Abstract
Hunger evokes stereotypic behaviors that favor the discovery of nutrients. The neural pathways that coordinate internal and external cues to motivate foraging behaviors are only partly known. Drosophila that are food deprived increase locomotor activity, are more efficient in locating a discrete source of nutrition, and are willing to overcome adversity to obtain food. We developed a simple open field assay that allows flies to freely perform multiple steps of the foraging sequence, and we show that two distinct dopaminergic neural circuits regulate measures of foraging behaviors. One group, the PAM neurons, functions in food deprived flies while the other functions in well fed flies, and both promote foraging. These satiation state-dependent circuits converge on dopamine D1 receptor-expressing Kenyon cells of the mushroom body, where neural activity promotes foraging independent of satiation state. These findings provide evidence for active foraging in well-fed flies that is separable from hunger-driven foraging.
Introduction
The neural mechanisms that regulate feeding motivation are ancient, fundamental for survival, and under complex regulation, and yet they remain partially defined and understood. Feeding motivation is classically divided into pre-ingestive and consummatory phases1,2. In the pre-ingestive phase, nutritional deficits cause release of hormonal signals that act on the brain to bias behavioral states towards seeking food, including heightened attention to food-related environmental cues, increased locomotion, and suppression of incompatible behaviors such as sleep. Once a nutritional source is encountered, homeostatic mechanisms in concert with sensory and nutrient detectors cause a cessation of locomotion and engagement of motor programs for food intake. Both pre-ingestive and consummatory phase behaviors are motivated and goal-directed. However, the goals and the conditions for their completion are different, suggesting that the neural circuits controlling each phase are also different. Defining the neural mechanisms of feeding motivation is important in part because the dysregulation of feeding behavior is intimately tied to obesity and eating disorders, as well as to other pathological alterations of motivation, including drug addiction3,4.
Simpler organisms such as Drosophila hold promise for uncovering the neural circuit mechanisms for motivated feeding behavior. In Drosophila, feeding behavior studies have focused mostly on the consummatory phase, and have revealed satiation state-dependent effects on sensory5–7, motor8–10, and central processing of feeding11–14. Appetitive associative conditioning with feeding has defined detailed neural circuits implicated in reward and reward learning15–18. Drosophila studies of the pre-ingestive phase have focused mostly on sensory perception of appetitive stimuli, including odor tracking, satiation state-dependent olfactory acuity, but also on search strategies19–23. The task-specific paradigms used in Drosophila feeding studies are critical for accurate assignment of circuit function. However, allowing an animal to perform only part of a behavioral sequence may cause circuits to be used inappropriately or in the wrong context. Here, we report the development of an open field assay for foraging behaviors in Drosophila. Flies search in an open arena for a discrete source of food, and can choose to occupy, taste, consume, or reject the source. Assays where animals can freely perform entire behavioral sequences compliment more task-specific assays in defining how complex information is processed to drive behavior. We demonstrate roles for distinct dopaminergic neural circuits in the well-fed and food-deprived states for regulating foraging behavior.
Results
Parametric Analysis of Drosophila Food Seeking Behavior
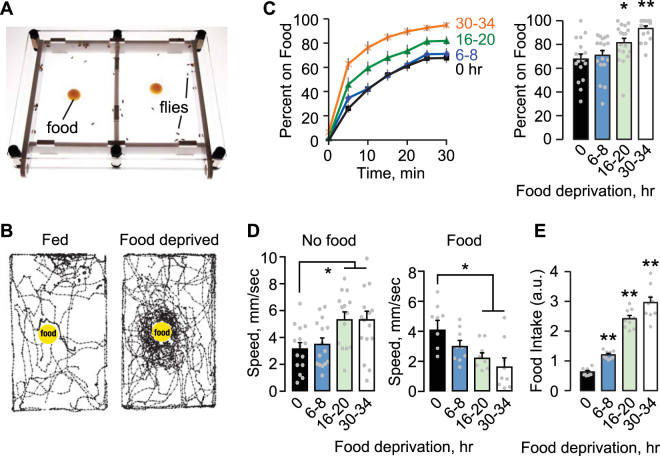
We developed an open field assay to measure various aspects of foraging in freely behaving flies. Flies placed into a translucent arena (Fig. 1A) are tracked with a video camera (Fig. 1B). After a set acclimation period, a small volume of food is introduced at the center of the arena. Longer periods of food deprivation (wet starvation with water only) increased the number of flies in contact with the food, the food occupancy rate (Fig. 1C). Locomotor speed in the absence of food increased with longer periods of food deprivation (Fig. 1D). Introduction of food into the arena rapidly decreased the locomotor speed of food deprived flies that were not in contact with the food source. Food intake also scaled with deprivation time, as measured in a separate assay that minimizes the effect of seeking time (Fig. 1E). For subsequent experiments, ‘food-deprived’ indicates 16–20 hr of a water only diet, unless otherwise noted.
Figure 1.
Food deprivation effect on foraging behavior. (A) Two-sided chamber for foraging assays. Flies and 100 ul of cornmeal molasses food on a Parafilm square placed in each chamber via sliding side doors. The chamber is lit from below. Fly locomotion is recorded from above. (B) 10 sec locomotor traces of 20 flies (fed and 20 hr food deprived) each filmed soon after addition of food (yellow dot). Tracking traces were generated with DIAS software. (C) Left: The percent of flies on food over time for a food deprivation time course. Right, food occupancy averaged at 25–30 min. P < 0.0001, ANOVA/Bonferroni comparison to 0 hr. n = 17–18 groups. (D) Locomotor speed. Left, speed at 20 min of acclimation, without food. Right, speed averaged over 0–10 min after food introduction. P = 0.0091 no food, P = 0.0066 food, ANOVA/Bonferroni compared to 0 hr. n = 9–15 groups. (E) Intake with increasing food deprivation time. P < 0.0001, ANOVA/Bonferroni comparison to 0 hr. n = 9 groups. *P < 0.05, **P < 0.01.
Sensory and Nutritional Inputs to Food Seeking
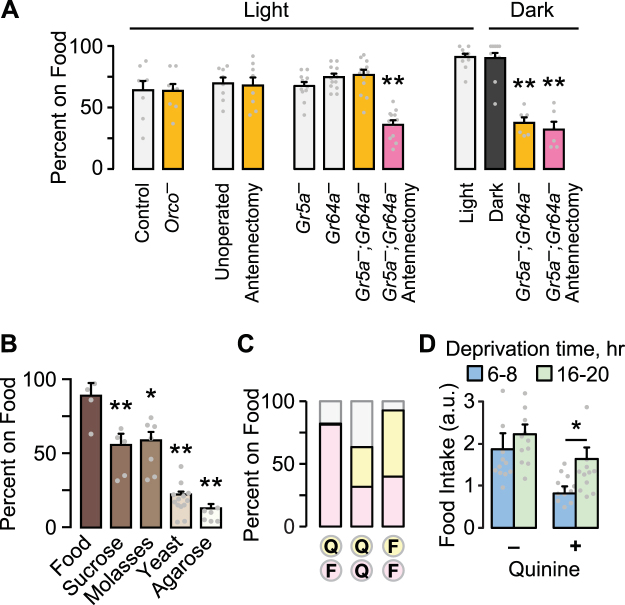
We tested for the role of olfaction, taste, and vision in foraging behavior in food-deprived flies (Fig. 2A). Neither genetic nor surgical ablation of food odor-detecting neurons - olfactory coreceptor mutant Orco1 or removal of the third antennal segment - affected food occupancy24,25. Similarly, flies lacking a subset of sugar sensing taste receptors showed normal food occupancy for sucrose. These experiments suggested that flies may use more than one sensory modality when seeking nearby food. Flies with both ablated antennae and taste receptor mutations showed decreased food occupancy, suggesting coordination between olfaction and taste. Food occupancy remained robust in complete darkness. However, taste receptor mutant flies showed reduced food occupancy in total darkness, and additionally removing olfactory input did not further reduce occupancy. These results indicate that flies use a combination of taste, olfactory, and visual cues to find and occupy a discrete food source.
Figure 2.
Environmental and sensory information in foraging. (A) Food occupancy following sensory ablations in 16–20 hr food deprived flies. Antennectomy is surgical removal of the third antennal segment. Orco− flies lack the Orco olfactory coreceptor; Gr5a− and Gr64a− are taste receptor mutants. P < 0.0001 for both Light and Dark, ANOVA/Bonferroni compared to control, n = 8–12 groups. Light/dark tests were performed in an incubator, where unknown environmental factors increased food occupancy overall. (B) Occupancy of 16–20 hr food deprived flies to agarose with the indicated food component. P < 0.0001, ANOVA/Bonferroni comparison to Food. n = 4–5 groups. (C) Food occupancy for flies given the choice between two closely apposed sources of food (yellow and pink): unadulterated food (F) and 10 mM quinine food (Q). n = 5 groups. (D) When presented with a single food source, flies consumed greater quantities of quinine food (3 mM) when food-deprived for 16–20 hr (long) versus 6–8 hr (short). P = 0.0251, Mann Whitney test, n = 12. *P < 0.05, **P < 0.01. See also Figure S1.
Flies may seek one or more food constituents. Food deprived flies were most attracted to complete food, then sugars, and then protein (Fig. 2B). In a binary choice competition where flies are presented with two closely apposed sources, flies preferred complete food over any other option, and sugars over yeast (Supplementary Fig. S1). Similarly, flies preferred nutritious and sweet sucrose more than sweet-only sucralose (Supplementary Fig. S1). Finally, nutrition appears to be important for switching the locomotor state of food deprived flies: when given a single source, flies slowed more in the presence of sucrose or D-glucose, compared to sweet only sucralose or L-glucose, respectively (Fig. S1D,E). These findings suggest that sweetness is a mechanism that captures flies on a food source, and that nutritional content is important for fully switching flies from the pre-ingestive to consummatory phase of foraging.
A characteristic of motivated behavior is the willingness to overcome negative consequences4. Flies will eat substantially less food when it is adulterated with bitter compounds, and this scales with satiation state13,26. In a binary choice competition, food deprived flies occupied quinine-containing food, but only if there was no better choice (Fig. 2C). Furthermore, food intake under one-choice conditions was less suppressed by quinine with a longer period of deprivation (Fig. 2D). We used a sucrose food source for all subsequent experiments.
Role of Dopaminergic Neurons in Food Seeking
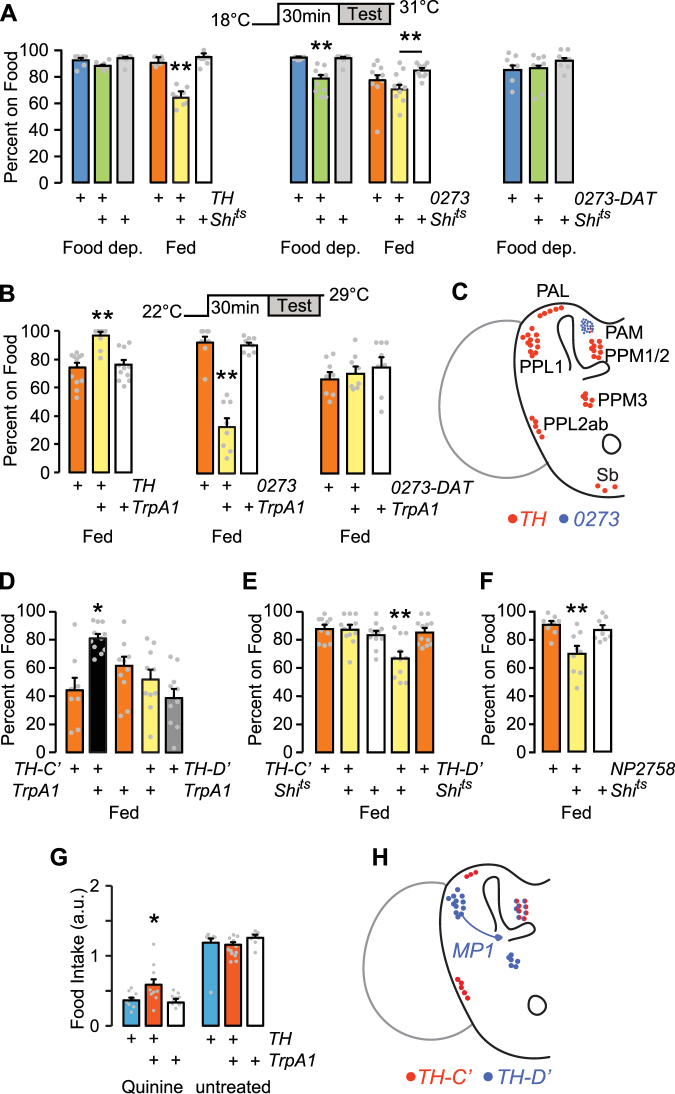
Dopaminergic neural circuits are critical for motivation, reward, and foraging in mammals, and for many similar functions in flies27. To test the role of dopamine in foraging in flies, we acutely inactivated and activated subsets of dopamine neurons in fed and food-deprived flies and assessed occupancy of sucrose. Dopamine neurons group into several discrete anatomical and functional clusters in the adult fly brain (Fig. 3C). TH-Gal4 labels most dopamine neuron clusters, but is largely absent from the PAM (protocerebral anterior medial) cluster of approximately 130 dopamine neurons28. 0273-Gal4 labels most dopamine neurons in the PAM cluster but not other dopamine neurons29. Acutely blocking transmitter release in TH-Gal4 neurons with the temperature-sensitive dynamin Shibire (Shits) had no effect on food occupancy in food deprived animals (Fig. 3A). Food occupancy was decreased when TH-Gal4 neurons were transiently inactivated in fed animals. There was no effect of inactivation on locomotor activity (Supplementary Fig. S2). Conversely, inactivation of 0273-Gal4 neurons decreased food occupancy in food deprived but not fed animals. DAT-Gal80 (R58E02-Gal80) expresses the GAL4 inhibitor GAL80 exclusively in PAM neurons: DAT-Gal80 blocked the 0273 > Shits food occupancy phenotype (Fig. 3A)17. Finally, chemical depletion of dopamine with 3-iodotyrosine also decreased food occupancy, indicating that dopamine is a neurotransmitter for foraging (Supplementary Fig. S2). Thus, dopamine neurons in the TH-Gal4 pattern promote food occupancy in fed animals, and PAM dopamine neurons in the 0273-Gal4 pattern promote food occupancy in food deprived animals.
Figure 3.
Satiation state-dependent effects of dopamine neuron activity on foraging. (A) Acute inactivation of dopamine neurons with Shibirets (Shits), food occupancy in fed and 16–20 hr food-deprived flies. P = 0.0012 ANOVA/Tukey’s, n = 8–11 groups with TH-Gal4. P = 0.0001 Kruskal-Wallis/Dunn’s, n = 8–10 groups food deprived; P = 0.0139 ANOVA/Tukey’s, n = 8–9 groups fed, with 0273-Gal4. 0273-DAT: 0273-Gal4 with R58E02-Gal80 to specifically block GAL4 activity in the PAM cluster dopamine neurons. n = 6 groups. (B) Acute activation of dopamine neurons in fed flies, food occupancy. P = 0.0002, ANOVA/Tukey’s, n = 8–11 groups with TH-Gal4. P = 0.0002, Kruskal-Wallis/Dunn’s, n = 8 groups with 0273-Gal4. 0273-DAT: n = 8 groups. (C) Dopamine neuron clusters in the adult brain that express TH-Gal4 and 0273-Gal4. (D) Acute activation of subsets of TH-Gal4 neurons, food occupancy in fed flies. P = 0.0002, ANOVA/Tukey’s, n = 8–11 groups. (E) Acute inactivation of subsets of TH-Gal4 neurons, food occupancy in fed flies. P < 0.0001, ANOVA/Tukey’s, n = 11–12 groups. (F) Acute inactivation of neurons with NP2758-Gal4. P = 0.001, ANOVA/Tukey’s, n = 8–9 groups. (G) Food intake in 4–6 hr food-deprived flies. P = 0.0053, ANOVA/Tukey’s, n = 15–19 groups. (H) Dopamine neurons that express TH-C’-Gal4 and TH-D’-Gal4. MP1: PPL1-γ1pedc neuron labeled by TH-D’-Gal4 and NP2758-Gal4. *P < 0.05, **P < 0.01. See also Figure S2.
To test if dopamine neurons are permissive or instructive, we acutely activated them using the temperature-sensitive cation channel TrpA1. Consistent with an instructive role, activating TH-Gal4 neurons in fed flies increased food occupancy (Fig. 3B). Fed 0273 > TrpA1 flies showed a marked decrease in food occupancy, and this was due to PAM dopaminergic activation in the 0273-Gal4 pattern.
To identify the relevant neurons in the TH-Gal4 pattern, we used transgenes that differentially label specific clusters of dopamine neurons (Fig. 3H)17. Activation of TH-C’ that included the PPL2ab, PPM2, and PAL, but not the PPL1, PPM1, or PPM3 dopamine neuron clusters increased food occupancy in fed flies (Fig. 3D). Conversely, inactivation of TH-D’ that includes PPL1, PPM2, and PPM3 neurons decreased food seeking in fed flies (Fig. 3E). The PPL1 neurons are particularly well-characterized for their roles in both appetitive and aversive learning and memory. Inactivation of neurons in the NP2758 pattern that includes PPL1-γ1pedc (MB-MP1) PPL1 and no other dopamine neurons decreased food occupancy in the fed state (Fig. 3F). To test if the identified dopaminergic neurons may regulate feeding motivation, we activated TH-Gal4 neurons in mildly (4 hr) food-deprived flies. Under these conditions, activation of TH-Gal4 neurons specifically increased consumption of quinine adulterated food (Fig. 3E).
Taken together, these experiments are consistent with dual roles for dopamine in foraging behavior: a PAM dopamine neuron-mediated promotion in the food-deprived state, and a TH-Gal4 dopamine neuron-mediated promotion in the fed state. PPL1-γ1pedc neurons in the TH-D’ pattern are necessary, and distinct neurons in the TH-C’ pattern are sufficient for promoting food occupancy in the fed state. PAM dopamine neurons can block foraging in the fed state.
Dopamine Receptor Regulation of Food Seeking
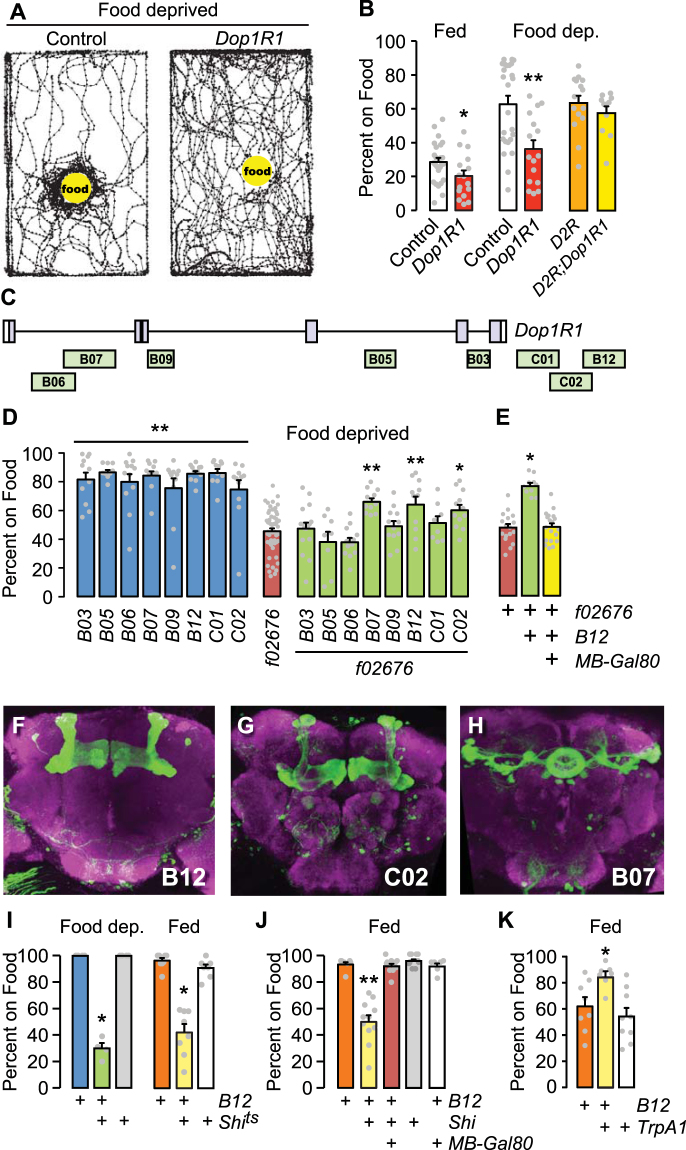
Dop1R1 encodes a D1-like dopamine receptor that functions in motivation-related behaviors, including arousal state, drug reward, and learning and memory30–32. We tested flies with strongly reduced expression of Dop1R1 for foraging behaviors. Food-deprived Dop1R1 mutant flies were hyperactive and appeared to ignore food (Fig. 4A). Moreover, Dop1R1 mutant food occupancy was reduced when fed or food deprived (Fig. 4B). Loss of the dopamine D2-like receptor D2R did not affect food occupancy, but did restore normal food occupancy to Dop1R1 mutants. The simplest explanation is that Dop1R1 promotes foraging, and that an opposite role for D2R is uncovered in the absence of Dop1R1. Food intake was unaffected in food-deprived flies of these genotypes (Supplementary Fig. S3).
Figure 4.
Dopamine receptor-expressing neurons in the mushroom body control foraging. (A) Locomotor traces of food-deprived flies 5 min after addition of food. Dop1R1 mutant f02676 vs. the Berlin genetic background control strain. (B) Food occupancy for the indicated genotypes that were fed or food deprived. t-test P = 0.0492 fed (n = 16–20 groups), P = 0.001 food deprived (n = 16–20 groups). D2R: the loss-of-function mutation f06521. (C) Location of Dop1R1 enhancer fragments. (D) Genetic rescue of Dop1R1 mutant food occupancy in 16–20 hr food deprived animals. Dop1R1-Gal4 strains (blue) were made heterozygous in f02676 homozygotes (rescuing configuration, green). P < 0.0001 ANOVA/Bonferroni’s comparison to f02676, n = 8–16 groups. (E) Inclusion of MB-Gal80, preventing GAL4 activity in the mushroom bodies blocks B12 rescue. P < 0.0001 ANOVA/Tukey’s, n = 10–19 groups. (F–H) Expression pattern of Dop1R1-Gal4 strains (CD8-GFP, green), and bruchpilot (magenta) to show the synaptic neuropil. (I) Acute silencing of B12 Dop1R1-Gal4 neurons with Shits, food occupancy, food deprived and fed. Food deprived: P < 0.0001 Kruskal-Wallis/Dunn’s, n = 4 groups. Fed: P = 0.0002 Kruskal-Wallis/Dunn’s, n = 7–8 groups. (J) Addition of MB-Gal80 in B12 Dop1R1-Gal4 > Shits fed flies, food occupancy. P < 0.0001 Kruskal-Wallis/Dunn’s, n = 6–10 groups. (K) Activation of B12 Dop1R1-Gal4 neurons in fed flies increased food occupancy. P = 0.0054, ANOVA/Tukey’s, n = 7–9 groups. *P < 0.05, **P < 0.01. See also Figure S3.
The Mushroom Bodies Promote Food Seeking Independent of Satiation State
We performed genetic rescue experiments to ask where Dop1R1 functions for foraging in food deprived flies. To bias the rescue towards functionally relevant brain regions, we utilized Dop1R1-Gal4 strains that expressed GAL4 under the control of short non-coding genomic DNA fragments cloned from the Dop1R1 locus (Fig. 4C)33. Food occupancy was partially rescued when Dop1R1 was expressed with three different Dop1R1-Gal4 strains in food-deprived Dop1R1 mutants: B07, B12, and C02 (Fig. 4D). Anatomical analysis of the expression patterns for the rescuing Dop1R1-Gal4 drivers revealed expression overlap. In the B12 and C02 strains, the mushroom bodies were prominently labeled, as were regions of the central complex, including the fan-shaped body and protocerebral bridge (Fig. 4F,G). The B07 strain prominently labeled the ellipsoid body of the central complex (Fig. 4H). We failed to rescue Dop1R1 mutant food occupancy using GAL4 drivers that label the ellipsoid body, fan-shaped body, or the protocerebral bridge (not shown). By contrast, decreasing GAL4 activity with mushroom body-specific expression of GAL80 (MB247-Gal80) eliminated B12 rescue of the Dop1R1 mutant food occupancy phenotypes (Fig. 4E)34. Moreover, restoring Dop1R1 with the mushroom body-specific driver MB247-Gal4 rescued Dop1R1 food occupancy (Supplementary Fig. S3). Thus, Dop1R1 expression in the mushroom bodies is sufficient to promote foraging in food deprived animals.
We next tested the role of neurotransmission in Dop1R1-expressing mushroom body neurons. Similar to loss of Dop1R1, acute blockade of synaptic output in B12 neurons with Shits decreased food occupancy in both fed and food-deprived flies (Fig. 4I). Importantly, this effect also localized to the mushroom bodies (Fig. 4J). B12 > Shits flies also showed reduced locomotion, however this phenotype persisted when the mushroom body neurons were subtracted from B12 (Supplementary Fig. S3), suggesting that distinct Dop1R1 neurons control food occupancy and locomotion. Finally, acute activation of B12 neurons in fed flies increased food occupancy (Fig. 4K). Taken together, these results indicate that the activity of Dop1R1-expressing mushroom body neurons promote foraging in both the fed and food-deprived state.
Discussion
Distinct dopaminergic circuitry promotes foraging under well fed and food deprived conditions. Dopamine neurons in the TH-C′ pattern promote foraging in well fed flies, and dopamine neurons in the PAM cluster promote foraging in food deprived flies. The PAM neurons likely function in a direct circuit with Dop1R1-expressing Kenyon cell neurons of the mushroom body that promote foraging in both the fed and food-deprived states. These circuits function under conditions where flies can freely perform many steps of foraging behavior. Understanding how these dopaminergic circuits contribute to discrete steps of feeding behavior, from local search through to repletion and disengagement from a food source, will help define how motivational states transition from task to task.
Roles of Dopamine in Appetitive Behaviors
Dopaminergic neurons are critical for many appetitive and aversive behavioral responses across animal species. Dopamine may act as a salience, arousal, or attention signal that gives importance to specific valence information arriving from other circuit elements27,35,36. In rodents, genetic, pharmacological, and lesioning studies indicate that striatal dopaminergic pathways can selectively function in the pre-ingestive phase to promote food seeking35,37,38. We found that acute activation of dopamine neurons in fed flies increased food occupancy, yet it did not cause increased food intake. Likewise, genetic elimination of the Dop1R1 receptor decreased food occupancy without affecting food intake. In contrast, inactivation of Dop1R1 receptor neurons decreased food intake in the food-deprived state, possibly reflecting their broader role in integrating sensory and internal state information (not shown). These findings suggest that dopaminergic pathways promote pre-ingestive food seeking. However, the role of dopamine is more complex. For example, the PAM dopamine neurons are activated by ingestion of sugar, and their activation is greater in food-deprived flies, indicating that dopaminergic neurons are engaged during the consummatory phase of feeding, and they may be sensitized to responding to input during the pre-ingestive phase17. Furthermore, specific dopamine neurons respond to other food-relevant environmental cues such as protein and water14,39,40.
Prior studies assigned dopamine to particular aspects of feeding behavior and also to motor functions that are critical to foraging14,23. In particular, dopamine neurons in the TH-Gal4 pattern are implicated in controlling motor output: TH-Gal4 neuron hyperpolarization, blocking synaptic input, interferes with motor performance and aspects of foraging behavior in food deprived flies23,28. We did not detect differences in unstimulated motor activity or in the magnitude of an olfactory-stimulated startle response when we blocked synaptic output from TH-Gal4 neurons, indicating that flies exhibited grossly normal motor behavior in our assay41. The differences in observed phenotypes may reflect the multifunctional roles of TH-Gal4 dopamine neurons that are revealed by specific types of manipulation.
Which dopamine neurons are responsible for foraging? In well-fed flies, neurons in the TH-Gal4 pattern are both necessary and sufficient to promote foraging. TH driver transgenes that express in a more restricted pattern allowed us to separate these roles. TH-C’ neurons are sufficient, but not necessary, to promote foraging. This pattern includes dopamine neurons in the PAL, PPM2, and PPL2 clusters. TH-C’ neurons were previously shown to promote protein consumption and, separately, egg-laying preference on sucrose17,42. Individual neurons in the PPM2 cluster, the DA-WED neurons, support protein consumption preference in protein deprived flies14. The DA-WED neurons synapse to Dop1R1 neurons in the B03 pattern, which did not support rescue of food seeking in our experiments. However, the B03 rescue was, by necessity, done in food deprived flies, when TH neurons were dispensable for foraging. Thus, it is possible that protein consumption preference and foraging are encoded by the same dopaminergic circuit that is used under different nutritional states and goals. Separately, dopamine neurons in the TH-D’ pattern are necessary, but not sufficient, to promote foraging. Inactivation of the PPL1-γ1pedc (MB-MP1) PPL1 neurons (using NP2758-Gal4), also decreased food occupancy, suggesting that these dopamine neurons are permissive for foraging in fed flies16. The PPL1-γ1pedc neurons are implicated in the formation of aversive memories in well-fed flies, and their activity is downregulated by food deprivation43–47. Our findings argue that there are distinct dopaminergic circuits in the TH-Gal4 pattern that control different aspects of food seeking in the well-fed state. The PAM neurons are also heterogeneous, sending projections that tile to well-defined regions of the mushroom body and to regions of the protocerebrum. Specific subsets of PAM neurons that are included in the 0273-Gal4 pattern have been implicated in various forms of appetitive learning and memory, however inactivation of these more specific PAM neuron subsets did not impact food seeking in food deprived flies (not shown)15,17,48–50. This suggests that there may be further segregation of PAM dopamine neuron function, possibly according to innate and learned appetitive responses.
Sensory Tuning of Food Seeking Motivation
Appetitive olfactory cues such as those emitted from palatable food elicit approach and can activate neurons important for feeding51,52. Olfactory receptor neurons that respond to appetitive odors increase sensitivity through the actions of the neuropeptides sNPF and SIFamide21,53. Further, neurons that release the neuropeptide NPF are activated to a greater extent in response to food odors in food-deprived flies; their activation promotes and inactivation inhibits odor attraction51. In well-fed larvae, the attractive odor pentyl acetate increases food intake through the actions of NPF and dopamine11. Therefore, food-related odors not only elicit approach behavior in a satiation state dependent manner, but also increase the activity of neurons expressing neuropeptides that regulate feeding behavior. Our results indicate that olfaction is important but apparently not crucial for food seeking in food-deprived flies: neither surgical nor genetic ablation of olfaction decreased food occupancy, and its role was only revealed by simultaneous partial ablation of taste responses. Further, flies were efficient in seeking odorless sucrose. Taken together, olfaction, hygrosensation, visual cues, and taste responses likely act in concert with internal cues to set the intensity of foraging when freely behaving flies are in close proximity to a food source.
Methods
Strains and Culturing
All strains were outcrossed for five generations to the Berlin genetic background prior to behavioral testing. Flies were raised on standard food containing agar (1.2% w/v), cornmeal (6.75% w/v), molasses (9% v/v), and yeast (1.7% w/v) at 25 °C and 70% humidity in a 16:8 light:dark cycle. For experiments with UAS-Shibire and UAS-TrpA1, flies were reared and held at 18 °C prior to testing. Dop1R1-Gal4 (R72B03, R72B05, R72B06, R72B07, R72B09, R72B12, R72C01, R72C02) strains were generated by the FlyLight project (Janelia Research Campus) and are available from the Bloomington Drosophila Stock Center (BDSC)33. Other BDSC stocks: UAS-TrpA1 (26264), UAS-CD8-GFP (32186), MB247-Gal4 (50742), UAS-Shits (66600). Harvard Medical School: Dop1R1f02676 and D2Rf06521. TH-Gal4 was from Jay Hirsh, TH-C’-Gal4 and TH-D’-Gal4 were from Mark Wu, Gr5aEP-5 and Gr64a1 were from Anupama Dahanukar, 0273-Gal4 was from Daryl Gohl and Thomas Clandinin, MB-Gal80 was from Scott Waddell, R58E02-Gal80 was from Hiromu Tanimoto, and Orco1 was from Leslie Vosshall.
Behavioral Measurements
Groups of 21 males were collected 1–2 days prior to the experiment. A group is an n = 1. For food deprivation, flies were placed into empty culture vials containing water saturated Whatman filter paper. For 3-iodotyrosine treatment, flies were cultured for 30 hr with 5% sucrose/2% yeast/10 mg/mL 3-iodotyrosine (3IY), and treated an additional 16 hr with 3IY in water for food deprivation. Standard fly food was used as the food source in the arenas for all experiments except where indicated. Approximately 100 μL of food or 1.25% agarose with additives was pipetted onto a small square of Parafilm and kept humidified. Thin-walled Plexiglas behavioral chambers were designed with two side-by-side arenas, each arena measuring 45 × 75 × 10 mm, or 85 × 135 × 10 mm for experiments with Shibirets. Chambers were designed and built by IO Rodeo; design files are available (Pasadena, CA). Chambers, food sources, and flies were acclimated to the testing temperature prior to introducing them into the behavioral arena. A Pelltier incubator was used for experiments performed at lowered and elevated temperatures (IN45, Torrey Pines Scientific). Flies were filmed from above at 10 fps with the arena placed on white light LED panel (Edmund Optics). Filmed flies were tracked with customized DIAS software as previously described54. For food occupancy, the number of flies off food was subtracted from the total number of flies and divided by total number of flies. In binary choice experiments, the food sources were deposited in direct apposition and placed at the center of the arena, and the number of flies on each source was manually counted. Percent on food was calculated as the average of the last two measured time points (20–30 min). Locomotor activity was the average speed of all flies in 20 sec bins measured for a 1 min interval at 20 and 30 min.
To measure food intake, 5 ml standard fly food with 2% erioglaucine (Sigma) with or without 3 mM quinine was striped onto 1/4 of the inner surface of a wide fly vial, and condensation removed. 30–50 flies were introduced and the vial laid on its side so that the food edge was at the apex. After 30 min, the flies were homogenized in a volume adjusted to the number of flies and consumption was determined spectrophotometrically.
Statistical measurements were made with Prism 6.0 (GraphPad). One-way ANOVA followed by Tukey’s post-hoc comparisons (or Bonferroni post-hoc planned comparison) were used when data did not show unequal variance by the Brown-Forsythe test, otherwise the Kruskal-Wallis test followed with Dunn’s post-hoc was used. t-tests were two-tailed. Error bars are the SEM. Data is available upon request.
Immunohistochemistry
Adult fly brains were fixed and immunostained as described previously41. Antibodies were rabbit anti-GFP (1:1000, Life Technologies), rabbit anti-Dop1R1 1:125041, and nc82 (1:25, Developmental Studies Hybridoma Bank, Iowa).
Electronic supplementary material
Acknowledgements
We thank the members of the laboratories of Fred Wolf and Michael Cleary for advice, and Daryl Gohl and Thomas Clandinin for unpublished strains. This work was supported by grants from the NIH (AA018799), The Hellman Fellowship Fund, and the University of California, Merced. The authors declare no competing interests. Data is made available upon request.
Author Contributions
D.L., D.S.F. and F.W.W. conceived of and carried out the experiments, and analyzed the results. F.W.W. wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Dan Landayan and David S. Feldman contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-24217-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benoit SC, Tracy AL. Behavioral controls of food intake. Peptides. 2008;29:139–47. doi: 10.1016/j.peptides.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig W. Appetites and Aversions as Constituents of Instincts. Proc Natl Acad Sci. 1917;3:685–8. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15:1330–5. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12:638–51. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 5.Jeong YT, et al. An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron. 2013;79:725–37. doi: 10.1016/j.neuron.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stafford JW, Lynd KM, Jung AY, Gordon MD. Integration of taste and calorie sensing in Drosophila. J Neurosci. 2012;32:14767–74. doi: 10.1523/JNEUROSCI.1887-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang YV, Raghuwanshi RP, Shen WL, Montell C. Food experience-induced taste desensitization modulated by the Drosophila TRPL channel. Nat Neurosci. 2013;16:1468–76. doi: 10.1038/nn.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flood TF, et al. A single pair of interneurons commands the Drosophila feeding motor program. Nature. 2013;499:83–7. doi: 10.1038/nature12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inagaki HK, et al. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–95. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mann K, Gordon MD, Scott K. A pair of interneurons influences the choice between feeding and locomotion in Drosophila. Neuron. 2013;79:754–65. doi: 10.1016/j.neuron.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Pu Y, Shen P. Neuropeptide-gated perception of appetitive olfactory inputs in Drosophila larvae. Cell Rep. 2013;3:820–30. doi: 10.1016/j.celrep.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Wu Q, et al. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–61. doi: 10.1016/S0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- 13.Wu Q, Zhao Z, Shen P. Regulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systems. Nat Neurosci. 2005;8:1350–5. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, et al. Branch-specific plasticity of a bifunctional dopamine circuit encodes protein hunger. Science. 2017;356:534–539. doi: 10.1126/science.aal3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke CJ, et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–7. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krashes MJ, et al. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–27. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, et al. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–6. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 18.Placais PY, Trannoy S, Friedrich AB, Tanimoto H, Preat T. Two pairs of mushroom body efferent neurons are required for appetitive long-term memory retrieval in Drosophila. Cell Rep. 2013;5:769–80. doi: 10.1016/j.celrep.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Duistermars BJ, Frye MA. Crossmodal visual input for odor tracking during fly flight. Curr Biol. 2008;18:270–5. doi: 10.1016/j.cub.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Frye MA, Tarsitano M, Dickinson MH. Odor localization requires visual feedback during free flight in Drosophila melanogaster. J Exp Biol. 2003;206:843–55. doi: 10.1242/jeb.00175. [DOI] [PubMed] [Google Scholar]
- 21.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–44. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim IS, Dickinson MH. Idiothetic Path Integration in the Fruit Fly Drosophila melanogaster. Curr. Biol. CB. 2017;27:2227–2238.e3. doi: 10.1016/j.cub.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson A, et al. Neuromodulatory circuit effects on Drosophila feeding behaviour and metabolism. Sci. Rep. 2017;7:8839. doi: 10.1038/s41598-017-08466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosjean Y, et al. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–40. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- 25.Steck K, et al. A high-throughput behavioral paradigm for Drosophila olfaction - The Flywalk. Sci Rep. 2012;2:361. doi: 10.1038/srep00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inagaki HK, Panse KM, Anderson DJ. Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila. Neuron. 2014;84:806–820. doi: 10.1016/j.neuron.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friggi-Grelin F, et al. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–27. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 29.Gohl DM, et al. A versatile in vivo system for directed dissection of gene expression patterns. Nat Methods. 2011;8:231–7. doi: 10.1038/nmeth.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebestky T, et al. Two different forms of arousal in drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–36. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A Drosophila model for alcohol reward. Nat Neurosci. 2011;14:612–9. doi: 10.1038/nn.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenett A, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–15. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–85. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaun KR, Rothenfluh A. Dopaminergic rules of engagement for memory in Drosophila. Curr. Opin. Neurobiol. 2017;43:56–62. doi: 10.1016/j.conb.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ilango A, et al. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J Neurosci. 2014;34:817–22. doi: 10.1523/JNEUROSCI.1703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin S, et al. Neural correlates of water reward in thirsty Drosophila. Nat. Neurosci. 2014;17:1536–1542. doi: 10.1038/nn.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjordal M, Arquier N, Kniazeff J, Pin JP, Léopold P. Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell. 2014;156:510–521. doi: 10.1016/j.cell.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 41.Kong EC, et al. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS One. 2010;5:e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang C-H, He R, Stern U. Behavioral and circuit basis of sucrose rejection by Drosophila females in a simple decision-making task. J. Neurosci. 2015;35:1396–1410. doi: 10.1523/JNEUROSCI.0992-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plaçais P-Y, et al. Upregulated energy metabolism in the Drosophila mushroom body is the trigger for long-term memory. Nat. Commun. 2017;8:15510. doi: 10.1038/ncomms15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim Y-K, et al. Repetitive aggressive encounters generate a long-lasting internal state in Drosophila melanogaster males. Proc Natl Acad Sci. 2018;115:1099–1104. doi: 10.1073/pnas.1716612115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aso Y, et al. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–51. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Placais PY, et al. Slow oscillations in two pairs of dopaminergic neurons gate long-term memory formation in Drosophila. Nat Neurosci. 2012;15:592–9. doi: 10.1038/nn.3055. [DOI] [PubMed] [Google Scholar]
- 47.Kirkhart C, Scott K. Gustatory learning and processing in the Drosophila mushroom bodies. J. Neurosci. 2015;35:5950–5958. doi: 10.1523/JNEUROSCI.3930-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aso Y, et al. The neuronal architecture of the mushroom body provides a logic for associative learning. eLife. 2014;3:e04577. doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamagata N, et al. Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proc Natl Acad Sci. 2015;112:578–583. doi: 10.1073/pnas.1421930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beshel J, Zhong Y. Graded encoding of food odor value in the Drosophila brain. J Neurosci. 2013;33:15693–704. doi: 10.1523/JNEUROSCI.2605-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ko, K. I. et al. Starvation promotes concerted modulation of appetitive olfactory behavior via parallel neuromodulatory circuits. eLife4 (2015). [DOI] [PMC free article] [PubMed]
- 53.Martelli C, et al. SIFamide Translates Hunger Signals into Appetitive and Feeding Behavior in Drosophila. Cell Rep. 2017;20:464–478. doi: 10.1016/j.celrep.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 54.Wolf FW, Rodan AR, Tsai LT, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22:11035–44. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.