Abstract
Acute traumatic coagulopathy (ATC) is an acute and endogenous mechanism triggered by the association of trauma and hemorrhage. Several animal models have been developed, but some major biases have not yet been identified. Our aim was to develop a robust and clinically relevant murine model to study this condition. Anesthetized adult Sprague Dawley rats were randomized into 4 groups: C, control; T, trauma; H, hemorrhage; TH, trauma and hemorrhage (n = 7 each). Trauma consisted of laparotomy associated with four-limb and splenic fractures. Clinical variables, ionograms, arterial and hemostasis blood tests were compared at 0 and 90 min. ATC and un-compensated shock were observed in group TH. In this group, the rise in prothrombin time and activated partial thromboplastin was 29 and 40%, respectively. Shock markers, compensation mechanisms and coagulation pathways were all consistent with human pathophysiology. The absence of confounding factors, such as trauma-related bleeding or dilution due to trans-capillary refill was verified. This ethic, cost effective and bias-controlled model reproduced the specific and endogenous mechanism of ATC and will allow to identify potential targets for therapeutics in case of trauma-related hemorrhage.
Introduction
Every year, 5.6 million people die worldwide as a result of trauma. The four leading causes of trauma are road traffic injuries, self-inflicted violence, inter personal violence and falls, responsible for 25, 16, 10 and 6% of death, respectively. Almost 50% of them affect young people aged between 15 and 44 years, explaining their social impact and financial burden to the healthcare system1,2. Uncontrolled bleeding is a major cause of preventable death in this setting3.
Major trauma with hemorrhage is associated with a specific pathophysiologic sequence. The initial phase is characterized by a macrocirculatory failure. The effects of hypovolemia are initially compensated by an activation of the sympathetic system4. Tachycardia and peripheral vasoconstriction maintain blood pressure and tissue perfusion in critical organs. This state is denominated “compensated shock”. If compensation mechanisms are overwhelmed, arterial blood pressure decreases and death occurs due to the inability to maintain tissue perfusion in critical organs. This state is called “un-compensated shock”. In parallel, microcirculation regulates the distribution of blood flow within the capillary network of each organ in order to match oxygen delivery and demand and optimize oxygen extraction4. In a later phase, after several days, activation of the immune system leads to similar patterns such as those described in sepsis. Microcirculatory dysfunction sustains tissue hypoperfusion and multiple organ failure. This phenomenon explains the occurrence of delayed mortality despite the restoration of a normal blood pressure5,6.
An acute traumatic coagulopathy (ATC) dramatically increases blood losses. This specific coagulation disorder develops extremely quickly in the first 60 minutes after trauma and is detected in approximately one third of multiple injured patients upon hospital admission7–9. Its presence is associated with higher mortality rates and transfusion requirements10. ATC is an endogenous phenomenon occurring independently from external factors such as dilution, hypothermia or anti-thrombotic treatments11. However, external factors aggravate this pre-existing coagulopathy12. Therefore, in this setting, current guidelines recommend limiting fluid resuscitation, maintaining normothermia and reversing antithrombotic therapies11. ATC is defined as an increase in prothrombin time (PT) and/or activated partial thromboplastin time (aPTT) due to the combination of trauma and hemorrhage. The coagulation disorder is correlated with the extent of tissue trauma and the severity of shock10. The difficulty to characterize this condition is reflected by the variety of designations proposed, such as “acute coagulopathy of trauma shock”13, “disseminated intravascular coagulation with the fibrinolytic phenotype”14 or “trauma induced coagulopathy”15. ATC is a complex phenomenon, involving coagulation factors, C protein, platelets and endothelial dysfunction but its physiopathology remains unclear16,17. An imbalance between procoagulant and fibrinolytic pathways is suspected to be a key component of this condition during the acute phase18. Pro and anti-inflammatory patterns are involved in the late phase19.
ATC’s physiopathology and the detection of potential targets for new therapeutics are a matter of great concern for trauma-teams. Thus, the “trans-agency consortium for trauma-induced coagulopathy” (TACTICS) in USA20 or “task force for advanced bleeding care in trauma” in Europe focused on this topic and made proposals for further works11,21. In addition, international experts emphasized a lack of relevant experimental models designed to study ATC22,23. An ideal model should have the following characteristics23: low cost, mechanisms and timeline similar to real-life patients, control of exogenous factors and potential biases. Several models were developed to study ATC22,23 but a recent review24 pointed out the difficulties in studying its endogenous mechanism. Indeed, among 27 distinct animal models, only 5 triggered a coagulopathy by using the combination of trauma and hemorrhage. For example, in one of them, ATC was triggered by a tissue factor infusion without trauma. This mechanism was therefore not similar to real-life patients25. Some models involved high-cost protocols on pigs26–30. In addition, pigs seem to be in a hypercoagulable state, with C protein levels lower than in humans31,32. Others studies used external factors such as dilution or hypothermia to trigger coagulopathy. They were therefore not focused on the endogenous mechanism26,27,33,34.
In addition, a confusion factor was neither identified nor controlled in many studies28,29,35,36. We called it “depletion bias”. Several animal studies on ATC compare groups subjected to an hemorrhage alone with groups subjected to an hemorrhage associated with trauma10. This last group presented higher coagulation disorders and studies conclude to the presence of ATC. However, trauma could induce an additional bleeding in injured tissues. Therefore, higher coagulation disorders detected in traumatized animals can rather be due to this additional bleeding than to a specific mechanism linked with the combination of trauma and hemorrhage. To control this depletion bias, the amount of bleeding due to trauma has to be assessed in a specific group. Unfortunately, bleedings due to trauma cannot be easily quantified. There is no measurement tool to evaluate the amount of blood in bone or liver fractures in rats. Therefore, hemorrhage due to trauma has to be indirectly assessed using clinical and biological variables such as heart rate, blood pressure, pH, blood lactates, base excess and hemoglobin5,15,37,38.
The objective of this study was to develop a clinically relevant and controlled biases model of ATC to study its pathophysiology and consequences during the early phase of a major trauma.
Materials and Methods
Animals
Twenty-eight adult Sprague-Dawley rats (430–650 g, Janvier SAS, Le Genest St Isla, France) were housed in a controlled environment (temperature 21 ± 1 °C, relative humidity 27 ± 16%, 12–12 h light-dark cycle) with ad libitum access to food and water. All procedures were conducted following a protocol approved by the French ministry of agriculture (APAFIS#3797_2016012211009124) and the local university animal research ethic committee. Procedures were in accordance with the guide for the care and use of laboratory animals published by the US National Institutes of Health39.
Preparation
After 1 week of housing, each rat was randomly assigned to an experimental group. Then, animals were anesthetized with an intra-peritoneal injection of ketamine (100 mg/Kg, Virbac, Carros, France) and xylazin (10 mg/Kg, Virbac, Carros, France). A temperature probe was inserted rectally and animals were placed in supine position on a warming pad (Z31SY, Ascon tecnologic, Italy) to maintain central body temperature in a normal range (37.5 ± 0.5 °C). A 2 cm cervical incision was performed, followed by a tracheostomy (2 mm diameter polyethylene tube). An arterial catheter (Leader Flex 22 G, 0,7 × 40 mm, Vygon, France) was inserted in the right carotid allowing blood sampling and continuous intra-arterial pressure monitoring. A venous catheter was inserted in the left jugular vein (Leader Flex 22 G, 0,7 × 40 mm, Vygon, France) followed by a continuous intravenous infusion of ketamin (1 mg/kg/h, Virbac inc., Carros, France). Ketamine was diluted in sodium chloride 0.9%, continuously infused at a rate of 1 ml/kg/h. Vital signs (heart rate, invasive arterial pressure and body temperature) were continuously recorded during all the procedure (MP35, Biopac systems inc. Varna, Bulgaria).
Experimental procedure
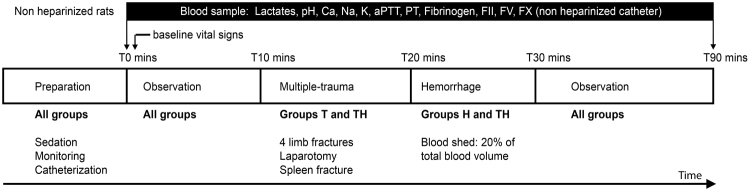
The experimental procedure was summarized in Fig. 1A total of 29 rats were included. One was excluded due to technical issue during blood collection (catheter misplacement). The 28 rats studied were allocated randomly to one of the 4 groups (n = 7 per group): Control (C) in which trauma and hemorrhage were not performed; Trauma (T) in which trauma was performed but not hemorrhage; Hemorrhage (H) in which hemorrhage was performed but not trauma; Trauma and hemorrhage (TH) in which trauma and hemorrhage were performed. In groups H and TH, 20% of total blood mass was gently collected in 10 min with a 10 ml syringe through the arterial catheter. Total blood mass was estimated at 5% of body weight. In groups T and TH, multiple traumas were performed as follows: 4 closed limb fractures on mid-height of the bone (2 femurs, 2 humerus) at 90 angular degrees with pliers (water pump pliers 250 mm, Facom, France). Then a 4 cm median laparotomy, and four spleen crushings of 1 cm on the inferior border of the spleen were done with surgical scissors and a needle holder, respectively (KS-463, Keysurgical, U.S.A.).
Figure 1.
Experimental protocol. Group C, without trauma without hemorrhage; T, trauma without hemorrhage; H, hemorrhage without trauma; TH, hemorrhage with trauma (n = 7 in each group).
Observation time
A very short observation time was chosen to investigate the endogenous mechanism of ATC. Indeed, transcapillary refill begins in the first minutes after hemorrhage. This compensatory mechanism is characterized by the transfer of interstitial fluid to the intravascular area, limiting the consequences of hypovolemia40–42. It also leads to dilution and constitutes a potential confounding factor. Thus, biological analyses at a later stage would have investigated the combined effect of both ATC and dilution coagulopathy due to transcapillary refill. This could have impaired the identification of the specific mechanisms related to ATC.
Blood Samples
No injection of heparin was performed during the whole experimentation. Blood samples were collected through the arterial catheter. Catheter was flushed with 50 μL of 0.9% NaCl without anticoagulant after each sampling, to prevent clot formation. A 1 ml syringe rinsed with heparin 500 UI/ml was used for point of care tests. A 1.4 ml 3.2% citrated tube (Monovette, Saerstedt France) was used for hemostasis tests. Citrated tubes were gently turned upside-down 5 times. Three 15 min centrifugations were performed: one at 1000 g and two at 3000 g (centrifuge 2–16 K, Sigma, Germany) to obtain poor platelet plasma. Plasma was frozen at −80 °C until measurements.
Blood analysis
Arterial blood pH, concentrations of hemoglobin, lactate, calcium, sodium and potassium were measured instantaneously after blood collection with a point-of care analyser (ABL80 FLEX, Radiometer, Copenhagen, Denmark). Hemostasis analysis (FII, FV, FX, fibrinogen, PT and aPTT) were performed on platelet poor thawed plasma on an automated analyser (STA-R Evolution, Stago, Asnieres sur Seine, France). PT, aPTT times and fibrinogen concentrations were measured with specific reagents, « neoplastine Cl + 10 », « triniclot aPTTb », and « STA liquid fib », respectively. Specific factor-depleted plasmas (Stago, Asnieres sur Seine, France) were used to determine coagulation factor times. Coagulation factor activities were expressed in time-to-clot instead of percentages in this study. Indeed, these percentages are expressed in comparison with the laboratory reference in humans and references for rats are not available. Therefore, we made the choice to express non-modified data: the time to clot in seconds. This time is inversely proportional to the factor activity.
Statistical analysis
Statistical analyses were performed with “SPSS statistics for Macintosh” software version 21 (I.B.M. corp., Armmonk, NY, 2012). Line graphs, boxplots and histograms were performed with “Prism 7 for Mac OS X” version 7.0a (Graphpad software, La Jolla, USA, 2016). Considering that the PT and aPTT values obtained at 0 min are reference values for each animal, ratios were calculated as follows: PT ratio = (PT at 90 min)/(PT at 0 min), aPTT ratio = (aPTT at 90 min)/(aPTT at 0 min). Two-way repeated measures analyses of variance with adequate post hoc tests were used to compare means over time within and between groups. Results were expressed as the mean ± standard error of mean (SEM), unless otherwise stated. A p-value of <0.05 was considered statistically significant.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Group comparability and effects of anesthesia
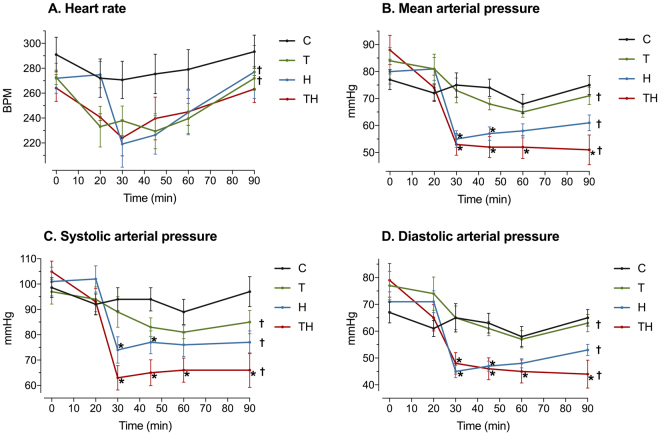
At 0 min, no statistical difference between groups was observed for all variables (p > 0.05). In addition, anesthetic agents did not interfere with hemodynamic because heart rate and MAP did not vary significantly over time in group C (p > 0.05, Fig. 2A,B). These results allow comparisons between groups at the end of the experimentation without doubt concerning potential bias due to the procedure.
Figure 2.
Hemodynamic variables. C, Control; T, Trauma; H, Hemorrhage; TH, Trauma and hemorrhage, n = 7 per group. Values represent mean ± SEM. *p < 0.05 compared with group C; †p < 0.05 for repeated measure ANOVA, meaning a statistically significant effect of time in concerned group.
ATC was reproduced in this model
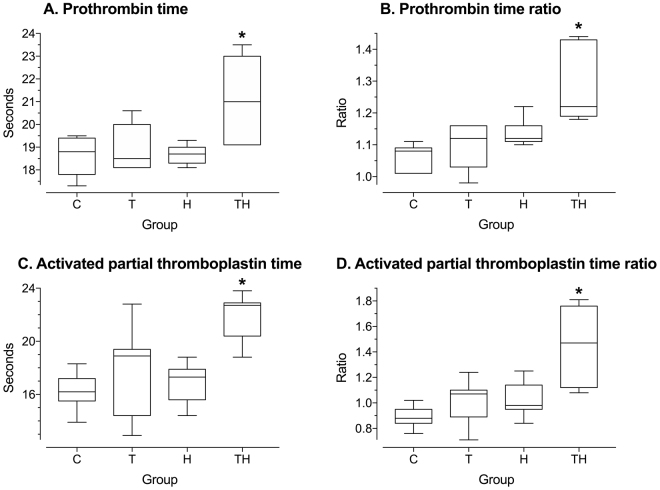
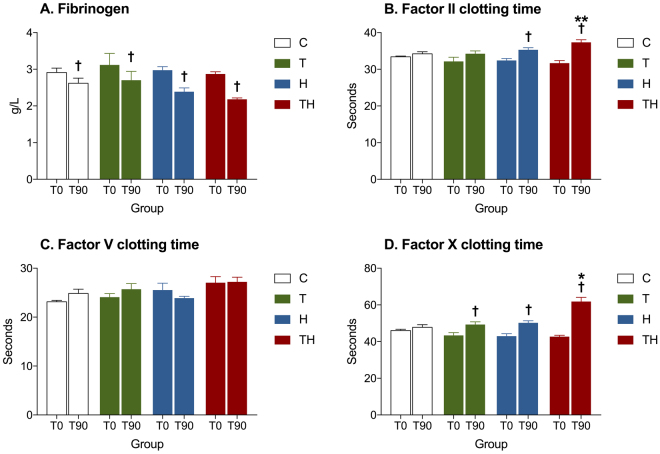
The main objective of this study was to reproduce the specific mechanism leading to ATC. However, trauma or hemorrhage alone could induce coagulation impairments. Thus, we studied their single effects on coagulation in group T and H, and compared them to their association in group TH. Mean PT, PTr, aPTT and aPTTr increased significantly in group TH compared to group C, T and H (p < 0.05, Fig. 3A–D). The four main biological markers of ATC were therefore present in the TH group10,11. This coagulation disorder was specifically induced by the association of trauma and hemorrhage because no statistical difference between C, T and H groups was retrieved for these variables (p > 0.05, Fig. 3A–D). The absence of potential confounding factors that may interfere with coagulation was also verified. No significant decrease in hemoglobin was observed in group H, suggesting the absence of transcapillary refill40. As mentioned in the “observation time” section, this compensatory mechanism leads to dilution and could have interfered with ATC. In addition, we neither used fluid replacement nor antithrombotic injection that could dilute coagulation factors or modify their activities. We also prevented hypothermia which is known to reduce clotting factor enzymatic protease activity in vivo. Coagulopathy was therefore due to a specific and endogenous mechanism, strictly corresponding to the definition of ATC.
Figure 3.
General coagulation assays. Box and whisker plot of general coagulation assays at 90 min in each experimental group. C, Control; T, Trauma; H, Hemorrhage; TH, Trauma and hemorrhage, n = 7 per group. Whiskers represent the 5th and 95th percentiles. *p < 0.05 versus C T and H. Ratios were calculated for each animal as follows: (value at 90 min)/(value at 0 min).
Un-compensated shock was triggered by ATC
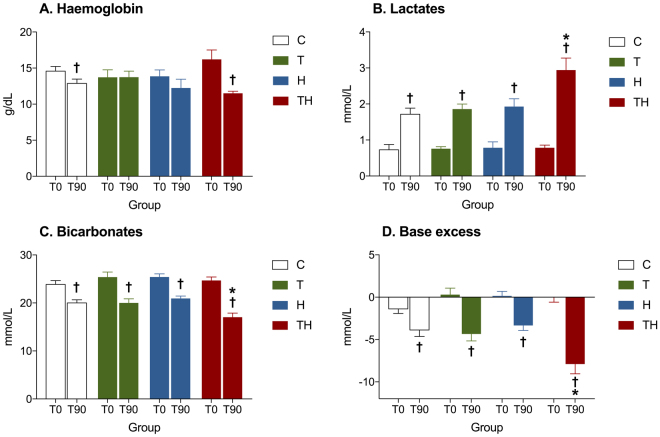
Next, we wanted to evaluate the clinico-biological consequences of ATC, which is a key point to explain higher mortality rates in case of trauma-related hemorrhage. In group TH, we observed a significant decrease in base excess concentrations (p < 0.05, Fig. 4D) associated with an increase in lactate (p < 0.05, Fig. 4B) and potassium concentrations (p < 0.05, Table 1). The rise in lactate concentration evidences the presence of cellular hypoxemia and the transition from aerobia to anaerobia. The lowering in base excess reflects a metabolic acidosis and the rise in potassium concentration indicates the presence of cellular leakage. These results indicate an energetic imbalance between cellular incomes and needs leading to cellular dysfunction, corresponding to the definition of shock: “a state in which the circulation is unable to deliver sufficient oxygen to meet the demands of the tissues”43. In addition, arterial pressure remained low until the end of the experimentation in group TH (p < 0.05, Fig. 2B–D). Compensatory mechanisms were therefore not sufficient to maintain blood pressure; this clinico-biological status is also known as un-compensated shock. In contrast, arterial pressures felt just after trauma/hemorrhage and raised slowly during the experimentation in group T and H (p > 0.05, Fig. 2B–D). Biological markers of shock were also negative in these groups (p > 0.05, Fig. 4B–D). Consequently, ATC induced a switch from compensated to un-compensated shock in this model.
Figure 4.
Shock variables. Mean values at 0 min and 90 min in each experimental group. C, Control; T, Trauma; H, Hemorrhage; TH, Trauma and hemorrhage, n = 7 per group. †p < 0.05 compared with 0 min in the same group, *p < 0.05 versus C T and H. Data are presented as mean ± SEM.
Table 1.
Arterial blood tests and ionogram assays.
| Group C | Group T | Group H | Group TH | p-value | |
|---|---|---|---|---|---|
| Arterial blood tests | |||||
| pH | |||||
| T0 | 7.35 ± 0.018 | 7.37 ± 0.016 | 7.36 ± 0.012 | 7.38 ± 0.005 | 0.441 |
| T90 | 7.38 ± 0.017 | 7.36 ± 0.006 | 7.37 ± 0.017 | 7.32 ± 0.033 | 0.412 |
| pO2 (mmHg) | |||||
| T0 | 69.2 ± 3.3 | 62 ± 2.5 | 66 ± 1.8 | 69.8 ± 2.5 | 0.158 |
| T90 | 83.8 ± 6.6 | 79.2 ± 2 | 71.8 ± 4.9 | 77.2 ± 5.6 | 0.437 |
| pCO2 (mmHg) | |||||
| T0 | 44.8 ± 3.3 | 44.5 ± 2.5 | 46 ± 1.8 | 42.4 ± 2.5 | 0.748 |
| T90 | 34.43 ± 1.6† | 36.1 ± 1.1† | 36.9 ± 1.8† | 34.5 ± 2.8† | 0.760 |
| Ionogram | |||||
| Calcium (mg/L) | |||||
| 0 min | 1.36 ± 0.46 | 1.42 ± 0.02 | 1.33 ± 0.02 | 1.29 ± 0.03 | 0.186 |
| 90 min | 1.38 ± 0.03 | 1.25 ± 0.02† | 1.3 ± 0.04 | 1.4 ± 0.11 | 0.336 |
| Sodium (mEq/L) | |||||
| 0 min | 140 ± 1.49 | 142 ± 0.72 | 141 ± 1 | 141 ± 1.01 | 0.740 |
| 90 min | 143 ± 0.77† | 144 ± 2.79 | 136 ± 3.5 | 142 ± 3.1 | 0.202 |
| Potassium (mmol/L) | |||||
| 0 min | 5.34 ± 0.24 | 5.14 ± 0.34 | 5.09 ± 0.13 | 5.57 ± 0.23 | 0.527 |
| 90 min | 5.39 ± 0.17 | 6.03 ± 0.26 | 5.80 ± 0.24† | 6.73 ± 0.26a | 0.005* |
Data are presented as mean ± SEM. C, Control; T, Trauma; H, Hemorrhage; TH, Trauma and hemorrhage, n = 7 per group. ap < 0.05 compared with C, †p < 0.05 compared with 0 min in the same group.
ATC was associated with a specific coagulation profile
We then studied specific coagulation factors to identify their role in case of ATC. A significant decrease in fibrinogen concentration was observed in all groups, probably due to clot formation after cervical incision and cannulation (Fig. 5A). Interestingly, a significant negative linear relationship between PT ratio and fibrinogen serum concentrations at 90 min was retrieved (intercept, 3.57; coefficient, −1.04; p, 0.0272; R2, 0.6561; adjusted R2, 0.5873). This result highlights a protective effect of fibrinogen against ATC. In addition, a significant increase of FII and FX times from 0 to 90 min was observed in this study (p < 0.05, Fig. 5B–D). These data suggest that specific coagulations pathways mediated by FII and FX are involved in ATC’s genesis.
Figure 5.
Coagulation factors. Histograms of specific coagulation factors at 0 min and 90 min in each experimental group. C, Control; T, Trauma; H, Hemorrhage; TH, Trauma and hemorrhage, n = 7 per group. †p < 0.05 compared with 0 min in the same group, *p < 0.05 versus C T and H. **p < 0.05 versus C and T. Data are presented as mean ± SEM.
Discussion
This model was particularly consistent with human ATC in terms of temporality, type of injuries, compensation mechanisms and coagulation impairments. Indeed, in most countries, patients are brought to hospital in approximately 60 min44 and injuries performed in this study were consistent with those retrieved after traffic accidents44. In addition, compensatory mechanisms were similar to those described in humans to maintain blood pressure and homeostasis. Concerning blood pressure, we observed a decrease in heart rate in group T, H ad TH, suggesting an activation of the Bezold Jarisch reflex (Fig. 2A). This reflex increases end-diastolic volumes and cardiac output by lengthening the time allowed to fill cardiac chambers45–47. It is triggered by left ventricle stretch receptors, endogenous catecholamins and serotonin. Serotonin is released after platelet activation, explaining bradycardia in T and TH groups. Stretch receptor and catecholamins are activated in case of hypovolemia, explaining bradycardia in H and TH groups. Concerning homeostasis, alveolar hyperventilation and bicarbonates consumption to maintain pH in normal range were retrieved in this model (Table 1). Moreover, the protective role of fibrinogen was observed48 and values for aPTT and PT ratios were comparable to those retrieved in humans when ATC occurs10.
The role of previously identified confounding factors was then assessed to guarantee the robustness of the model. The absence of transcapillary refill was established by comparing hemoglobin concentrations at 0 and 90 min in the H group to ensure the absence of spontaneous dilution due to hemorrhage (Fig. 4A). The “depletion bias” was assessed by comparing markers of hemorrhage to ensure the absence of significant bleeding in the trauma group. In our model, mean arterial pressure, base excess, hemoglobin, bicarbonates and lactate concentrations were similar in group C and T at the end of the experimentation and did not vary during time in group T (Figs 2B and 4A–D). Therefore, trauma alone did not lead to any significant bleeding. As far as we know, this study is the first to identify and verify the absence of these two major bias.
Then, we wanted to illustrate the usefulness of our model to explore ATC’s pathophysiology. To this purpose, analysis of coagulation factors concentrations were performed and allowed us to hypothesize that TFPI could play a role in case of trauma-related hemorrhage. Indeed, activation of C-protein pathway is known to be one of the key drivers of ATC49–51. This activation leads to a decrease in FV52,53. Interestingly, in our study, ATC occurred in group TH without any change in FV time. On the contrary, FX time raised, indicating a decrease of FX activity. These data suggest that APC’s activation is not the only mechanism to trigger ATC. Another pathway inhibiting specifically FX appears to be involved in this context. A valid theory could be that FX was repressed by an inappropriate secretion of tissue factor pathway inhibitor (TFPI). TFPI is a serine protease inhibitor54. One of its major isoform, TFPIα, is localized on endothelium and platelets in humans, and only in platelet in murines55. It is externalized after platelet activation and has 3 Kunitz domains56–58. The first domain binds to the active site of FVIIa, the second to the active site of FX and the third to protein S59,60. This structure explain why TFPI induces a specific repression of FX61–63. Consequently, an increase in TFPI plasma concentration could explain the inhibition of FX observed in this experimentation. These findings reinforce the theory that platelets and/or endothelium play a role in ATC’s genesis.
In addition, our model has particularities that could be of interest for further studies. As far as we know, it is the first to demonstrate and reproduce the switch from compensated to un-compensated shock due to ATC. This makes possible to explain why higher mortality rates are observed in this condition. Moreover, it is the first to show that ATC can arise even in case of moderate hemorrhage. Indeed, ATC occurred with only a loss of 20% of total blood mass whereas 40% were usually necessary in previous studies. In addition, no animal died before the end of the procedure. This small mammalian model is therefore ethic, time and cost-effective.
The main limitation of this model is that average total blood mass of rats is about 25 ml. Volumes were therefore limited to avoid hemodynamic disturbance due to blood samples. High volumes or repeated blood samples, for example to investigate the time course of multiple coagulation factors in the same time, are therefore excluded. This constraint applies to all small mammalian models. In further studies we should consider that one general assay such as PT or aPTT will be sufficient to conclude to the presence of ATC. The remaining blood will be available to explore ATC’s pathophysiology. Others limitations should be considered: Clotting factors serum concentrations are not similar among species; quantitative results are therefore not strictly transferable to humans. However, the role of coagulation in survival is so crucial that key mechanisms were probably conserved between species during evolution.
In conclusion, this model reproduced the sequence and timeline retrieved in humans in case of severe trauma associated with hemorrhage. The combination of these two factors induced a specific and endogenous coagulopathy fitting the definition of ATC. A complete consistence with humans was observed concerning clinical and biological markers for shock, compensation mechanism, protective factors and coagulation disorders involved in this condition. We identified and controlled two new bias: majored bleedings due to trauma and dilution due to transcapillary refill. Moreover, we reproduced the switch from compensated to un-compensated shock when ATC occurs, corresponding to the time when compensation mechanisms are overwhelmed. This could help to understand the pathophysiology of ATC at this key moment and to identify potential targets for therapeutics to decrease mortality. In addition, we demonstrated that ATC can occur even in case of moderate hemorrhage and we made the hypothesis that TFPI could repress FX in this case. This model is therefore effective to explore coagulation patterns involved in the genesis of ATC and its consequences on tissue perfusion.
Acknowledgements
Kerri Moore, Jean Alain Martignole. This work was supported by public funding provided by the French ministry of research.
Author Contributions
C.G., K.P., M.T. and O.G. designed the experiment. C.G. performed the experiments. C.G. and M.T. performed statistical analysis. C.G. wrote the manuscript. K.P., M.T., F.M., H.G. and Y.O. revised the manuscript. All authors read and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet: GBD 2013 Mortality and Causes of Death Collaborators385, 117–171 (2015). [DOI] [PMC free article] [PubMed]
- 2.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31:1507–11. doi: 10.1007/s00268-007-9087-2. [DOI] [PubMed] [Google Scholar]
- 3.Stanworth, S. J. et al. Mortality from trauma haemorrhage and opportunities for improvement in transfusion practice. Br J Surg10.1002/bjs.10052 (2016). [DOI] [PubMed]
- 4.Libert N, Harrois A, Duranteau J. Haemodynamic coherence in haemorrhagic shock. Best Pract Res Clin Anaesthesiol. 2016;30:429–435. doi: 10.1016/j.bpa.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao W, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theusinger OM, et al. Changes in Coagulation in Standard Laboratory Tests and ROTEM in Trauma Patients Between On-Scene and Arrival in the Emergency Department. Anesthesia & Analgesia. 2015;120:627–635. doi: 10.1213/ANE.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 8.Engels PT, et al. The Natural History of Trauma-Related Coagulopathy: Implications for Treatment. The Journal of Trauma: Injury, Infection, and Critical Care. 2011;71:S448–S455. doi: 10.1097/TA.0b013e318232e6ac. [DOI] [PubMed] [Google Scholar]
- 9.Maegele M, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Frith D, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8:1919–25. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 11.Rossaint R, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100. doi: 10.1186/s13054-016-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hess JR, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 13.Oshiro A, et al. Hemostasis during the early stages of trauma: comparison with disseminated intravascular coagulation. Crit Care. 2014;18:R61. doi: 10.1186/cc13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gando S, et al. Differentiating disseminated intravascular coagulation (DIC) with the fibrinolytic phenotype from coagulopathy of trauma and acute coagulopathy of trauma-shock (COT/ACOTS) J Thromb Haemost. 2013;11:826–35. doi: 10.1111/jth.12190. [DOI] [PubMed] [Google Scholar]
- 15.Neal MD, et al. Clinical assessment of trauma-induced coagulopathy and its contribution to postinjury mortality: A TACTIC proposal. J Trauma Acute Care Surg. 2015;79:490–2. doi: 10.1097/TA.0000000000000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White, N. J. et al. Early Hemostatic Responses to Trauma: Identified Using Hierarchical Clustering Analysis. J Thromb Haemost10.1111/jth.12919 (2015). [DOI] [PMC free article] [PubMed]
- 17.Dobson GP, Letson HL, Sharma R, Sheppard FR, Cap AP. Mechanisms of early trauma-induced coagulopathy: The clot thickens or not? J Trauma Acute Care Surg. 2015;79:301–9. doi: 10.1097/TA.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Darlington DN, Cap AP. Procoagulant and fibrinolytic activity after polytrauma in rat. Am J Physiol Regul Integr Comp Physiol. 2016;310:R323–9. doi: 10.1152/ajpregu.00401.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darlington DN, et al. Trauma-Induced Coagulopathy Is Associated with a Complex Inflammatory Response in the Rat. Shock. 2015;44(Suppl 1):129–37. doi: 10.1097/SHK.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 20.Mann KG, Freeman K. TACTIC: Trans-Agency Consortium for Trauma-Induced Coagulopathy. J Thromb Haemost. 2015;13(Suppl 1):S63–71. doi: 10.1111/jth.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spahn DR, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17:R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frith D, Cohen MJ, Brohi K. Animal models of trauma-induced coagulopathy. Thromb Res. 2012;129:551–6. doi: 10.1016/j.thromres.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 23.Parr MJ, et al. Traumatic coagulopathy: where are the good experimental models? J Trauma. 2008;65:766–71. doi: 10.1097/TA.0b013e31818606d2. [DOI] [PubMed] [Google Scholar]
- 24.van Zyl, N., Reade, M. C. & Fraser, J. F. Experimental Animal Models of Traumatic Coagulopathy: A Systematic Review. Shock10.1097/SHK.0000000000000372 (2015). [DOI] [PubMed]
- 25.Hayakawa M, et al. Massive amounts of tissue factor induce fibrinogenolysis without tissue hypoperfusion in rats. Shock. 2013;39:514–9. doi: 10.1097/SHK.0b013e318293980d. [DOI] [PubMed] [Google Scholar]
- 26.Doran CM, et al. Targeted resuscitation improves coagulation and outcome. J Trauma Acute Care Surg. 2012;72:835–43. doi: 10.1097/TA.0b013e318248347b. [DOI] [PubMed] [Google Scholar]
- 27.Mohr J, et al. Induced hypothermia does not impair coagulation system in a swine multiple trauma model. J Trauma Acute Care Surg. 2013;74:1014–20. doi: 10.1097/TA.0b013e3182826edd. [DOI] [PubMed] [Google Scholar]
- 28.Duan K, et al. A time course study of acute traumatic coagulopathy prior to resuscitation: from hypercoagulation to hypocoagulation caused by hypoperfusion? Transfus Apher Sci. 2014;50:399–406. doi: 10.1016/j.transci.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Hagemo JS, et al. Changes in fibrinogen availability and utilization in an animal model of traumatic coagulopathy. Scand J Trauma Resusc Emerg Med. 2013;21:56. doi: 10.1186/1757-7241-21-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesperance RN, et al. Recombinant factor VIIa is effective at reversing coagulopathy in a lactic acidosis model. J Trauma Acute Care Surg. 2012;72:123–9. doi: 10.1097/TA.0b013e318224e24a. [DOI] [PubMed] [Google Scholar]
- 31.Velik-Salchner C, et al. Normal values for thrombelastography (ROTEM) and selected coagulation parameters in porcine blood. Thromb Res. 2006;117:597–602. doi: 10.1016/j.thromres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Siller-Matula JM, Plasenzotti R, Spiel A, Quehenberger P, Jilma B. Interspecies differences in coagulation profile. Thromb Haemost. 2008;100:397–404. doi: 10.1160/TH08-02-0103. [DOI] [PubMed] [Google Scholar]
- 33.Martini J, Cabrales P, Fries D, Intaglietta M, Tsai AG. Effects of fibrinogen concentrate after shock/resuscitation: a comparison between in vivo microvascular clot formation and thromboelastometry*. Crit Care Med. 2013;41:e301–8. doi: 10.1097/CCM.0b013e31828a4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwamoto S, Takasu A, Sakamoto T. Therapeutic mild hypothermia: effects on coagulopathy and survival in a rat hemorrhagic shock model. J Trauma. 2010;68:669–75. doi: 10.1097/TA.0b013e3181a0fbb3. [DOI] [PubMed] [Google Scholar]
- 35.White NJ, Martin EJ, Brophy DF, Ward KR. Coagulopathy and traumatic shock: characterizing hemostatic function during the critical period prior to fluid resuscitation. Resuscitation. 2010;81:111–6. doi: 10.1016/j.resuscitation.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulier KE, Greenberg JG, Beilman GJ. Hypercoagulability in porcine hemorrhagic shock is present early after trauma and resuscitation. J Surg Res. 2012;174:e31–5. doi: 10.1016/j.jss.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Maegele M, et al. Revalidation and update of the TASH-Score: a scoring system to predict the probability for massive transfusion as a surrogate for life-threatening haemorrhage after severe injury. Vox Sang. 2011;100:231–8. doi: 10.1111/j.1423-0410.2010.01387.x. [DOI] [PubMed] [Google Scholar]
- 38.Mutschler M, et al. The ATLS((R)) classification of hypovolaemic shock: a well established teaching tool on the edge? Injury. 2014;45(Suppl 3):S35–8. doi: 10.1016/j.injury.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Guide for the Care and Use of Laboratory Animals. National Research Council of the National Academies Available at: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf (2011).
- 40.Sallum EA, et al. Blood loss and transcapillary refill in uncontrolled treated hemorrhage in dogs. Clinics. 2010;65:67–78. doi: 10.1590/S1807-59322010000100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lloyd, S., Boulanger, B. & Johnston, M. The lymphatic circulation plays a dynamic role in blood volume and plasma protein restitution after hemorrhage. Shock doi:8799953 (1996). [DOI] [PubMed]
- 42.Prist R, et al. A quantitative analysis of trancapillary refill in severe hemorrhagic hypotension in dogs. Shock. 1994;1:188–195. doi: 10.1097/00024382-199403000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Aya, H. D. et al. From cardiac output to blood flow auto-regulation in shock. Anaesthesiol Intensive Ther47Spec No, s56–62 (2015). [DOI] [PubMed]
- 44.Nance, M., Steward, R., Rotondo, M. & Nathens, A. B. NTDB annual report 2015. American College of Surgeons Committee on Trauma Leadership (2015).
- 45.Wisbach G, Tobias S, Woodman R, Spalding A, Lockette W. Preserving cardiac output with beta-adrenergic receptor blockade and inhibiting the Bezold-Jarisch reflex during resuscitation from hemorrhage. J Trauma. 2007;63:26–32. doi: 10.1097/TA.0b013e31806864e2. [DOI] [PubMed] [Google Scholar]
- 46.Aviado DM, Guevara Aviado D. The Bezold Jarisch Reflex. Ann N Y Acad Sci. 2001;940:48–58. doi: 10.1111/j.1749-6632.2001.tb03666.x. [DOI] [PubMed] [Google Scholar]
- 47.Verberne AJ, Saita M, Sartor DM. Chemical stimulation of vagal afferent neurons and sympathetic vasomotor tone. Brain Res Brain Res Rev. 2003;41:288–305. doi: 10.1016/S0165-0173(02)00269-2. [DOI] [PubMed] [Google Scholar]
- 48.Stinger, H. K. et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma64, S79–85; discussion S85 (2008). [DOI] [PubMed]
- 49.Davenport RA, et al. Activated Protein C Drives the Hyperfibrinolysis of Acute Traumatic Coagulopathy. Anesthesiology. 2017;126:115–127. doi: 10.1097/ALN.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Zyl N, et al. Activation of the protein C pathway and endothelial glycocalyx shedding is associated with coagulopathy in an ovine model of trauma and hemorrhage. J Trauma Acute Care Surg. 2016;81:674–84. doi: 10.1097/TA.0000000000001190. [DOI] [PubMed] [Google Scholar]
- 51.Howard BM, et al. Inducing Acute Traumatic Coagulopathy In Vitro: The Effects of Activated Protein C on Healthy Human Whole Blood. PLoS One. 2016;11:e0150930. doi: 10.1371/journal.pone.0150930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dahlback B. Pro- and anticoagulant properties of factor V in pathogenesis of thrombosis and bleeding disorders. Int J Lab Hematol. 2016;38(Suppl 1):4–11. doi: 10.1111/ijlh.12508. [DOI] [PubMed] [Google Scholar]
- 53.Dahlback B, Villoutreix BO. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol. 2005;25:1311–20. doi: 10.1161/01.ATV.0000168421.13467.82. [DOI] [PubMed] [Google Scholar]
- 54.Mast AE. Tissue Factor Pathway Inhibitor: Multiple Anticoagulant Activities for a Single Protein. Arterioscler Thromb Vasc Biol. 2016;36:9–14. doi: 10.1161/ATVBAHA.115.306607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maroney SA, et al. Temporal expression of alternatively spliced forms of tissue factor pathway inhibitor in mice. J Thromb Haemost. 2009;7:1106–13. doi: 10.1111/j.1538-7836.2009.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novotny WF, Girard TJ, Miletich JP, Broze GJ. Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein associated coagulation inhibitor. Blood. 1988;72:2020–5. [PubMed] [Google Scholar]
- 57.Maroney SA, Cooley BC, Ferrel JP, Bonesho CE, Mast AE. Murine hematopoietic cell tissue factor pathway inhibitor limits thrombus growth. Arterioscler Thromb Vasc Biol. 2011;31:821–6. doi: 10.1161/ATVBAHA.110.220293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maroney SA, Mast AE. Expression of tissue factor pathway inhibitor by endothelial cells and platelets. Transfus Apher Sci. 2008;38:9–14. doi: 10.1016/j.transci.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hackeng TM, Sere KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci USA. 2006;103:3106–11. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Girard TJ, et al. Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–20. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- 61.Novotny WF, Girard TJ, Miletich JP, Broze GJ. Purification and characterization of the lipoprotein-associated coagulation inhibitor from human plasma. J Biol Chem. 1989;264:18832–7. [PubMed] [Google Scholar]
- 62.Camire RM. Rethinking events in the haemostatic process: role of factor V and TFPI. Haemophilia. 2016;22(Suppl 5):3–8. doi: 10.1111/hae.13004. [DOI] [PubMed] [Google Scholar]
- 63.Peraramelli S, et al. Role of exosite binding modulators in the inhibition of Fxa by TFPI. Thromb Haemost. 2016;115:580–90. doi: 10.1160/th15-04-0354. [DOI] [PubMed] [Google Scholar]