | 1 (2) | Lung | – | – | – | 18 | 1.93 |
| 9 | 1 (1) | Lung | Dexamethasone | Cisplatin | 93 | 6 | 11.40 |
| 14 | 3 (3) | Lung | Dexa/prednisolone | Erlotinib | 377 | 16 | 0.11 |
| 0.27 | |||||||
| 1.87 | |||||||
| 15 | 1 (6) | Melanoma | – | – | 39 | 0.07 | |
| 20 | 2 (2) | – | Cisplatin | 217 | 13 | 17.89 | |
| Lung | Dexamethasone | 2.78 | |||||
| 21 | 3 (3) | Cisplatin | 289 | 9 | 0.24 | ||
| Lung | Dexamethasone | 0.37 | |||||
| 5.47 | |||||||
| 25 | 1 (1) | Lung | – | – | 12 | 5.42 |
Discussion
In this study we showed that in a substantial number of patients with brain metastases significant tumor shifts occur in short time frames. Largest tumor shifts occurred for tumors with a change in peritumoral edema of more than 3 cc whereby the median DCoM was 1.4 mm and the median DMRD-MRRT 2.6 mm. Because of the steep dose gradients in SRS treatments, tumor shifts may therefore have a significant impact on treatment accuracy.
In our study we evaluated the occurrence of a tumor shift in the time between the diagnostic MRI and radiotherapy planning MRI, assuming that this interval can be used as a surrogate for tumor shifts between the planning MRI and radiation therapy delivery. In our clinic the time lapse between the radiotherapy planning MRI and SRS is approximately one week whereas the time interval between MRD and MRRT was longer. Importantly, in our analysis we assumed isotropic growth and corrected the DMRD-MRRT for tumor growth. We observed that larger tumor volumes were at higher risk for greater DCoM and DMRD-MRRT, which can be explained by the increased edema changes in these lesions. Our results reflect clinically relevant shifts by correcting the DMRD-MRRT for isotropic tumor growth. Nonetheless, larger tumors may have a higher probability of detectable non-isotropic growth (e.g. due to necrosis), which was not taken into account in our analysis.
Steroids are often used in patients with brain metastases to reduce edema. Some studies suggest that steroids should be administered to all patients for minimizing the risk of complications [8]. Because of the shown correlation between tumor shifts and chances in edema volume, it should be questioned whether the MRRT images remain representative for the tumor geometry and spatial localization at treatment start when steroids are initiated within the time period of MRRT and SRS delivery. Moreover, we found that patients without edema on the diagnostic MRI were likely to have an increase in edema. Therefore, this group is also at risk for significant tumor shifts. In other words, all patients are at risk having larger tumor shifts related to edema changes (increase or decrease) and only a short time interval between MRRT and treatment could account for this phenomenon.
We observed tumor volume shrinkage in patients taking steroids, which can be the result of loss of interstitial fluid in the tumor or restoration of the blood–brain barrier [9]. Although the role of chemotherapy in the treatment for brain metastases is still unclear [10], a review of the literature showed that several studies demonstrated objective responses with systemic chemotherapy [11]. We did not exclude patients receiving systemic treatment for their primary tumor. Systemic therapy did not seem to influence the tumor growth in our study. However, the time between the last gift of systemic treatment and the MRRT varied from 0 to 577 days and the number of patients limited our capability of conducting subgroup analyses.
For Linac-based SRS, safety margins are often used incorporating the uncertainties of the MRI imaging, registration errors, lesion delineation, and patient set-up variability but not tumor position variability. A recent randomized controlled trial showed no significant difference in 12-month rate of local control for brain metastases (BM) with a 1 mm or 3 mm PTV expansion (91% vs 95%) [12]. All patients were treated with a linear accelerator-based radiosurgery platform, but the time between the pretreatment planning MRI and RT was not reported in this study. In addition, this study was not designed to take tumor shifts into account. We expect however that under-treatment of the tumor may occur with longer time intervals resulting in decreased local control as was shown by Seymour et al. [13]. Our limited follow up time and sample size preclude a tumor control outcomes analysis.
We found that the number of brain metastases found on the MRRT was higher than on MRD. This can be the consequence of tumor outgrow in time and due to a larger slice thickness in the MRD protocol for some patients and a fairly long time interval between the MRD and the MRRT. Differences in slice thickness can also result in registration- and delineation biases influencing the tumor and edema volumes, DCoM and DMRD-MRRT. Delineations were executed by 2 observers to limit the variability. Due to the variation in MR scanners and protocols, differences in shifts may occur due to differences in bandwidths and in the amount of non-linearity of the gradients. However, considering the magnitude of our reported differences on tumor position over time, we estimate that these factors, if present, did not substantially contribute, and patients should be considered at risk for significant tumor shifts.
We did not find a correlation between edema changes or tumor shifts and time. The shortest time difference between the two MR examinations was 7 days, which might be too long to observe such correlations. From clinical practice we know that the clinical onset (or disappearance after dexamethasone prescription) of neurologic symptoms caused by peritumoral edema can occur within 72 h. Due to logistical limitations of Linac-based SRS this treatment modality is traditionally encompassing multiple days (in contrast to for example Gamma-Knife based SRS). Following our results we were able to limit the time interval between the SRS workup and delivery to a maximum of 2 days for single metastasis and 3 days for multiple metastases. We deliberately introduced this workflow instead of performing a prospective follow up study evaluating the effect of time, the use of steroids and edema changes in a larger cohort of patients.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ctro.2016.12.007.
Appendix A. Supplementary data
References
- 1.Oncoline guidelines brain metastases version 3.0 2011. <http://www.oncoline.nl/hersenmetastasen>.
- 2.Tsao M.N., Rades D., Wirth A. Radiotherapeutic and surgical management for newly diagnosed brain metastasis (es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linskey M.E., Andrews D.W., Asher A.L. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto M., Serizawa T., Shuto T. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 5.Pinkman M.B., Whitfield G.A., Brada M. New developments in intracranial stereotactic radiotherapy for metastases. Clin Oncol. 2015;27:316–323. doi: 10.1016/j.clon.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Ruschin M., Komljenovic P.T., Ansell S. Cone beam DCoMputed tomography image guidance system for a dedicated intracranial radiosurgery treatment unit. Int J Radiat Oncol Biol Phys. 2013;85(1):243–250. doi: 10.1016/j.ijrobp.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Ryken T.C., McDermott M., Robinson P.D. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:103–114. doi: 10.1007/s11060-009-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suh J.H. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. 2010;362:1119–1127. doi: 10.1056/NEJMct0806951. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich J., Krithika R., Pastorino S., Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233–242. doi: 10.1586/ecp.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta M.P., Paleologos N.A., Mikkelsen T. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:71–83. doi: 10.1007/s11060-009-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walbert T., Gilbert M.R. The role of chemotherapy in the treatment of patients with brain metastases from solid tumors. Int J Clin Oncol. 2009;14:299–306. doi: 10.1007/s10147-009-0916-1. [DOI] [PubMed] [Google Scholar]
- 12.Kirkpatrick J.P., Wang Z., Sampson J.H. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91(1):100–108. doi: 10.1016/j.ijrobp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Seymour Z.A., Fogh S.E., Westcott S.K., Braunstein S., Larson D.A., Barani I.J., Nakamura J., Sneed P.K. Interval from imaging to treatment delivery in the radiation surgery age: how long is too long? Int J Radiat Oncol Biol Phys. 2015 Sep 1;93(1):126–132. doi: 10.1016/j.ijrobp.2015.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.