Highlights
-
•
Immunotherapy is being established as forth pillar of cancer therapy.
-
•
Besides immune checkpoint inhibition several other immunotherapy approaches are tested.
-
•
Radiation induces intratumoural changes that might lead to anti-tumour immunity.
-
•
CAR T cells/bispecific antibodies, vaccines and immunocytokines might synergize with radiation.
Abbreviations: bsAb, bispecific antibody; CAR, chimeric antigen receptor; CDN, cyclic dinucleotides; CTL, cytotoxic T lymphocyte; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; GM-CSF, granulocyte-monocyte colony stimulating factor; IR, irradiation; PD-1, Programmed cell death protein 1 receptor; PD-L1, PD-1 ligand; scFv, single chain variable fragment; TCR, T cell receptor; Treg, regulatory T cells
Keywords: Immunotherapy, Radiotherapy, Vaccination, Immunocytokines, CAR-T-cells, Bispecific antibodies
Abstract
The revival of cancer immunotherapy has taken place with the clinical success of immune checkpoint inhibition. However, the spectrum of immunotherapeutic approaches is much broader encompassing T cell engaging strategies, tumour-specific vaccination, antibodies or immunocytokines. This review focuses on the immunological effects of irradiation and the evidence available on combination strategies with immunotherapy. The available data suggest great potential of combined treatments, yet also poses questions about dose, fractionation, timing and most promising multimodal strategies.
1. Immune checkpoint inhibition
The use of immune checkpoint inhibitors for the treatment of advanced melanoma [1] and other solid tumour entities [2], [3], [4], [5] is establishing immunotherapy as a fourth pillar besides systemic anticancer treatments (conventional chemotherapy and targeted therapies), surgery and radiotherapy. Numerous clinical trials and preclinical projects have been started and CTLA4 (cytotoxic T-lymphocyte-associated protein 4) and PD-1 (Programmed cell death protein 1 receptor)/PD-L1 (PD-1 ligand) antibodies have been FDA approved for the treatment of malignant melanoma and non-small cell lung cancer as well as other cancer types. The combination of immunotherapy with radiation is based on promising preclinical data and supported by a strong theoretical rationale [6], [7], [8]. To date, several clinical trials testing such combinations have been started for multiple cancer types [9] and the first results should be reported within the next years.
2. Immunotherapy beyond checkpoint inhibition
Yet, the spectrum of immunotherapy is much broader than immune checkpoint inhibition. Anti-tumour vaccination [10], [11], cytokine based therapies [12], chimeric antigen receptor (CAR) T cells [13] and bispecific antibodies (bsAbs) [14], [15] are only a few examples. Other strategies include toll-like receptor agonists [16], TGFβ blockade [17], NK cell based therapy [18] and immune modulation of macrophages [19] among others. Some of these strategies have been developed well before the clinical use of checkpoint inhibitors, yet with limited success. However, most of the clinical studies have been performed with immunotherapies as monotherapy, which leaves the question, if patients might benefit from combination therapies.
The last 2–3 decades have seen the development of two approaches that utilize polyclonal cytotoxic T lymphocytes (CTL) independently of T cell receptor (TCR)-mediated recognition of MHC (major histocompatibility complex) bound peptides for the elimination of tumours. Both, CAR T cells and bsAbs typically make use of a specificity-conferring single-chain variable fragment (scFv) derived from a monoclonal antibody to target a specific surface antigen on tumour cells. To generate CAR T cells, T cells are transduced with a recombinant fusion protein, in which such an scFv is fused to intracellular signalling components of the TCR and – at least in newer-generation CAR T cells – co-stimulatory domains usually derived from CD28 or 4-1BB are also incorporated [20]. T cell-recruiting bsAbs consist of two scFvs, one of which is directed against a tumour cell surface antigen. The other scFv is specific for the invariant CD3 signalling chain of the TCR and is able to recruit and activate tumour infiltrating T cells [21]. One of the biggest advantages of both approaches is the fact that every T cell, independent of its inherent specificity (through the α/β TCR), can be converted into a CTL for the specific lysis of tumour cells.
Vaccination strategies include “off-the-shelf” peptide vaccines as evaluated for renal cell carcinoma [22], [23], [24], personalized peptide vaccination approaches [25], as well as RNA vaccines [26] and strategies using dendritic cells [27] or whole inactivated tumour cells [28], [29]. These therapies have the advantage of inducing tumour-specific immune responses targeting tumour-specific or tumour-associated antigens. Yet, vaccination as monotherapy, even in patients with minimal disease burden such as biochemical recurrence of prostate cancer after radical prostatovesiculectomy slowed PSA kinetics but did not control the disease in most patients [30], [31]. The reason for that is most probably, that the tumour-inherent immunosuppression via Th2 polarization and intratumoural regulatory T cells (Tregs) do not allow the cytotoxic T cells primed by the vaccination to enter the tumour microenvironment [32] or exert their cytolytic function.
Cytokines have been established in oncological therapies for several years, e.g. IL-2 in melanoma [33], [34]. Yet, systemic application can cause severe inflammation and even led to grade 5 toxicities in the case of IL-12 [35], [36]. Therefore, recent efforts were focused on the development of tumour targeted cytokine application e.g. by coupling the active component with a tumour targeting antibody [37], [38], [39], [40] creating so-called immunocytokines or complexing IL-2 with antibodies for altering binding specificities [41], [42]. These therapies are able to overcome the general immunosuppression in the tumour microenvironment by converting the stroma into Th1 polarization, thus enabling T cells to enter the tumour and recognize their cognate antigens in context with co-stimulation on mature APCs. However, some cytokine effects are dependent on spatial distribution and exact concentrations. IL-2 is known to have dual effects depending on the concentration. It can either foster Th1 polarization and thus prime naïve T cells for anti-tumour responses or support Th2 polarization and Treg differentiation leading to a protumourigenic effect [43]. Thus, the effects of cytokine therapies might not be predictable and even heterogeneous in different patients and tumours depending on the tumour microenvironment.
3. Immune activation through tumour irradiation
During the last decade a paradigm shift has taken place acknowledging that besides the direct or indirect interaction of ionizing radiation with the radiosensitive DNA, secondary radiation responses additionally occur. In close proximity bystander effects and in distal sites of the irradiated area systemic, abscopal effects have been observed [44]. Distinct tumour cell death forms accompanied by the release of danger signals by IR-stressed cells and/or phenotypical cell alterations foster immune cell activation, thereby contributing to such non-DNA-targeted radiation-effects [7], [45], [46], [47]. The so called immunogenic cancer cell death was originally linked to certain chemotherapeutic agents such as anthracyclines [48] and has been expanded to many stressors like radiation during the last years [49], [50]. Characteristics and detection of immunogenic cancer cell death are discussed in the recently published consensus guidelines [51]. The key outcome is that tumour cells should be killed in a way that they become an intrinsic (in situ) cancer-specific vaccine and secrete danger signals to activate the innate immune system [7], [52]. This can also be achieved by interfering with cell death pathways and consecutive induction of immunogenic necrosis [53].
The tumour microenvironment, too, can be modulated by radiation. Irradiation (IR) generates novel peptide sequences and enhances MHC class I expression [54]. Neoantigen-specific CD8+ T cell responses have been shown to go along with tumour regression [55]. Radiation further enhances the diversity of the T-cell receptor repertoire of intratumoural T cells [56]. Some of the mutations that create neoantigens influence the response of patients to immune checkpoint inhibition. One pre-requisite for anti-tumour immune reactions is the infiltration of immune cells into the tumour tissue [57]. Neoadjuvant local IR with a single dose of 2 Gy causes inflammation and normalization of tumour vasculature and consecutively enables the recruitment of tumour-specific T cells. This was shown in the RIP1-Tag5 (RT5) transgenic mouse model expressing the simian virus 40 derived T antigen (Tag) as a model tumour antigen. M1 polarized macrophages in the tumour micro-milieu mediated the tumour infiltration of T cells by producing nitric oxide (NO) [58]. Currently, a randomized phase II study of radiation-induced immune boost in operable non-small cell lung cancer (RadImmune trial) evaluates the impact of low dose neoadjuvant irradiation in particular on CD8+ T cell infiltration and secondarily on the association between CD8+ T cell counts and progression free survival [59]. However, tumour irradiation has also been described to enhance tumour infiltration by Treg cells and immune system exhaustion [60] and to have a negative influence on anti-tumour immunity. In line with this, low dose IR can have anti-inflammatory effects including on macrophages [61], exploited for the treatment of benign, autoimmune T cell-driven inflammatory or degenerative diseases [62]. Additionally, the dynamics of the immunomodulation have to be considered, since the time window of radiation-induced infiltration of high numbers of immune cells in the tumour is narrow [63], [64]. A close-meshed monitoring of the individual patients’ immune status should be performed after radiation to define opportune moments for addition of selected immunotherapies. For that purpose, liquid biopsies might be useful [65]. Multicolour flow-cytometry-based assays allow for the detection of many immune cells and subsets and additionally give information about the activation status of these cells circulating in the peripheral blood [66]. Liquid biopsies and flow cytometry have been evaluated in cancer patients for purposes of diagnosis and monitoring of different treatments.
4. Car T cells, bispecific antibodies and irradiation
CAR T cells as well as bsAbs have shown spectacular results for the treatment of CD19+ hematological neoplasms, particularly acute B-lymphoblastic leukemia [67], [68], [69]. However, the treatment of solid tumour malignancies by these approaches is more challenging. Currently, only few cell surface antigens are known that are truly tumour-specific or whose targeting would cause acceptable (“off-tumour on-target”) side effects [70]. Undifferentiated, stem-like tumour cells might express a different set of antigens. Thus, the antigen targeted by CAR-T-cells might have to be chosen explicitly to target tumour initiating cells [71]. A potential advantage of CAR T cells is the possibility to artificially manipulate cytokine secretion and other parameters (to generate so-called “armored T cells” or TRUCKs) [72], [73] to improve both passive and active immunotherapy with or without radiotherapy. In the case of cytokine-secreting CAR T cells, it remains to be determined if this approach can also reduce the risk of inducing unwanted side effects of systemic cytokine application. In solid tumours, the number of infiltrating CAR T cells or bsAb-recruited T cells has to reach a certain threshold to be effective. This can only be achieved if sufficient T cell extravasation occurs and the immunosuppressive tumour microenvironment is disarmed. Tumour infiltration by T cells has been described to be fostered by total body irradiation before transfer of in vitro expanded lymphocytes [57], [74] as well as local tumour IR [58], [64], [75], [76]. These findings point toward a possible rationale for the combination of CAR T cells and IR.
Mechanistically, enhanced T cell infiltration after tumour IR is largely due to the induction of adhesion molecules, cytokines, and chemokines involved in the recruitment of effector T cells [57], [75], [76]. Furthermore, particularly high-dose hypofractionated irradiation, in addition to reducing tumour cell numbers and inducing immunogenic cell death, is also capable of eliminating immunosuppressive cell populations in tumours, such as Tregs and myeloid-derived suppressor cells, at least transiently, either directly or indirectly via the induced T cells [77]. Low-dose IR with one fraction in the range of 0.5–2 Gy, although not capable of depleting myeloid cells, has been reported to change the polarization of macrophages from proangiogenic and protumourigenic M2 to anti-tumourigenic, iNOS+ M1 macrophages, which support tumour infiltration by T cells through vascular normalization and the upregulation of chemokines [58]. In addition, it was shown that radiotherapy can have an enhancing effect on the cell surface expression of certain target antigens [78], [79].
Contrarily to CAR T cells, the generation of which is still time consuming, expensive, and more vulnerable to errors, T cell-recruiting bsAbs have the crucial benefit to be readily available off-the-shelf. Recently, it has been reported that the combination of IR and a T cell-recruiting bsAb yielded additive effects compared to the respective monotherapies in the treatment of small tumours in mice. However, contrarily to the combination of IR and anti-PD-1 antibodies, the anti-tumour effects were not durable and no complete remissions were observed [64]. Surprisingly, large tumours treated with IR and bsAb relapsed even faster than after IR alone. Mechanistic analyses revealed, that this was caused by massive apoptotic depletion of tumour-resident T cells induced by repetitive strong T cell stimulation by the relatively large numbers of remaining tumour cells decorated with the T cell-recruiting bsAb. This study clearly outlines the risk of induction of specific immunological tolerance caused by bsAb-mediated overstimulation of tumour-specific T cells, reflected by worsened tumour control. However, it is conceivable that this hazard of bsAbs could be overcome by design optimization (including affinity alteration of the scFvs or the addition of co-stimulatory domains) [80], refined application schemes or other measures. Future studies have to show to what extent CAR T cells and/or T cell-recruiting bsAbs can contribute to successful tumour therapy in combination with radiotherapy.
5. Tumour vaccination in combination with irradiation
Radiotherapy is capable of creating a tumour micro- and macroenvironment that complements therapeutic cancer vaccines in several aspects. Besides the possible creation of neoantigens through mutations induced by IR triggered DNA damage, IR triggers the tumour tissue to release danger signals that attract and activate innate immune cells robustly which in turn leads to efficient antigen-presentation by local APCs and priming of T cells thus establishing long lasting T cell immunity [81]. In patients who lack a natural tumour response the latter should be triggered by thoughtfully designed vaccines. Type I interferon and pro-inflammatory cytokine responses necessary for maturation of APC, efficient presentation of antigen and co-stimulation, and the attraction and priming of T cells can be achieved through diverse adjuvants. Both exogenous and endogenous cytosolic cyclic dinucleotides (CDN), used as adjuvants bind to the cytosolic receptor stimulator of interferon genes (STING) and consecutively trigger immune cell infiltration [82]. These bacteria-derived CDN can be coupled to GVAX, an allogeneic, granulocyte–macrophage colony-stimulating factor (GM-CSF)-secreting cellular immunotherapy comprising of irradiated tumour cells transfected with the GM-CSF gene [83]. This example highlights that the generation of vaccines is complex but always based on provision of a true tumour-associated antigen and a potent trigger for Th1 polarization of the microenvironment which allows the presentation of the antigen in context with a strong costimulatory signal.
That radiation does synergize with vaccination in the induction of anti-tumour immune responses is based on the mechanisms described by Tang et al., such as radiation-induced release of antigen and the possible generation of neo-antigens (see above), that in concerted action with vaccination-induced immune stimulation do result in strong and long-lasting anti-tumour immune effects [84]. As shown in pre-clinical models for head and neck cancer, combination of IR with 7.5 Gy and human papilloma virus (HPV)-vaccination enhanced intra-tumour vascular permeability, which correlated with anti-tumour response [85]. One draw-back of IR in multimodal settings is that the expression of checkpoint-molecules such as PD-L1 might be increased [86]. Even though local IR combined with vaccination increases CD8+ T cell infiltration e.g. in pancreatic tumours, only modest inhibition of tumour growth was observed. However, addition of anti-PD-L1 antibody enhanced the effector function of tumour-infiltrating T cells and significantly increased the survival of the mice. This calls for a triple combination of IR, vaccination and checkpoint-blockade, which converts non-T cell-inflamed cancers to T cell-inflamed cancers, revealing one underlying mechanism of induction of efficient anti-tumour immune responses [87]. Recent research suggests that interfering with immune checkpoint molecules also depends on the gut microbiome [88], provoking very personalized multimodal cancer therapies in the future [89].
6. Immunocytokines in combination with irradiation
Immunocytokines are fusion proteins of cytokines with anti-tumour efficiency and tumour targeting antibodies. The cytokine mostly used is IL-2, a cytokine which can polarize the tumour micromilieu and the intratumoural T cells to a Th1 phenotype [90]. IL-2 is mainly produced by T cells, dendritic cells and natural killer (NK) cells and it has differential effects on effector T cells, Treg cells and NK cells [90], [91]. Interleukin 12 (IL-12), too, can induce tumour regression and affects innate and adaptive immunity [92], [93]. Besides its role in T-cell priming, IL-12 efficiently leads to Th1 polarization in the tumour microenvironment [94]. Systemic administration of IL-12 has already shown efficacy against solid tumours but induced dose-limiting toxicity [36], [95]. The use in the form of immunocytokines refuelled the interest in the use of IL-12 for cancer therapy.
IL-2-based immunocytokines that have been used in preclinical and clinical studies include ch14.18-IL2 [96], L19-IL2 [97] and NHS-IL2 [98]. L19-IL2 has also been evaluated in combination with IR [97]. The antibody part of this immunocytokine targets altered tumour vasculature by binding to extradomain B of fibronectin (ED-B) [99]. Preclinically, anti-tumour efficacy has been shown against mantle cell lymphoma [100] and B cell lymphoma in combination with rituximab [101] as well as pancreatic cancer [102]. A novel biologic combination showing preclinical efficiency is the combination with syndecan-1 targeting antibodies [103]. Clinical studies were performed for a cohort of patients with mixed solid tumours and renal cell carcinoma in phase I [104] and intralesionally in stage III melanoma. In this study, complete responses were observed in 7 of 13 patients [105], [106]. L19-IL2 has been described to be effective in the clinical setting in combination with dacarbazine for malignant melanoma [107] and checkpoint inhibition [108]. The combination of L19-IL2 and IR has shown anti-tumour efficacy in a CD8+ T cell dependent manner according to ED-B expression of tumour vasculature in different cancer models [97]. Even in the absence of MHC-I expression in the tumour with immune effects being dependent on NK cell responses, treatment with IR and L19-IL2 led to additive anti-tumour effects [38].
A different antibody used for the construction of immunocytokines is NHS-76, an antibody binding to DNA-histone complexes and thus targeting necrotic tumour regions that provide extracellular access to the target [109]. The fusion protein with IL-2 has been shown to have anti-tumour effects in vivo [110] and has been introduced to the clinic in a phase I study [111], [112]. The combination of NHS-IL2 with IR showed promising results in preclinical experiments and was evaluated in a phase Ib trial leading to long term cancer control in 1 of 13 patients [98]. The fusion protein of NHS-76 with IL-12 has shown anti-tumour effects in vivo with decreased toxicity compared to IL-12 in a non-human primate model [37]. Mice reconstituted with a human immune system showed long-lasting tumour control of rhabdomyosarcoma xenografts through senescence and differentiation [40]. Dogs with malignant melanoma treated with NHS-IL12 showed signs of clinical response [113]. In addition to the described immunological effects of radiation the rationale for combining necrosis targeting immunocytokines with IR also includes necrosis induction and enhanced intratumoural bioavailability of the compound through IR [114]. Unpublished data revealed additive effects of the combination as well as abscopal effects by combining NHS-IL12 with local tumour IR (Eckert et al., unpublished data).
7. Conclusion
Whereas immune checkpoint inhibition is the most advanced immunotherapeutic strategy already established in several different indications [2], [115], [116], [117] and is FDA approved for advanced melanoma, non-small cell lung cancer and other cancer entities such as urothelial bladder cancer [118], other immunotherapeutic approaches also merit further attention. Immune checkpoint inhibition might be more effective in combination with other immunotherapy strategies or anti-cancer treatment as indicated by the findings of Badoual et al. [119].
The challenges of combination therapies are manifold. The cellular effects of the combination of IR and immunotherapy are at least in part different than the combinatorial effects of IR and chemotherapy [40], [114]. Thus, “common knowledge” in radiation biology might have to be reconsidered. As shown for bispecific antibodies, combination therapies might even decrease anti-tumour effects, e.g., in the case of large tumours. There are intense discussions about radiotherapy dose, fractionation, timing as well as therapy sequencing [120], [121], [122]. The common notion is that hypofractionated, stereotactic radiation might be of advantage, yet this leaves the question of how immunotherapy can be combined with primary or neoadjuvant normofractionated radiotherapy of larger treatment volumes encompassing adjuvant lymph node regions. Current clinical trials mostly start immunotherapy after curative radiation e.g. in non-small cell lung cancer or they combine stereotactic body radiotherapy with immunotherapy regimens. Yet, the most successful fractionation and sequencing might differ depending on the applied immunotherapy, tumour type and patient inherent factors. Radiotherapy might also be used in immunotherapy refractory disease to induce a secondary response with continuation of the treatment. Furthermore, combinatorial regimens might induce abscopal effects in non-irradiated lesions in oligometastastatic patients.
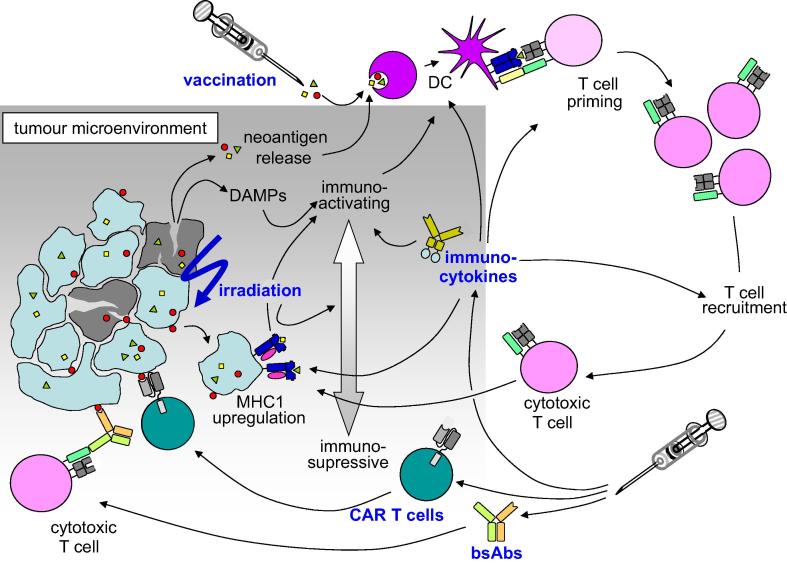
In summary, the combination of immunotherapy and radiotherapy might be a way to long-term cancer control and survival for a large number of cancer patients and might avoid aggressive systemic therapies. The range of immunotherapeutics that might be combined with IR is much broader than checkpoint inhibition (Fig. 1). The further developments in this fascinating area will have to focus on the regimens and immunotherapeutics used in different clinical settings of metastatic or localized disease in different cancer types.
Fig. 1.
Tumour irradiation leads to cell death and a release of danger molecules. The tumour microenvironment and cytokine milieu is also altered by irradiation. T cell priming and T cell recruitment is enhanced through irradiation. This is the rationale for combining irradiation with CAR T cells and bispecific antibodies. Vaccination might be enhanced by irradiation through neoantigen release and triggers anti-tumour immune responses. Immunocytokines are able to alter the tumour microenvironment to enhance anti-tumour immune responses and lead to T cell recruitment into the tumour.
References
- 1.Ascierto M.L., Melero I., Ascierto P.A. Melanoma: from incurable beast to a curable bet. The success of immunotherapy. Front Oncol. 2015;5:152. doi: 10.3389/fonc.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alatrash G., Daver N., Mittendorf E.A. Targeting immune checkpoints in hematologic malignancies. Pharmacol Rev. 2016;68(4):1014–1025. doi: 10.1124/pr.116.012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Osta H. Immune checkpoint inhibitors: the new frontier in non-small-cell lung cancer treatment. Onco Targets Ther. 2016;9:5101–5116. doi: 10.2147/OTT.S111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong J. Advances in treatment of metastatic renal cell carcinoma. Curr Opin Urol. 2016;26(5):439–446. doi: 10.1097/MOU.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 5.Lynch D., Murphy A. The emerging role of immunotherapy in colorectal cancer. Ann Transl Med. 2016;4(16):305. doi: 10.21037/atm.2016.08.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Formenti S.C., Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey B. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63(1):29–36. doi: 10.1007/s00262-013-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharabi A.B. Stereotactic radiation therapy combined with immunotherapy: augmenting the role of radiation in local and systemic treatment. Oncology (Williston Park) 2015;29(5):331–340. [PMC free article] [PubMed] [Google Scholar]
- 9.Zavala V.A., Kalergis A.M. New clinical advances in immunotherapy for the treatment of solid tumours. Immunology. 2015;145(2):182–201. doi: 10.1111/imm.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melief C.J. Therapeutic cancer vaccines. J Clin Invest. 2015;125(9):3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rammensee H.G., Singh-Jasuja H. HLA ligandome tumor antigen discovery for personalized vaccine approach. Expert Rev Vaccines. 2013;12(10):1211–1217. doi: 10.1586/14760584.2013.836911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neri D., Sondel P.M. Immunocytokines for cancer treatment: past, present and future. Curr Opin Immunol. 2016;40:96–102. doi: 10.1016/j.coi.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haji-Fatahaliha M. CAR-modified T-cell therapy for cancer: an updated review. Artif Cells Nanomed Biotechnol. 2016;44(6):1339–1349. doi: 10.3109/21691401.2015.1052465. [DOI] [PubMed] [Google Scholar]
- 14.Lameris R. Bispecific antibody platforms for cancer immunotherapy. Crit Rev Oncol Hematol. 2014;92(3):153–165. doi: 10.1016/j.critrevonc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Weidle U.H., Kontermann R.E., Brinkmann U. Tumor-antigen-binding bispecific antibodies for cancer treatment. Semin Oncol. 2014;41(5):653–660. doi: 10.1053/j.seminoncol.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Shi M. Application potential of toll-like receptors in cancer immunotherapy: systematic review. Medicine (Baltimore) 2016;95(25):e3951. doi: 10.1097/MD.0000000000003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris J.C. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE. 2014;9(3):e90353. doi: 10.1371/journal.pone.0090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker P.S. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol Immunother. 2016;65(4):477–484. doi: 10.1007/s00262-016-1792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ries C.H. CSF-1/CSF-1R targeting agents in clinical development for cancer therapy. Curr Opin Pharmacol. 2015;23:45–51. doi: 10.1016/j.coph.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Sadelain M., Brentjens R., Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez P. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985;316(6026):354–356. doi: 10.1038/316354a0. [DOI] [PubMed] [Google Scholar]
- 22.Kirner A., Mayer-Mokler A., Reinhardt C. IMA901: a multi-peptide cancer vaccine for treatment of renal cell cancer. Hum Vaccin Immunother. 2014;10(11):3179–3189. doi: 10.4161/21645515.2014.983857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noguchi M. A randomized phase II clinical trial of personalized peptide vaccination with metronomic low-dose cyclophosphamide in patients with metastatic castration-resistant prostate cancer. Cancer Immunol Immunother. 2016;65(2):151–160. doi: 10.1007/s00262-015-1781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshitake Y. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res. 2015;21(2):312–321. doi: 10.1158/1078-0432.CCR-14-0202. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X. Personalized cancer vaccines: targeting the cancer mutanome. Vaccine. 2016 doi: 10.1016/j.vaccine.2016.05.073. [Epub ahead of print]. pii: S0264-410X(16)30399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebastian M. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive(R)) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer. 2014;14:748. doi: 10.1186/1471-2407-14-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Constantino J. Antitumor dendritic cell-based vaccines: lessons from 20 years of clinical trials and future perspectives. Transl Res. 2016;168:74–95. doi: 10.1016/j.trsl.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Weiss E.M. High hydrostatic pressure treatment generates inactivated mammalian tumor cells with immunogeneic features. J Immunotoxicol. 2010;7(3):194–204. doi: 10.3109/15476911003657414. [DOI] [PubMed] [Google Scholar]
- 29.Weiss E.M. Selected anti-tumor vaccines merit a place in multimodal tumor therapies. Front Oncol. 2012;2:132. doi: 10.3389/fonc.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feyerabend S. Novel multi-peptide vaccination in Hla-A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate. 2009;69(9):917–927. doi: 10.1002/pros.20941. [DOI] [PubMed] [Google Scholar]
- 31.Widenmeyer M. Promiscuous survivin peptide induces robust CD4+ T-cell responses in the majority of vaccinated cancer patients. Int J Cancer. 2012;131(1):140–149. doi: 10.1002/ijc.26365. [DOI] [PubMed] [Google Scholar]
- 32.Ostrand-Rosenberg S. Tolerance and immune suppression in the tumor microenvironment. Cell Immunol. 2016;299:23–29. doi: 10.1016/j.cellimm.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dillman R.O. Should high-dose interleukin-2 still be the preferred treatment for patients with metastatic melanoma? Cancer Biother Radiopharm. 2012;27(6):337–343. doi: 10.1089/cbr.2012.1220. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg S.A. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Car B.D. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999;27(1):58–63. doi: 10.1177/019262339902700112. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270(5238):908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 37.Fallon J. The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget. 2014;5(7):1869–1884. doi: 10.18632/oncotarget.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rekers N.H. Long-lasting antitumor effects provided by radiotherapy combined with the immunocytokine L19-IL2. Oncoimmunology. 2015;4(8):e1021541. doi: 10.1080/2162402X.2015.1021541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rekers N.H. Combination of radiotherapy with the immunocytokine L19-IL2: additive effect in a NK cell dependent tumour model. Radiother Oncol. 2015 doi: 10.1016/j.radonc.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Schilbach K. Cancer-targeted IL-12 controls human rhabdomyosarcoma by senescence induction and myogenic differentiation. Oncoimmunology. 2015;4(7):e1014760. doi: 10.1080/2162402X.2015.1014760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krieg C. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci USA. 2012;107(26):11906–11911. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Letourneau S. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proc Natl Acad Sci USA. 2010;107(5):2171–2176. doi: 10.1073/pnas.0909384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malek T.R., Bayer A.L. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4(9):665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 44.Kadhim M.A., Hill M.A. Non-targeted effects of radiation exposure: recent advances and implications. Radiat Prot Dosimetry. 2015;166(1–4):118–124. doi: 10.1093/rpd/ncv167. [DOI] [PubMed] [Google Scholar]
- 45.Chakraborty M. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 46.Galluzzi L. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2016 doi: 10.1038/nri.2016.107. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Kulzer L. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells. J Immunotoxicol. 2014;11(4):328–336. doi: 10.3109/1547691X.2014.880533. [DOI] [PubMed] [Google Scholar]
- 48.Casares N. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apetoh L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 50.Perez C.A. Radiation induces an antitumour immune response to mouse melanoma. Int J Radiat Biol. 2009;85(12):1126–1136. doi: 10.3109/09553000903242099. [DOI] [PubMed] [Google Scholar]
- 51.Kepp O. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3(9):e955691. doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanpouille-Box C. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33(51):7415–7422. doi: 10.1016/j.vaccine.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Werthmoller N. Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T-cell-dependent manner. Cell Death Dis. 2015;6:e1761. doi: 10.1038/cddis.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reits E.A. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rizvi N.A. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Twyman-Saint Victor C. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ganss R. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62(5):1462–1470. [PubMed] [Google Scholar]
- 58.Klug F. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 59.Safi S. A randomized phase II study of radiation induced immune boost in operable non-small cell lung cancer (RadImmune trial) BMC Cancer. 2015;15:988. doi: 10.1186/s12885-015-2006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mendes F. The role of immune system exhaustion on cancer cell escape and anti-tumor immune induction after irradiation. Biochim Biophys Acta. 2016;1865(2):168–175. doi: 10.1016/j.bbcan.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Wunderlich R. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol. 2015;179(1):50–61. doi: 10.1111/cei.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trott K.R., Kamprad F. Radiobiological mechanisms of anti-inflammatory radiotherapy. Radiother Oncol. 1999;51(3):197–203. doi: 10.1016/s0167-8140(99)00066-3. [DOI] [PubMed] [Google Scholar]
- 63.Frey B. Modulation of inflammation by low and high doses of ionizing radiation: implications for benign and malign diseases. Cancer Lett. 2015;368(2):230–237. doi: 10.1016/j.canlet.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 64.Hettich M. Checkpoint antibodies but not T cell-recruiting diabodies effectively synergize with TIL-inducing gamma-irradiation. Cancer Res. 2016;76(16):4673–4683. doi: 10.1158/0008-5472.CAN-15-3451. [DOI] [PubMed] [Google Scholar]
- 65.Sloan A.E. Liquid biopsy can distinguish recurrent glioblastomas from pseudoprogression and radiation necrosis after concurrent radiochemotherapy. Neurosurgery. 2016;63(Suppl. 1):185–186. [Google Scholar]
- 66.Ruhle P.F. Development of a modular assay for detailed immunophenotyping of peripheral human whole blood samples by multicolor flow cytometry. Int J Mol Sci. 2016;17(8) doi: 10.3390/ijms17081316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maude S.L. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Topp M.S. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 69.Topp M.S. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 70.Fesnak A.D., June C.H., Levine B.L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16(9):566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu X. Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget. 2015;6(1):171–184. doi: 10.18632/oncotarget.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chmielewski M., Abken H. CAR T cells transform to trucks: chimeric antigen receptor-redirected T cells engineered to deliver inducible IL-12 modulate the tumour stroma to combat cancer. Cancer Immunol Immunother. 2012;61(8):1269–1277. doi: 10.1007/s00262-012-1202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khalil D.N. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13(5):273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goff S.L. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol. 2016;34(20):2389–2397. doi: 10.1200/JCO.2016.66.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lugade A.A. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 76.Matsumura S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deng L. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao N. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res. 2009;171(1):9–21. doi: 10.1667/RR1472.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hassan R. Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts. Clin Cancer Res. 2006;12(16):4983–4988. doi: 10.1158/1078-0432.CCR-06-0441. [DOI] [PubMed] [Google Scholar]
- 80.Cartellieri M. TCR/CD3 activation and co-stimulation combined in one T cell retargeting system improve anti-tumor immunity. Oncoimmunology. 2013;2(12):e26770. doi: 10.4161/onci.26770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kwilas A.R. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol. 2012;2:104. doi: 10.3389/fonc.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fu J. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7(283):283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eager R., Nemunaitis J. GM-CSF gene-transduced tumor vaccines. Mol Ther. 2005;12(1):18–27. doi: 10.1016/j.ymthe.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 84.Tang C. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831–838. doi: 10.1158/2326-6066.CIR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mondini M. Synergy of radiotherapy and a cancer vaccine for the treatment of HPV-associated head and neck cancer. Mol Cancer Ther. 2015;14(6):1336–1345. doi: 10.1158/1535-7163.MCT-14-1015. [DOI] [PubMed] [Google Scholar]
- 86.Dovedi S.J., Illidge T.M. The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunology. 2015;4(7):e1016709. doi: 10.1080/2162402X.2015.1016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng W. Combination of radiotherapy and vaccination overcome checkpoint blockade resistance. Oncotarget. 2016;7(28):43039–43051. doi: 10.18632/oncotarget.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vetizou M. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dolgin E. Using DNA, radiation therapy gets personal. Science. 2016;353(6306):1348–1349. doi: 10.1126/science.353.6306.1348. [DOI] [PubMed] [Google Scholar]
- 90.Xu H.M. Th1 cytokine-based immunotherapy for cancer. Hepatobiliary Pancreat Dis Int. 2014;13(5):482–494. doi: 10.1016/s1499-3872(14)60305-2. [DOI] [PubMed] [Google Scholar]
- 91.Sim G.C., Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014;25(4):377–390. doi: 10.1016/j.cytogfr.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 92.Nastala C.L. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153(4):1697–1706. [PubMed] [Google Scholar]
- 93.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 94.Kerkar S.P. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121(12):4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lasek W., Zagozdzon R., Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother. 2014;63(5):419–435. doi: 10.1007/s00262-014-1523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morris Z.S. In situ tumor vaccination by combining local radiation and tumor-specific antibody or immunocytokine treatments. Cancer Res. 2016;76(13):3929–3941. doi: 10.1158/0008-5472.CAN-15-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zegers C.M. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin Cancer Res. 2015;21(5):1151–1160. doi: 10.1158/1078-0432.CCR-14-2676. [DOI] [PubMed] [Google Scholar]
- 98.van den Heuvel M.M. NHS-IL2 combined with radiotherapy: preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med. 2015;13:32. doi: 10.1186/s12967-015-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carnemolla B. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99(5):1659–1665. doi: 10.1182/blood.v99.5.1659. [DOI] [PubMed] [Google Scholar]
- 100.Borschel N. Potentiating the activity of rituximab against mantle cell lymphoma in mice by targeting interleukin-2 to the neovasculature. Leuk Res. 2015;39(7):739–748. doi: 10.1016/j.leukres.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 101.Schliemann C. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood. 2009;113(10):2275–2283. doi: 10.1182/blood-2008-05-160747. [DOI] [PubMed] [Google Scholar]
- 102.Wagner K. The targeted immunocytokine L19-IL2 efficiently inhibits the growth of orthotopic pancreatic cancer. Clin Cancer Res. 2008;14(15):4951–4960. doi: 10.1158/1078-0432.CCR-08-0157. [DOI] [PubMed] [Google Scholar]
- 103.Orecchia P. Targeting Syndecan-1, a molecule implicated in the process of vasculogenic mimicry, enhances the therapeutic efficacy of the L19-IL2 immunocytokine in human melanoma xenografts. Oncotarget. 2015;6(35):37426–37442. doi: 10.18632/oncotarget.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johannsen M. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46(16):2926–2935. doi: 10.1016/j.ejca.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 105.Danielli R. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64(8):999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weide B. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014;2(7):668–678. doi: 10.1158/2326-6066.CIR-13-0206. [DOI] [PubMed] [Google Scholar]
- 107.Eigentler T.K. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17(24):7732–7742. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 108.Schwager K. The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol. 2013;133(3):751–758. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 109.Sharifi J. Characterization of a phage display-derived human monoclonal antibody (NHS76) counterpart to chimeric TNT-1 directed against necrotic regions of solid tumors. Hybrid Hybridomics. 2001;20(5–6):305–312. doi: 10.1089/15368590152740707. [DOI] [PubMed] [Google Scholar]
- 110.Gillies S.D. A low-toxicity IL-2-based immunocytokine retains antitumor activity despite its high degree of IL-2 receptor selectivity. Clin Cancer Res. 2011;17(11):3673–3685. doi: 10.1158/1078-0432.CCR-10-2921. [DOI] [PubMed] [Google Scholar]
- 111.Gillessen S. A phase I dose-escalation study of the immunocytokine EMD 521873 (Selectikine) in patients with advanced solid tumours. Eur J Cancer. 2013;49(1):35–44. doi: 10.1016/j.ejca.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 112.Laurent J. T-cell activation by treatment of cancer patients with EMD 521873 (Selectikine), an IL-2/anti-DNA fusion protein. J Transl Med. 2013;11:5. doi: 10.1186/1479-5876-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paoloni M. Defining the pharmacodynamic profile and therapeutic index of NHS-IL12 immunocytokine in dogs with malignant melanoma. PLoS ONE. 2015;10(6):e0129954. doi: 10.1371/journal.pone.0129954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eckert F. Enhanced binding of necrosis-targeting immunocytokine NHS-IL12 after local tumour irradiation in murine xenograft models. Cancer Immunol Immunother. 2016;65(8):1003–1013. doi: 10.1007/s00262-016-1863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Postow M.A. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vansteenkiste J. Immunotherapy for non-small-cell lung cancer: the past 10 years. Future Oncol. 2015:1–15. doi: 10.2217/fon.15.116. [DOI] [PubMed] [Google Scholar]
- 117.De Felice F. Immunotherapy of ovarian cancer: the role of checkpoint inhibitors. J Immunol Res. 2015;2015:191832. doi: 10.1155/2015/191832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.La-Beck N.M. Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacotherapy. 2015;35(10):963–976. doi: 10.1002/phar.1643. [DOI] [PubMed] [Google Scholar]
- 119.Badoual C. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73(1):128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 120.Demaria S., Formenti S.C. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gandhi S.J. Awakening the immune system with radiation: optimal dose and fractionation. Cancer Lett. 2015;368(2):185–190. doi: 10.1016/j.canlet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 122.Levy A. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: single centre subset analysis from a phase 1/2 trial. Eur J Cancer. 2016;68:156–162. doi: 10.1016/j.ejca.2016.09.013. [DOI] [PubMed] [Google Scholar]