Abstract
The DREB (dehydration-responsive element binding)-type transcription factors are classified into six subgroups, named A-1 to A-6. The members of DREB A-1 and A-2 subgroups have been reported to be involved in response to various abiotic stresses. However, there were only a few genes belonging to A-3 to A-6 subgroups to be reported. In this study, we cloned a DREB A-4 subgroup gene from maize (Zea mays), ZmDREB4.1, and analyzed its characteristics and functions. ZmDREB4.1 was expressed in roots, stems, and leaves at very low levels. It was not induced by any biotic or abiotic treatment. ZmDREB4.1 was located in the nucleus, could directly bind to the DRE element and functioned as a transcriptional activator. The constitutive expression of ZmDREB4.1 in tobacco (Nicotiana tabacum L.) repressed leaf extension and hypocotyl, petiole and stem elongation. In maize, overexpression of ZmDREB4.1 repressed calli growth and regeneration. Further analysis showed that the smaller leaves of transgenic tobacco resulted from inhibition of cell division. The contents of cytokinin and auxin in transgenic leaves were severely decreased. The shorter hypocotyls, stems and petioles of transgenic tobacco were caused by inhibition of cell elongation. The transgenic hypocotyls, stems and petioles contained reduced gibberellin levels. Application of exogenous GA3 rescued the shorter hypocotyls, stems and petioles, but not the smaller leaves. These results demonstrated that ZmDREB4.1 plays an important role in the negative regulation of plant growth and development.
Keywords: DREB, ZmDREB4.1, transcriptional activator, dwarfism, maize, tobacco
Introduction
The APETALA2 (AP2)/ethylene-responsive factor (ERF) family is unique to plants. The transcription factors of AP2/ERF family possess one or two AP2 DNA-binding domains comprised of approximately 58 residues (Nakano et al., 2006; Velasco et al., 2010). The first cDNA encoding AP2/ERF protein, APETALA2, was identified from Arabidopsis (Jofuku et al., 1994). Since then, 147, 164 and 167 AP2/ERF genes have been identified from Arabidopsis, rice and maize, respectively. These genes are classified into four subfamilies, DREB, ERF, AP2, and RAV, according to the number of copies of the AP2 domain and their sequence similarity (Zhuang et al., 2010). Extensive studies have shown that AP2/ERF genes play important roles in plant flower (Elliott et al., 1996), seed (Ohto et al., 2009), and root (Kitomi et al., 2011) development, in response to biotic (Zhang et al., 2008) and abiotic (Stockinger et al., 1997) stresses and in the regulation of cell division (Mehrnia et al., 2013).
The DRE element was first identified in the rd29A promoter, which contains a DRE core sequence (ACCGAC) (Yamaguchi-Shinozaki and Shinozaki, 1994). The DREB-type transcription factors DREB1A and DREB2A were first identified using yeast one-hybrid screening by the DRE element (Liu et al., 1998). The DREB transcription factors were classified into six subgroups, named A-1 to A-6, based on sequence similarities of their AP2 domains (Sakuma et al., 2002). Previous studies have shown that most DREB1-type and DREB2-type genes are involved in abiotic stress responses (reviewed by Mizoi et al., 2012). Most DREB1/CBF genes are involved in response to low temperatures in plants (Akhtar et al., 2012). Overexpression of DREB1B/CBF1 or DREB1A/CBF3 enhanced cold and dehydration tolerance in transgenic Arabidopsis (Stockinger et al., 1997; Gilmour et al., 2000). DREB2-type genes were shown to be induced by osmotic stresses, such as drought and high salinity. Overexpression of an active form of DREB2A (DREB2A-CA), whose negative regulatory region was deleted, improved transgenic Arabidopsis tolerance to drought (Sakuma et al., 2006). In addition, several DREB A-1 or A-2 subgroup genes, SiARDP from foxtail millet (Li et al., 2014), ZmDREB2A from maize (Qin et al., 2007), OsDREB1A and OsDREB2B from rice (Dubouzet et al., 2003), GmDREB2 from soybean (Chen et al., 2007) and PeDREB2 from populous (Chen et al., 2009) were also reported.
Several genes belonging to A-3 to A-6 subgroups were also reported to function in response to abiotic stress or plant growth and development. Overexpression of GhDBP3 (Huang and Liu, 2006), MnDREB4 (Liu et al., 2015) and StDREB1 (Bouaziz et al., 2013) of DREB A-4 subgroup; PeDREB2a (Zhou et al., 2012), StDREB2 (Bouaziz et al., 2012) and ScDREB8 (Liang et al., 2017) of DREB A-5 subgroup; and GhDBP2 (Huang et al., 2008) and SsDREB (Zhang et al., 2015) of DREB A-6 subgroup can improve plant tolerance to abiotic stresses. ABI4, a member of DREB A-3 subgroup, was identified from Arabidopsis. It is involved in abscisic acid (ABA) signal transduction and seed development (Finkelstein et al., 1998; Söderman et al., 2000). The ABI4 homologous gene from maize was also identified and analyzed (Niu et al., 2002). TINY identified from Arabidopsis was classified into the DREB A-4 subgroup. Its expression was induced by both abiotic stresses (drought and cold) and biotic stresses (ethylene and methyl jasmonate). TINY-overexpressing Arabidopsis displayed a partial constitutive ethylene triple response and dwarf phenotype (Wilson et al., 1996).
Plant morphology is affected by internal signals and external environmental stimuli. Plant hormones are a large class of small signal molecules and play an important role in the regulation of multiple aspects of plant growth and development (Seif El-Yazal et al., 2015). Gibberellic acids (GAs) have been reported to be involved in the regulation of cell elongation and division, stem elongation and seed germination (Richards et al., 2001). GA20ox, GA3ox and GA2ox are three major oxidase gene families that participate in GA synthesis and degradation (Hedden and Phillips, 2000). The levels of endogenous GAs are homeostatically modulated by negative feedback regulation of the GA20ox and GA3ox genes and positive feedforward regulation of GA2ox genes (Chiang et al., 1995; Xu et al., 1995; Cowling et al., 1998; Thomas et al., 1999; Hedden and Phillips, 2000). Many mutants that are deficient in GA biosynthesis or GA response exhibit a dwarf phenotype, such as ga4, ga5 (Talon et al., 1999) and gid1a gid1b gid1c mutants (Griffiths et al., 2006). Cytokinins (CKs) were first identified for their ability to promote cell division in cultured plant cells (Miller et al., 1955). They are involved in regulation of the apical meristem maintenance, seed germination, flower and fruit development and leaf senescence (Mok and Mok, 2001). Many genetic and molecular biological studies have revealed the two-component regulatory system of CK perception and signal transduction (Hwang et al., 2002). In addition, it is reported that some components of CK signaling are involved in abiotic stress response (Tran et al., 2007).
In the present study, ZmDREB4.1 belonging to the DREB A-4 subgroup was cloned from maize. ZmDREB4.1 is a constitutively expressed gene, even though its expression level is very low. It is not induced either by abiotic (drought, salt, or cold) or by biotic (wound, NAA, 6-BA, GA, or ACC) stresses. ZmDREB4.1 has the characteristics of a typical transcription factor. It is located in the nucleus of maize protoplasts and young stem tissue, specifically bound to the DRE element, and activates the expression of downstream genes. We analyzed its function by overexpressing ZmDREB4.1 in both tobacco and maize. ZmDREB4.1-overexpressing tobacco exhibited smaller leaves and shorter hypocotyls, petioles and stems. ZmDREB4.1-overexpressing maize calli grew slowly and failed to regenerate seedlings. Our work provides evidence that ZmDREB4.1 plays a negative role in regulation of plant growth and development. In addition, its mechanisms were analyzed and discussed.
Materials and Methods
Plant Materials and Stress Treatments
Maize B73 inbred seeds were germinated on moist gauze overnight and the germinated seeds were then grown in mixtures of half vermiculite and half soil at 28°C under a long-day photoperiod (16 h light, 8 h dark) in an artificial illumination incubator with 70% relative humidity. The three-leaf seedlings were selected and cleaned. The seedlings were then fastened to plastic foam, transferred to 1/4 Hoagland solution and grown hydroponically with oxygen for 3 days. The 1/4 Hoagland solution was replaced every day during culture. The seedlings were then exposed to various treatments. For salt, drought, ABA, auxin, CK, GA and ethylene treatments, the seedlings were immersed in 1/4 Hoagland solution containing 100 mM NaCl, 20% PEG-6000, 10 μM ABA, 1 μM IAA, 1 μM 6-BA, 10 μM GA3, and 10 μM ACC, respectively. For cold treatment, seedlings in the soil were placed at 4°C. For wound treatment, the leaves were cut with a blade. The seedlings were collected after treatment and frozen in liquid nitrogen for RNA extraction. Meanwhile, seedlings without any treatment were used as controls.
For protoplast isolation, the germinated maize seeds were grown in vermiculite at 28°C under a long-day photoperiod (16 h light, 8 h dark) in an artificial illumination incubator with 70% relative humidity for 3 days and were then shifted to a completely dark artificial illumination incubator for 7–10 days until the second leaf was approximately 10–15 cm. The middle section (6–8 cm) of the second leaf was used for protoplast isolation.
The wild-type and ZmDREB4.1-overexpression tobacco seeds were sterilized by chlorine for 2 h and then grown on MS medium at 28°C under a long-day photoperiod (16 h light, 8 h dark) in an artificial illumination incubator with 70% relative humidity. The seedlings were used for RNA extraction and phenotype observation. For drought treatment, seeds were grown in mixtures of half vermiculite and half soil at 28°C under a long-day photoperiod (16 h light, 8 h dark) in a greenhouse and the germinated seedlings were not watered for 2 weeks, then rewatered and grew for 1 week.
RNA Extraction and Quantitative PCR Analysis
Total RNA was extracted using TRIzol reagent (Ambion, United States), and first-strand cDNA synthesis was performed using 5 μg of total RNA and 20-bp poly (dT) oligonucleotide according to the protocol of the Reverse Transcription System (Promega, United States). Quantitative RT-PCR analysis was performed using the SYBR green method with the CWBIO Fast SYBR Mixture real-time PCR system (CWBIO, China). The primers used in this study were showed in Table 1. The expression levels of relevant genes were determined by the ΔΔCT method.
Table 1.
Primer sequences used in this study.
| Primer name | Sequence (5′–3′) | Restriction sites |
|---|---|---|
| ZmDREB4.1-F | ATGGAGCACGAGCAGGTGTCG | |
| ZmDREB4.1-R | TCAATGTTCCCAAAGCAGCAAGG | |
| ZmDREB4.1-ORF-F | TCTAGAATGGAGCACGAGCAGGTGTCG | Xba I |
| ZmDREB4.1-ORF-R | GAGCTCTCAATGTTCCCAAAGCAGCAAGG | Sac I |
| ZmDREB4.1-qRT-F | CATTGGAGTTGGACTGGGCTATACG | |
| ZmDREB4.1-qRT-R | ATGGTCAATGTTCCCAAAGCAGCAA | |
| ZmDREB4.1-SL-F | TCTAGAATGGAGCACGAGCAGGTGTCG | Xba I |
| ZmDREB4.1-SL-R | CCCGGGATGTTCCCAAAGCAGCAAGGGCT | Sma I |
| ZmDREB4.1-TA-F | GAATTCATGGAGCACGAGCAGGTGTCG | EcoR I |
| ZmDREB4.1-TA-R | GTCGACTCAATGTTCCCAAAGCAGCAAGGG | Sal I |
| ZmDREB4.1-YH-F | GAATTCATGGAGCACGAGCAGGTGTCG | EcoR I |
| ZmDREB4.1-YH-R | GGATCCTCAATGTTCCCAAAGCAGCAAGG | BamH I |
| ZmDREB4.1-PE-F | GAATTCCTCAACGGCTCGTCGTCGCC | EcoR I |
| ZmDREB4.1-PE-R | GTCGACTCAAGCCGGCACAGTGGCCGC | Sal I |
| mini35S-F | AAGCTTATCTCCACTGACGTAAGGGATG | Hind III |
| mini35S-R | CCATGGCGTGTTCTCTCCAAATGAAATG | Nco I |
| DRE element | GATATACTACCGACATGAGTTC | |
| mDRE element | GATATACTAAAAAAATGAGTTC | |
| IPT3-qRT-F | CCAAGAACTTCTGTGACATGGCTTCA | |
| IPT3-qRT-R | ACCTCGTTGACAAGTCCTTTCTCGA | |
| IPT5-qRT-F | AAGCAGAGGTTGTGAACAGTGACAA | |
| IPT5-qRT-R | TCGACCCTCCAGCAATTATCGGTAG | |
| YUCCA9-qRT-F | TTATTGTAGGTGCAGGTCCATCGGG | |
| YUCCA9-qRT-R | TGTCTCTTTGTTGGGTACTCAGGGT | |
| YUCCA10-qRT-F | TTCGATGCCCAAAACATAGCCACAG | |
| YUCCA10-qRT-R | AATCACATCCGATCGACCCCTTCAT | |
| YUCCA11-qRT-F | AGGATTAGATGGCTTTGGTGGGACA | |
| YUCCA11-qRT-R | ACAGGGCTACGAACAATAATGGAGGT | |
| GA2ox2-qRT-F | AGCAGATTGGATGTAATGGCGATTCC | |
| GA2ox2-qRT-R | TCTCACACGCCATTTTCTTCACTGC | |
| GA2ox3-qRT-F | TCAGCAATGAAGAAAATGGCATGTGA | |
| GA2ox3-qRT-R | GCACCAAATCCAATCAAATTTCTGCCA | |
| GA2ox4-qRT-F | AAAGTCGTCAACCATAGCGTCCCTA | |
| GA2ox4-qRT-R | TTCGACCCAACCCAAATCACCATTG | |
| GA3ox2-qRT-F | GATGGTATTGCTGGTTATGGCGTGG | |
| GA3ox2-qRT-R | CCACATCAGTCTTCCGGCTAGTTTT | |
| GA20ox1-qRT-F | GCGTAGGGAGGGAACACTTCAAAGA | |
| GA20ox1-qRT-R | AAACTTGAAGCCCGCCAACACTATC | |
| GA20ox2-qRT-F | AGGCAATGAAGAGGGTAGGACTTGT | |
| GA20ox2-qRT-R | GTGCAGTTCCAAGGGCTAAATCAGG | |
| GA20ox3-qRT-F | CCCTTGAGGCTTGGACAAATGGAAG | |
| GA20ox3-qRT-R | AGATGCCTCAGTTCAGCCCATGTAA | |
| ZmActin-F | GTTGGGCGTCCTCGTCA | |
| ZmActin-R | TGGGTCATCTTCTCCCTGTT | |
| NtActin-F | GAGCGGGAAATTGTCAGGGA | |
| NtActin-R | GAAACGCTCAGCACCAATGG |
Gene Isolation and Phylogenetic Analysis
ZmDREB4.1 was isolated from maize roots with primer pair ZmDREB4.1-F/R (Table 1), and the PCR product was then cloned into the pMD19T vector. The amino acid sequences of ZmDREB4.1 and other DREB A-4 subgroup transcription factors in this study were obtained from Phytozome1 or NCBI2. Multiple sequence alignment was performed using ClustalX software, and the phylogenetic tree was generated using MEGA5.1 software.
Subcellular Localization
The CDS sequence of ZmDREB4.1 without termination codon was cloned into the 5′ terminus of the enhanced green fluorescent protein (eGFP) gene sequence in pUC-eGFP vector via XbaI/SmaI sites (primer pair: ZmDREB4.1-SL-F/R; Table 1). Protoplasts of etiolated maize mesophyll cells were isolated using macerozyme R-10 and cellulase R-10 (Yakult, Japan) and the fusion construct pUC-ZmDREB4.1-eGFP and the control pUC-eGFP were transformed into the protoplasts independently using PEG-Ca2+ method according to the protocol described by Sheen (Sheen, 2002). After culture in W1 solution (0.6 M mannitol, 4 mM MES (pH 5.7), 4 mM KCl) in the dark for 14 h, the green fluorescence signals were observed under a confocal microscope (Zeiss 510, Carl Zeiss, Germany). The young stems were cut into thin slices and then treated on NB medium containing 0.4 M mannitol for 4–6 h. The plasmids mentioned above were transformed into young stem tissue independently by particle bombardment with the PDS-1000 system (Bio-Rad, United States) according to the protocol reported by Wang et al. (2006). GFP fluorescence was observed after further culture overnight.
Yeast One-Hybrid Assay
The 3 × DRE sequence (DRE: GATATACTACCGACATGAGTTC, between positions -174 and -153 in the rd29A promoter; Table 1) and 3 × mDRE sequence (The DRE core sequence ACCGAC was substituted with AAAAAA; Table 1) were inserted into the pAbAi vector via the HindIII/XhoI sites to create the bait and mutant bait vectors. The vectors were transformed into yeast strain Y1HGold independently and they were then screened on SD/-Ura medium. The positive yeast was grown on SD/-Ura medium which contains Aureobasidin A (AbA) at different concentrations to examine activity of self activation according to the protocol of the MatchmakerTM gold Yeast One-Hybrid Library Screening System (PT4087-1). The CDS sequence of ZmDREB4.1 was constructed into the pGADT7-AD vector via EcoRI/BamHI sites as prey vector (Primer pair: ZmDREB4.1-YH-F/R; Table 1). It was transformed into bait and mutant bait strains, respectively, and then grown on SD/-Leu medium containing 800 ng mL-1 AbA for 3 days at 30°C.
Transcriptional Activation Assay in Yeast
The CDS of ZmDREB4.1 was cloned into the 3′ terminus of the GAL4 binding domain sequence in pBD-GAL4 vector via EcoRI/SalI sites (Primer pair: ZmDREB4.1-TA-F/R; Table 1). The plasmids pBD-ZmDREB4.1, pGAL4 (positive control) and pBD-GAL4 (negative control) were independently transformed into yeast strain YRG-2 which contains the HIS3 and lacZ reporter genes. These transformed yeast cells were grown on SD/-Trp or SD/-Trp/-His medium for 3 days at 30°C. The β-galactosidase activity of the transformed yeast cells was also analyzed according to the protocol described in the Yeast Protocols Handbook (PT3024-1).
Transcriptional Activation Assay in Tobacco
For the transcriptional activation assay of ZmDREB4.1 in tobacco, the full-length CDS of ZmDREB4.1 was inserted into the pCambia1300 vector to construct the effector plasmid (Primer pair: ZmDREB4.1-ORF-F/R; Table 1). The reporter plasmid was constructed by placing the cascade of 3 × DRE and mini 35S upstream of the GUS reporter gene in pCambia1381 (Primer pair: mini35S-F/R; Table 1). The effector plasmid (pSuper::ZmDREB4.1), reporter plasmid (p3 × DRE:mini35S::GUS), internal control plasmid (p35S::LUC), enhancer plasmid (p19) and control plasmid (pCambia1300) were transformed into Agrobacterium tumefaciens strain GV3101 independently. The tobacco seedlings were grown at 23°C under a long-day photoperiod (16 h light, 8 h dark) in a greenhouse for 4–5 weeks until the six-leaf stage. Three separate tobacco leaves were infected with the mixed Agrobacterium solution (pSuper::ZmDREB4.1: p3 × DRE:mini35S::GUS: p35S::LUC:p19 = 800:100:4:300) as three biological replicates. The mixed solution in which pSuper::ZmDREB4.1 was substituted with pCambia1300 served as the control. After inoculation for 3 days, total protein was extracted from these transformed leaves to measure GUS and LUC activities. The LUC activities were quantified using the Luciferase Assay System (Promega, United States) and the GUS activities were measured by spectrofluorometry (F-4500, HITACHI, Japan). The relative GUS/LUC activity was used to represent promoter activity. The column diagram was generated by GraphPad Prism software using the results from three biological replicates and the statistically significant differences were evaluated by Student’s t-test (∗P-value < 0.05, ∗∗P-value < 0.01, ∗∗∗P-value < 0.001).
Electrophoretic Mobility Shift Assay (EMSA)
The full-length ZmDREB4.1 protein fused with 6 × His tag, GST tag or MBP tag was not obtained in the prokaryotic expression system. Therefore, the truncated sequence containing the coding sequence of the conserved AP2 domain of ZmDREB4.1 (31-147 amino acids) was cloned into the pMAL-C2x vector via EcoRI/BamHI sites (Primer pair: ZmDREB4.1-PE-F/R; Table 1) and expressed in Escherichia coli strain TB1. The recombinant protein was purified using MBP Sepharose High Performance (GE Healthcare, United States) following the manufacturer’s protocol and then used for the EMSA. The probe (3 × DRE labeled with biotin at the 5′ terminus), the competitor1 (3 × DRE) and the competitor2 (3 × mDRE) were synthesized (Sangon Biotech, China). The double-stranded probes were obtained by incubating at 95°C for 5 min and cooling gradually overnight. The gel-shift assay was carried out using the LightShift® Chemiluminescent EMSA Kit (Thermo, United States) following the manufacturer’s instructions.
Generation of Transgenic Tobacco Plants
The full-length CDS of ZmDREB4.1 was amplified with primer pairs ZmDREB4.1-ORF-F/R (Table 1) and then constructed into the binary vector pBI121. The recombinant vector pBI121-ZmDREB4.1 was transformed into A. tumefaciens strain LBA4404. The positive clone was used for tobacco transformation with the leaf disk method. The transformed leaves were cultured on MS medium in the dark for 3 days and then transferred to differentiation medium (MS medium containing 0.2 mg L-1 NAA, 3 mg L-1 6-BA and 50 mg L-1 kanamycin) under the light. The mediums were replaced every 2 weeks. The regenerated buds rooted on MS medium to generate T0 plants. PCR amplification was used to confirm the positive lines with primer pair ZmDREB.41-F/R (Table 1). The expression levels of ZmDREB4.1 in transgenic lines were examined by qRT-PCR using primer pair ZmDREB4.1-qRT-F/R (Table 1).
Phenotype Observation and Measurement of Transgenic Tobacco
The sterile seeds of WT and ZmDREB4.1-overexpression lines were sown on MS medium. The WT and transgenic seedlings at the two-leaf stage were chosen for hypocotyl observation and length measurement. The length of the stems and second petioles and the area of the second leaves of seedlings at the four-leaf stage were observed and measured. The data were collected from 25 seedlings of each of three independent transgenic lines. A scanning electron microscope (SEM) was used for fine structure observation (Olympus 6000, Japan). The hypocotyls and petioles were consistently observed in the mid-region. For leaves, adaxial epidermis was observed. The numbers of leaf epidermal cells and hairs were counted from 10 leaves. For measurements of cell length of the hypocotyl and petiole, data was collected from at least 40 cells of two different hypocotyls and petioles. The column diagram was generated by GraphPad Prism software and the statistically significant differences were evaluated by Student’s t-test (∗P-value < 0.05, ∗∗P-value < 0.01, ∗∗∗P-value < 0.001) and corrected by Bonferroni correction.
Analysis of Callus Formation
The top leaves and second full-length petioles of seedlings at the four-leaf stage were obtained. The leaves were cut into small pieces of similar size. About 60 pieces from 30 separate plants were precultured on four callus-induced medium (CIM, MS medium containing 1 mg L-1 2,4-D, and 0.2 mg L-1 6-BA) for 4 days. Also, about 20 full-length petioles from 20 separate seedlings were precultured on two CIM for 4 days. They were then transferred to new CIM for further culture for 2 weeks. The calli induced from leaves and petioles were observed and the calli induced from leaves were measured. The column diagram was generated by GraphPad Prism software using the fresh weight of the 60 calli and the statistically significant differences were evaluated by Student’s t-test (∗P-value < 0.05, ∗∗P-value < 0.01, ∗∗∗P-value < 0.001). The protocol was reported by Mehrnia et al. (2013).
Measurement of Endogenous Phytohormone Levels
The sterile seeds of WT and ZmDREB4.1-overexpression tobacco were grown on MS medium at 28°C for 10–14 days until the four-leaf stage. The leaves and petioles of WT and OE9 were obtained. The leaves and petioles were divided into three parts, respectively, and the fresh weight of every part was about 0.2 g. Then they were frozen in liquid nitrogen and homogenized to a fine powder. The powder was immersed in 3 mL of 80% methanol for 3 h and then extracted with another 1 mL of 80% methanol for 1 h. The IAA, ZR, DHZR, IPA, GA3, and GA4 contents were measured with their monoclonal antibodies using the indirect competitive enzyme-linked immunosorbent assay (icELISA) as described by Deng et al. (2008). The column diagrams were generated by GraphPad Prism software using the results from three biological replicates and the statistically significant differences were evaluated by Student’s t-test (∗P-value < 0.05, ∗∗P-value < 0.01, ∗∗∗P-value < 0.001).
Hormonal Treatment
The WT and ZmDREB4.1-overexpressing tobacco seedlings were grown on MS medium for 5 days. The WT seedlings were transplanted into new MS medium. The transgenic seedlings were transplanted into new MS medium or medium containing GA3, NAA, or 6-BA at different concentrations. The hypocotyl, stem and petiole length and leaf size of WT and transgenic seedlings grown on different media was observed and measured after culture for 2 weeks. The data was collected from 15 separate seedlings of WT and OE9. The column diagrams were generated by GraphPad Prism software and multiple means were compared using Tukey’s multiple comparison test (α = 0.05).
Callus Transformation in Maize
The bar gene expression cassette was inserted into the pUC-ZmDREB4.1-eGFP and pUC-eGFP vector via HindIII site to construct the overexpression and control vectors. The calli were treated on NB medium containing 0.4 M mannitol for 4–6 h. These two vectors were transformed into treated calli by particle bombardment independently. The bombarded calli were further cultured overnight and transferred to NB medium containing 0.2 mg L-1 2,4-D for 2 weeks. A fluorescence microscope was used to examine GFP fluorescence. The calli with green fluorescence were observed and measured. Then, they were cultured on selection medium [NB medium containing 0.2 mg L-1 2,4-D and 3 mg L-1 bialaphos (Kyocera, Japan)] for 2 months. The medium was replaced every 2 weeks. Subsequently, the resistant calli were recovered on NB medium containing 50 g L-1 sucrose for 1 week and then transferred to differentiation medium to regenerate seedlings.
Accession Numbers
The GenBank accession number of each gene in this study is as follows: ZmDREB4.1 (GRMZM2G028969), DBF2 (GRMZM5G889719), TINY (AT5G25810), TINY2 (AT5G11590), GhDBP3 (DQ224382), StDREB1 (JN125862), MnDREB4A (KF678389), YUCCA9 (XM_016635159), YUCCA10 (XM_016642753), YUCCA11 (XM_016649400), IPT3 (XM_016647510), IPT5 (XM_016584942), GA2ox2 (AB125233), GA2ox3 (EF471117), GA2ox4 (KC568201), GA3ox2 (EF471116), GA20ox1 (NM_001326160), GA20ox2 (XM_016615759), and GA20ox3 (XM_016648485).
Results
Isolation and Expression Analysis of ZmDREB4.1
ZmDREB4.1 was cloned by PCR from maize root cDNA. The ZmDREB4.1 gene contains an open reading frame of 759 bp encoding a protein composed of 252 amino acid residues. It is reported that the transcription factors of DREB subfamily possess a conserved AP2 DNA-binding domain and YRG, WLG, and RAHD motifs (Okamuro et al., 1997). The DREB transcription factors were divided into six subgroups according to the sequence specificity localized from the 14th to 19th residues of the AP2 DNA-binding domain (Sakuma et al., 2002). DBF2 (Kizis and Pagès, 2002), TINY (Wilson et al., 1996), TINY2 (Wei et al., 2005), GhDBP3 (Huang and Liu, 2006), MnDREB4A (Liu et al., 2015), and StDREB1 (Bouaziz et al., 2013) belonging to DREB A-4 subgroup have been reported. The sequence alignment between ZmDREB4.1 and these transcription factors was performed. As shown in Figure 1A, ZmDREB4.1 had a conserved AP2 domain; YRG, WLG, and RAHD motifs; and VSEIRE sequence at the 14th to 19th position, which was consistent with these known DREB A-4 subgroup transcription factors. The results indicated that ZmDREB4.1 was classified into the A-4 subgroup of the DREB subfamily.
FIGURE 1.
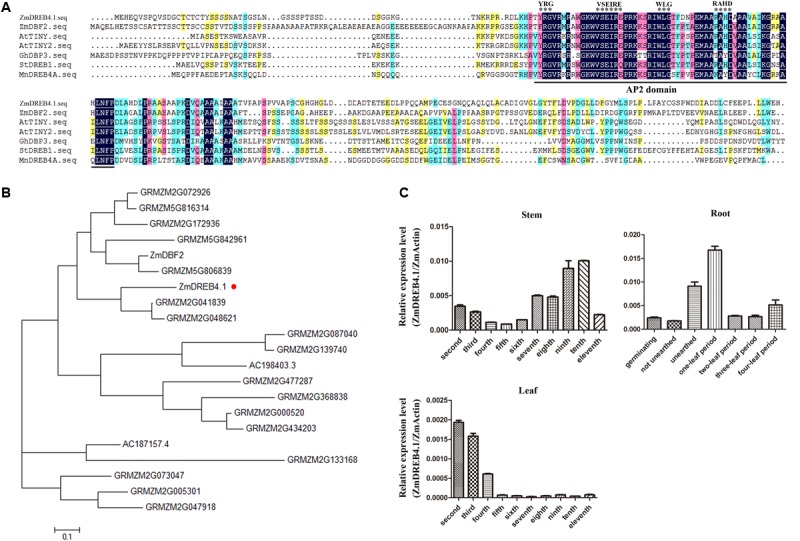
Multiple sequence alignment and expression analysis of ZmDREB4.1. (A) Multiple sequence alignment between ZmDREB4.1 and several reported DREB A-4 subgroup members from various species. The conserved AP2 domain is marked by a black line and the VSEIRE/Q, YRG, RAYD, and WLG motifs are marked by black asterisks. (B) Phylogenetic analysis of the DREB A-4 subgroup transcription factors from maize. The tree was generated by the neighbor-joining method using the full-length protein sequences. ZmDREB4.1 is highlighted by a round red dot. (C) The relevant expression levels of ZmDREB4.1 (the expression level of ZmDREB4.1/the expression level of ZmActin) analyzed by qRT-PCR in maize roots, stems and leaves.
Furthermore, 21 DREB A-4 subgroup transcription factors were identified from maize according to the conserved AP2 DNA-binding domain and VSEIRE/Q sequence. A phylogenetic tree was generated using amino acid sequences of these 21 proteins by the MEGA program and the neighbor-joining method (Figure 1B). DBF2, a DREB A-4 subgroup gene from maize, has been reported to play a negative role in the regulation of the promoter activity of rab17 (Kizis and Pagès, 2002). Phylogenetic tree analysis placed ZmDREB4.1 and DBF2 into two different branches. The AP2 DNA-binding domains of ZmDREB4.1 and DBF2 shared 89.66% amino acid identity. They shared low amino acid identity outside of the AP2 DNA-binding domain.
The expression levels of ZmDREB4.1 in different maize tissues were analyzed using qRT-PCR. The roots at germinating, not unearthed, unearthed, one-leaf, two-leaf, three-leaf, and four-leaf stage and second to eleventh stems and leaves of V11-stage maize were selected. The results showed that ZmDREB4.1 was expressed in roots of different stages and that the expression of ZmDREB4.1 peaked at the V1 stage. ZmDREB4.1 was also expressed in the stems and leaves of V11-stage maize. The expression of ZmDREB4.1 decreased gradually from the second to fifth stems and then rose from the sixth to tenth stems. Transcript levels of ZmDREB4.1 decreased from the second to fourth leaves and were almost not examined from the fifth to eleventh leaves (Figure 1C). It is reported that the expression levels of most DREB genes are induced by various abiotic stresses (Agarwal et al., 2017). We examined the expression levels of ZmDREB4.1 under various abiotic and biotic stresses. Three-leaf seedlings were treated with 20% PEG6000, 100 mM NaCl, cold (4°C), wound, 1 μM NAA, 10 μM 6-BA, 10 μM GA3, or 10 μM ACC. ZmDREB4.1 expression was not induced by any of the above treatments (Supplementary Figure S1).
ZmDREB4.1 Is Located in the Nucleus and Functions as a Transcriptional Activator
We examined the subcellular localization of ZmDREB4.1. The ZmDREB4.1-GFP fusion gene, under the control of the CaMV 35S promoter, was transiently expressed in maize mesophyll protoplasts and young stem tissue. The p35S::GFP plasmid was used as a control and transfected independently. As shown in Figure 2A, the green fluorescent signals accumulated only in the nucleus of cells transfected with ZmDREB4.1-GFP, while the signals were observed in both the nucleus and cytoplasm of the control. These results confirmed that ZmDREB4.1 was located in the nucleus.
FIGURE 2.
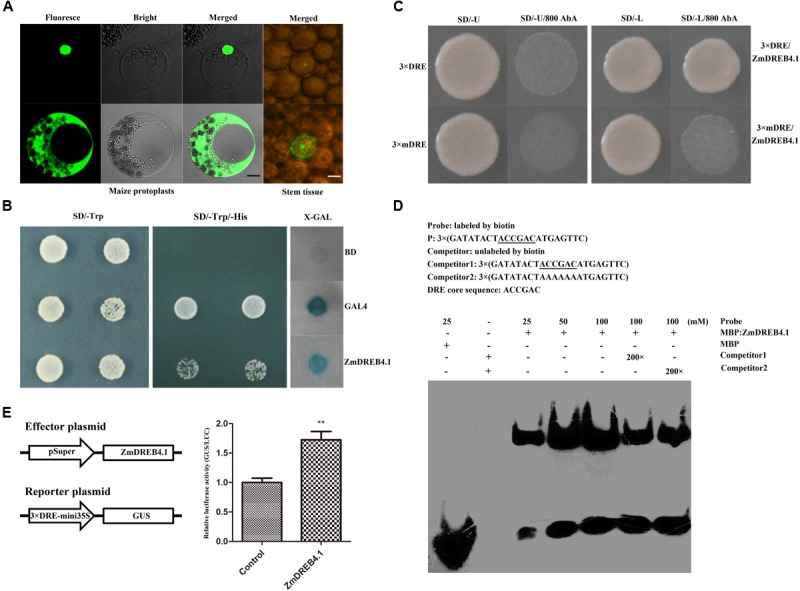
Transcription factor characteristics of ZmDREB4.1. (A) Subcellular localization of the ZmDREB4.1 transcription factor in maize mesophyll protoplasts and young stem tissue. The black and white scale bars indicate 5 and 10 μm, respectively. (B) Transcriptional activation analysis of ZmDREB4.1 in the yeast. GAL4 and BD indicate the positive control and negative control, respectively. (C) Yeast one-hybrid assay. ZmDREB4.1 binds to the DRE element, but not the mDRE element. (D) EMSA. The DRE core sequence is marked by underlines. ZmDREB4.1 specifically binds to the DRE element. (E) Transcriptional activation analysis of ZmDREB4.1 in tobacco leaves. GUS/LUC value indicated ZmDREB4.1 activates the expression of GUS gene driven by the fusion promoter of 3 × DRE and mini 35S. The leaves co-transformed with the empty p1300Super effector, the GUS reporter, p19 and p35S::LUC severed as the control. The GUS/LUC value of the control was set to 1. The values represent the mean ± SD from three biological replicates and the statistically significant differences were evaluated by Student’s t-test (∗∗P-value < 0.01).
According to previous reports, the members of the DREB subfamily can bind to the DRE element (Yamaguchi-Shinozaki and Shinozaki, 1994). The yeast one-hybrid system and EMSA were used to determine the binding characteristics of ZmDREB4.1 to the DRE element. The bait (pABAi-3 × DRE) and mutant bait (pABAi-3 × mDRE) plasmids were transformed into yeast strain Y1HGold independently to generate bait and mutant bait strains. The bait stain transfected with prey vector (pGADT7-ZmDREB4.1) could grow on SD/-Leu medium containing 800 ng ml-1 AbA, while mutant bait stain could not (Figure 2C). For EMSA, the MBP-ZmDREB4.131-147 aa fusion protein containing the AP2 DNA-binding domain was obtained by the prokaryotic expression system (Supplementary Figure S2). The 3 × DRE oligonucleotide was labeled with biotin at the 5′ terminus and used as the probe. The retardant band indicated that ZmDREB4.131-147 aa could bind to the DRE element directly (Figure 2D).
Yeast and tobacco assay systems were used to determine whether ZmDREB4.1 was able to activate transcription. The pBD-ZmDREB4.1, pGAL4 (positive control) and pBD-GAL4 (negative control) plasmids were transfected into yeast strain YRG-2 individually. The yeast transfected with pBD-ZmDREB4.1 and pGAL4 could grow on the SD/-Trp/-His, but the negative control could not. In addition, blue color was observed when the extract of yeast transfected with pBD-ZmDREB4.1 and pGAL4 was incubated with X-gal (Figure 2B). In tobacco leaves, the relative GUS/LUC activity of the extract of leaves transfected with pSuper::ZmDREB4.1 and p3 × DRE:mini35S::GUS was approximately 1.7 times as high as the control (Figure 2E), indicating that ZmDREB4.1 promoted the activity of the p3 × DRE:mini35S promoter. These results demonstrated that ZmDREB4.1 could bind to the DRE element directly and functioned as a transcriptional activator.
The Differences Between Transgenic Lines and WT in Tobacco
ZmDREB4.1 was overexpressed in tobacco to investigate the function of ZmDREB4.1. The transgenic tobacco plants were examined by PCR, and 19 transgenic lines were obtained. The expression levels of ZmDREB4.1 were analyzed by qRT-PCR. Three lines (OE6, OE9 and OE13) with high expression levels were selected for further investigations (Supplementary Figure S3).
The transgenic and WT plants were grown on MS medium at 28°C under a long-day photoperiod (16 h light, 8 h dark) to explore whether there were some phenotypes in ZmDREB4.1-overexpressing lines. Compared with WT, the overexpression of ZmDREB4.1 gave rise to obvious changes in morphology. ZmDREB4.1-overexpressing plants exhibited a dwarf phenotype with shorter hypocotyls, stems and petioles and smaller leaves (Figures 3A–C). The average hypocotyl length of OE6, OE9, and OE13 at the two-leaf stage was 3.7, 3.2, and 3.9 mm which were 55.58, 47.51, and 58.71% as long as the control (6.7 mm) (Figure 3D). The average stem length of OE6, OE9, and OE13 at the four-leaf stage was 0.65, 0.53, and 0.62 cm which were significantly shorter than the control (1.4 cm) (Figure 3E). The second petiole lengths of OE6, OE9, and OE13 at the four-leaf stage (3.12, 3.14, and 3.47 mm) were 49.89, 50.09 and 55.35% as long as the control (6.26 mm) (Figure 3G). The areas of the second leaves of OE6, OE9, and OE13 (0.72, 0.72, and 0.74 cm2) at the four-leaf stage were significantly smaller than those of the control (1.65 cm2) (Figure 3F). However, the number of the leaves of OE6, OE9, or OE13 was not influenced during the entire life cycle (Supplementary Figure S4). In addition, there were some differences at reproductive stage such as the shorter pedicel and earlier abscission of flowers (Supplementary Figure S5). We also examined ZmDREB4.1-overexpressing tobacco tolerance to drought. The transgenic plants displayed reduced drought tolerance compared with WT after they were subjected to drought treatment for 2 weeks (Supplementary Figure S6). These results demonstrated that overexpression of ZmDREB4.1 repressed plant growth and development and resulted in reduced drought tolerance in tobacco.
FIGURE 3.
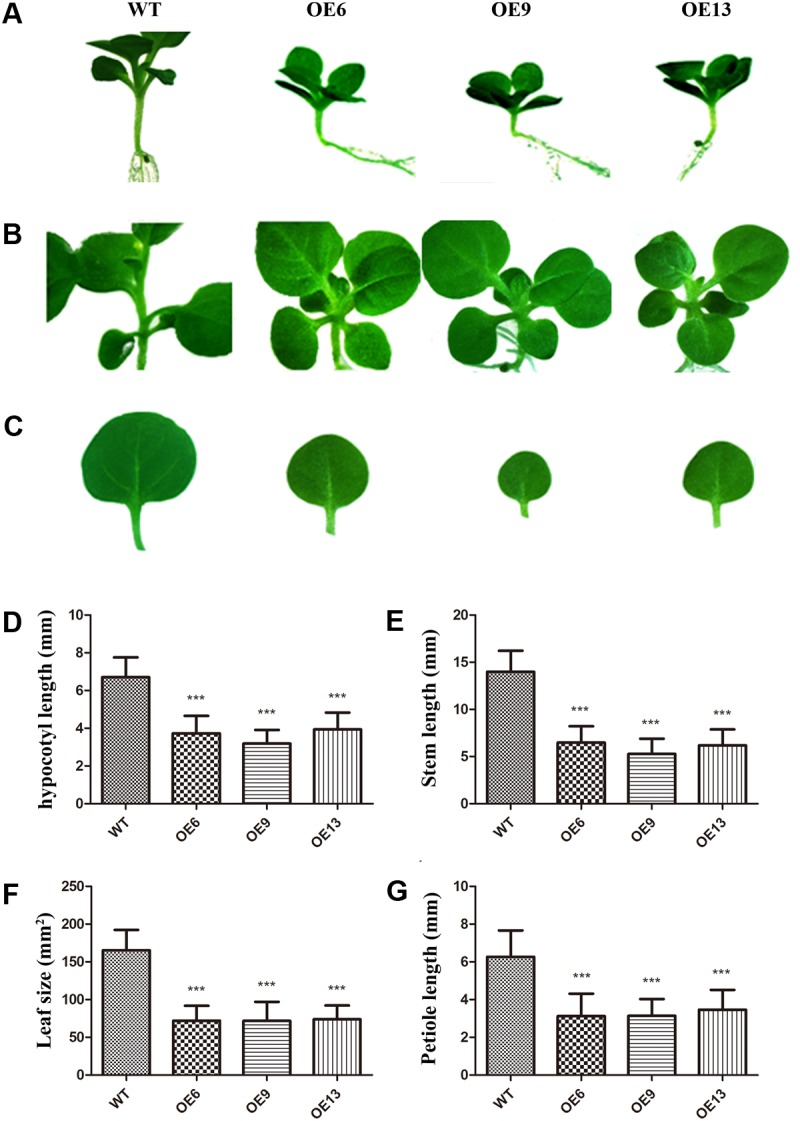
Phenotypes of ZmDREB4.1-overexpressing tobacco. Compared with WT, ZmDREB4.1-overexpressing tobacco exhibited shorter hypocotyls (A), stems (B) and petioles and smaller leaves (C). The tobacco plants at the two-leaf stage were chosen for hypocotyl observation, and tobacco plants at the four-leaf stage were chosen for observation of stem, petiole, and leaf. Scale bar: 5 mm. Column diagrams in (D–G) showed quantitative determination of hypocotyl length, plant height, petiole length and leaf size, respectively. For (D–G), the values represent the mean ± SD from 25 separate plants of each of three independent transgenic lines and the statistically significant differences were evaluated by Student’s t-test and corrected by Bonferroni correction (∗∗∗P-value < 0.001/3).
Overexpression of ZmDREB4.1 Repressed Cell Division in Transgenic Leaves
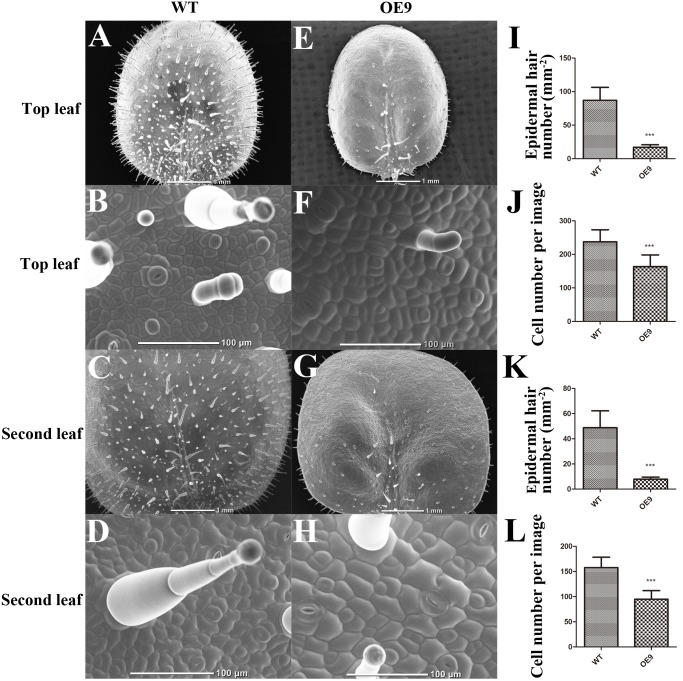
Fewer cells or smaller cells can give rise to smaller leaves. To determine the exact reason, a SEM was used to observe the structure and cell size of OE9 and WT. The cell sizes of the top and second leaves of OE9 seedlings (Figures 4F,H) at the four-leaf stage were larger than those of the WT (Figures 4B,D). There was approximately 163 and 237 cells per image in the top leaves of OE9 and WT, respectively (Figure 4J), and the numbers of cells were 95 and 158 in the second leaves (Figure 4L). In addition, we observed that the number of epidermal hairs of OE9 seedlings (Figures 4E,G) was significantly lower than that of WT (Figures 4A,C). The average numbers of epidermal hairs of OE9 were approximately 19.54 and 15.98% as great as those of WT in the top and second leaves, respectively (Figures 4I,K). The initiation of epidermal hairs relies on reactivation of endoreduplication (Payne et al., 1999). The fewer cells and epidermal hairs in OE9 indicated that overexpression of ZmDREB4.1 repressed cell proliferation in transgenic leaves.
FIGURE 4.
Scanning electron microscope (SEM) observation and calculation of the leaves. SEM observation of adaxial leaves of WT and OE9. (A,E) Top leaf. (C,G) Second leaf. (B,F) The enlargement of (A,E), respectively. (D,H) The enlargement of (C,G). The epidermal hair number per mm2 of the top (I) and second leaves (K) and the average cell number per image of the top (J) and second leaves (L) were calculated. Scale bar: 1 mm in (A,C,E,G) and 100 μm in (B,D,F,H). For (I–L), the values represent the mean ± SD from 10 separate plants of WT and OE9 and the statistically significant differences were evaluated by Student’s t-test (∗∗∗P-value < 0.001).
ZmDREB4.1 Overexpression Repressed Callus Growth in Tobacco
To further analyze the function of ZmDREB4.1 in cell proliferation, a callus-induction assay was performed. The top leaves of WT and OE9 seedlings at the four-leaf stage were cut into small pieces of similar size. After being cultured for 2 weeks, the calli induced from leaves of OE9 grew slowly and were significantly smaller than those of WT (Figure 5A). The results from the calli cultured on four CIM were coincident. Accordingly, the fresh weight of calli induced from OE9 leaves was on average 39.81 mg, while this value was 86.22 mg in WT (Figure 5B). Similarly, the second petioles from 20 separate plants of OE9 at four-leaf stage generated fewer calli compared with WT (Figure 5C). These results indicated that overexpression of ZmDREB4.1 inhibited cell proliferation during callus induction in tobacco.
FIGURE 5.
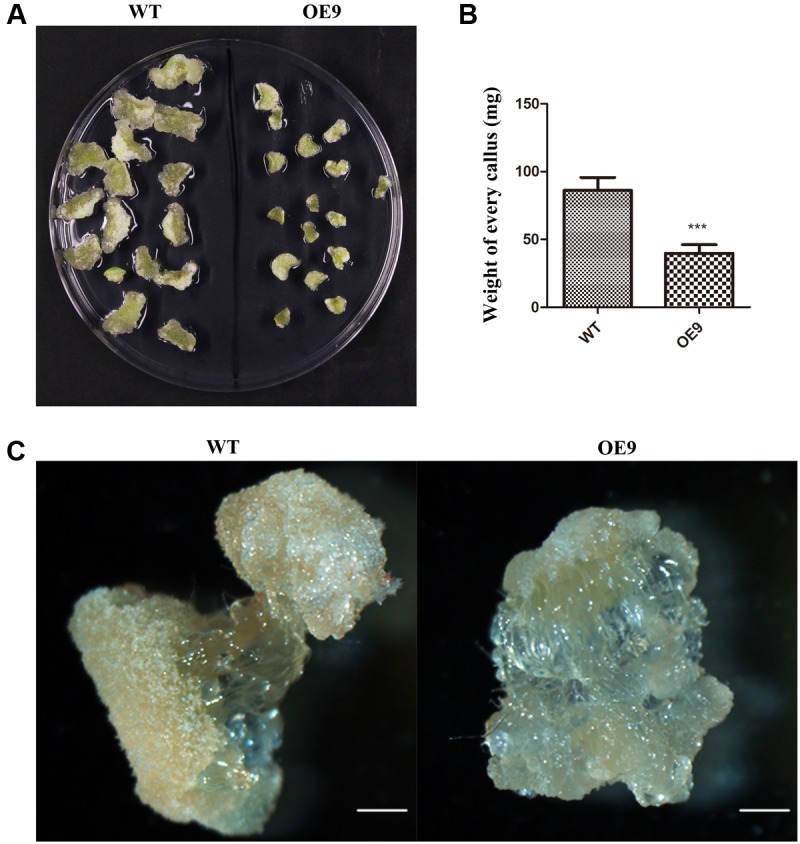
Callus-induction assay of tobacco leaf and petiole. (A) Morphology of calli induced from top leaf of the four-leaf-stage plants after culture for 2 weeks. (B) The average fresh weight of calli in (A). The values represent the mean ± SD from 60 calli and the statistically significant differences were evaluated by Student’s t-test (∗∗∗P-value < 0.001). (C) The calli induced from second petioles of the four-leaf-stage plants after culture for 2 weeks. OE9 showed smaller callus. Scale bar: 1 mm in (C).
Shorter Hypocotyls and Petioles Were Because of Inhibition of Cell Elongation
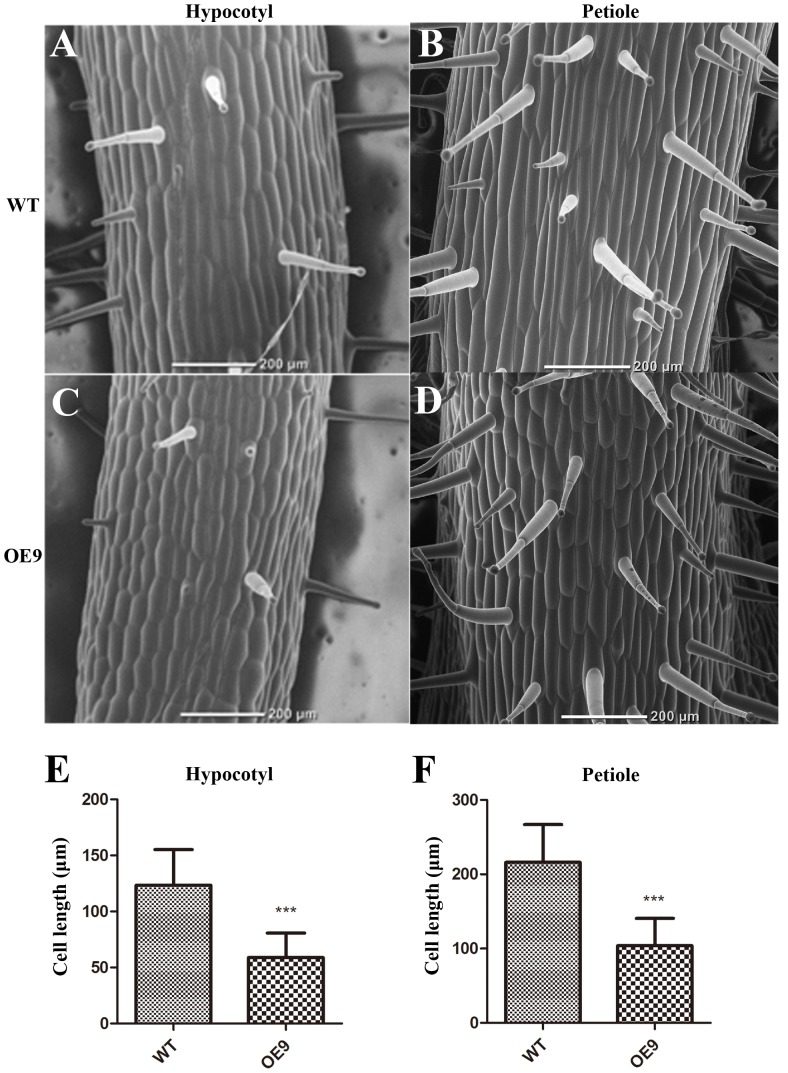
We also examined why the length of hypocotyls and petioles of OE9 became shorter. The hypocotyls at the two-leaf stage and second petioles at the four-leaf stage of OE9 and WT were selected to be observed. The cell sizes of OE9 hypocotyls (Figure 6C) were shorter than those of WT (Figure 6A). The average cell length of OE9 was 58.98 μm, while this value of WT was 123.48 μm (Figure 6E). Similar results were observed in petioles of OE9 (Figure 6D) and WT (Figure 6B). The average cell length of OE9 was approximately 48.03% as long as that of WT (Figure 6F). The hypocotyl and petiole length in OE9 decreased by 52.49% (Figure 3D) and 49.91% (Figure 3G), respectively, which was close to the reduced degree of cell length of the hypocotyl and petiole (52.24 and 51.97%). These results indicated that the shorter hypocotyls and petioles of OE9 resulted from inhibition of cell elongation rather than cell division.
FIGURE 6.
Scanning electron microscope observation and the statistics of cell length of the hypocotyls and petioles. (A,C) Indicated the cell morphology of hypocotyls of the two-leaf-stage plants. (B,D) Indicated cell morphology of the second petioles of the four-leaf-stage plants. The average cell length of the hypocotyls (E) and petioles (F) were calculated. OE9 possessed shorter cells in both hypocotyl and petiole. For (E) and (F), the values represent the mean ± SD of the data from at least 40 cells of two different hypocotyls and petioles and the statistically significant differences were evaluated by Student’s t-test (∗∗∗P-value < 0.001).
Overexpression of ZmDREB4.1 Decreased Auxin, CK and GA Levels and Application of GA3 Rescued the Dwarf Phenotype in Transgenic Tobacco
Some mutants deficient in phytohormone biosynthesis and response exhibit dwarfism (Choe et al., 1998; Talon et al., 1999; Griffiths et al., 2006) which resembles the phenotype of ZmDREB4.1-overexpressing tobacco. This reminded us to pay attention to the change in endogenous phytohormone levels. We obtained the leaves and petioles of OE9 and WT. They were divided into three parts and used to measure endogenous phytohormone levels by icELISA. Compared with WT, the IAA, ZR, DHZR, and IPA contents were reduced by 35.52, 50.55, 42.60, and 28.51% in OE9 leaves (Figure 7A). Further analysis showed that the expression levels of YUCs (YUCCA9, YUCCA10, and YUCCA11) involved in biosynthesis of auxin (Stepanova et al., 2011) and IPTs (IPT3 and IPT5) involved in biosynthesis of CK (Lindner et al., 2014) were significantly down-regulated in OE9 leaves as well (Figure 7B). In OE9 petioles, the GA3 and GA4 contents were reduced by 15.75 and 50.47%, while the IAA and ZR contents were not significantly different from WT (Figure 7C). The GA2ox genes (GA2ox2, GA2ox3, and GA2ox4) involved in the conversion of bioactive GAs to inactive forms in GA metabolism (Thomas et al., 1999) were obviously up-regulated in OE9. However, the genes (GA3ox2, GA20ox1, and GA20ox2) involved in biosynthesis of bioactive GA (Xu et al., 1995) were also up-regulated (Figure 7D). Therefore, we proposed that the reduced bioactive GA levels in OE9 were because of up-regulation of GA2ox genes, whereas up-regulation of GA20ox and GA30ox genes might result from feedback mechanism in GA homeostasis regulation.
FIGURE 7.
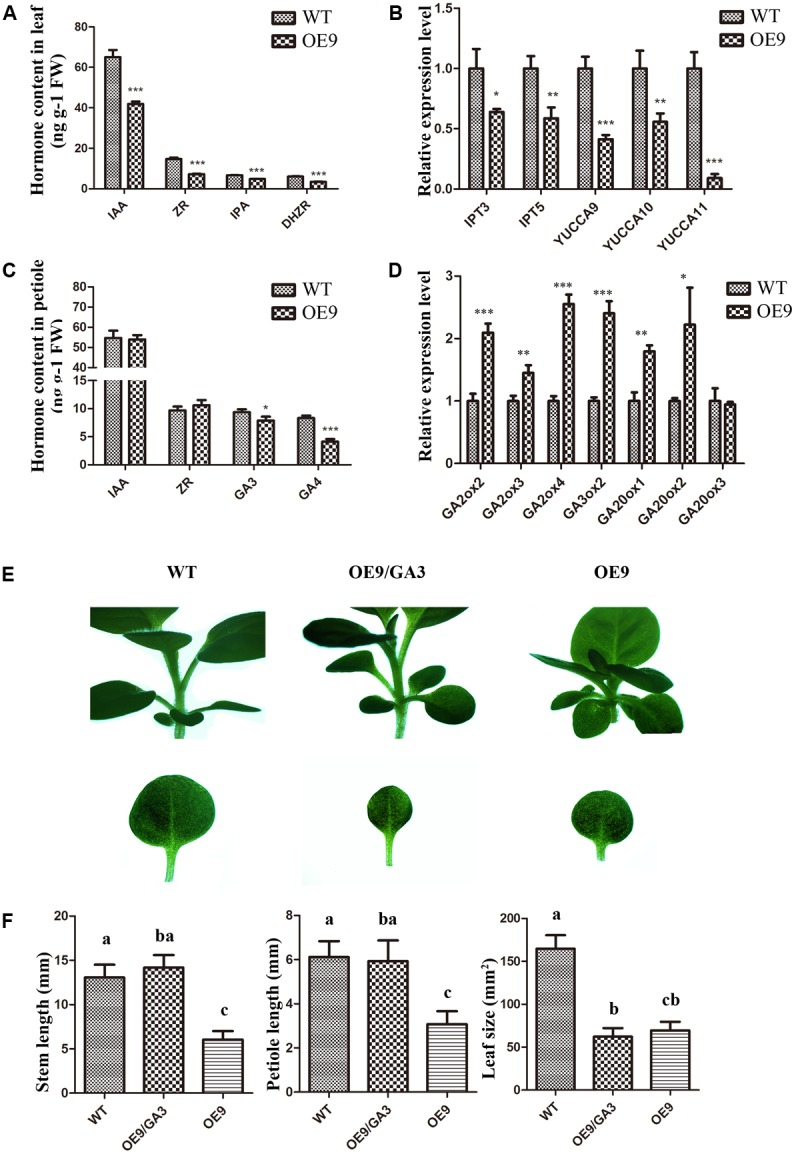
Analysis of the endogenous phytohormone levels and the expression levels of relative genes and effects of GA3 on phenotypic restoration. (A) IAA, ZR, DHZR, and IPA contents in WT and OE9 leaves. (B) The expression levels of YUCCA9, YUCCA10, and YUCCA11 which were involved in the biosynthesis of auxin and IPT3 and IPT5 which were involved in the biosynthesis of cytokinin. (C) GA3 and GA4 contents in WT and OE9 petioles. (D) The expression levels of GA2ox2, GA2ox3, and GA2ox4 which were involved in the biosynthesis of GA, and GA3ox2, GA20ox1, GA20ox2, and GA20ox3 which were involved in conversion to inactive forms in GA metabolism. For (A–D), the values represent the mean ± SD of the results from three biological replicates and the statistically significant differences were evaluated by Student’s t-test (∗P-value < 0.05, ∗∗P-value < 0.01, ∗∗∗P-value < 0.001). (E) The shorter-hypocotyl, -stem and -petiole phenotypes were rescued by application of exogenous GA3 at 1 μM. Scale bar: 5 mm. (F) The stem and petiole length and leaf size of WT, OE9 and OE9 cultured on CIM containing 1 μM GA3. The valves represent the mean ± SD from 15 separate plants of WT and OE9 and multiple means were compared using Tukey’s multiple comparison test (α = 0.05). The letters (a,b,c) represent the significant difference.
Since ZmDREB4.1-overexpressing tobacco contained reduced auxin, CK and GA contents, we investigated whether exogenous hormone could rescue phenotypes in ZmDREB4.1-overexpressing tobacco. The OE9 and WT seedlings were grown on MS medium or MS medium containing NAA, 6-BA or GA3 at different concentrations for 2 weeks. The results showed that the shorter stems and petioles were rescued by 1 μM GA3 (Figure 7E). The stem and petiole length of OE9 when they were cultured on the medium containing GA3 was close to those of WT cultured on MS medium, respectively (Figure 7F). However, the exogenous GA3 failed to rescue the smaller-leaf phenotype in OE9 (Figures 7E,F). In addition, we did not observe recovery of the smaller-leaf phenotype by NAA or 6-BA. These results demonstrated that the dwarf phenotype of ZmDREB4.1-overexpressing tobacco might result from deficiency of GA.
Overexpression of ZmDREB4.1 Repressed Callus Growth and Regeneration in Maize
To further analyze the function of ZmDREB4.1, the full-length coding sequence of ZmDREB4.1 fused to GFP under the control of the CaMV 35S promoter was transformed into maize immature embryo by particle bombardment (Figure 8A). These transformed calli were successively cultured on selection medium, recovery medium and differentiation medium. After being cultured on differentiation medium for 2 weeks, we obtained many regenerative seedlings, but they were all negative. We then examined the calli that grew slowly under a fluorescence microscope, and GFP was observed in a callus. We also detected the expression of bar protein in this callus by QUICKStix Kit (EnviroLogix, United States), and the positive band was observed. However, this callus failed to regenerate seedlings (Figure 8B).
FIGURE 8.
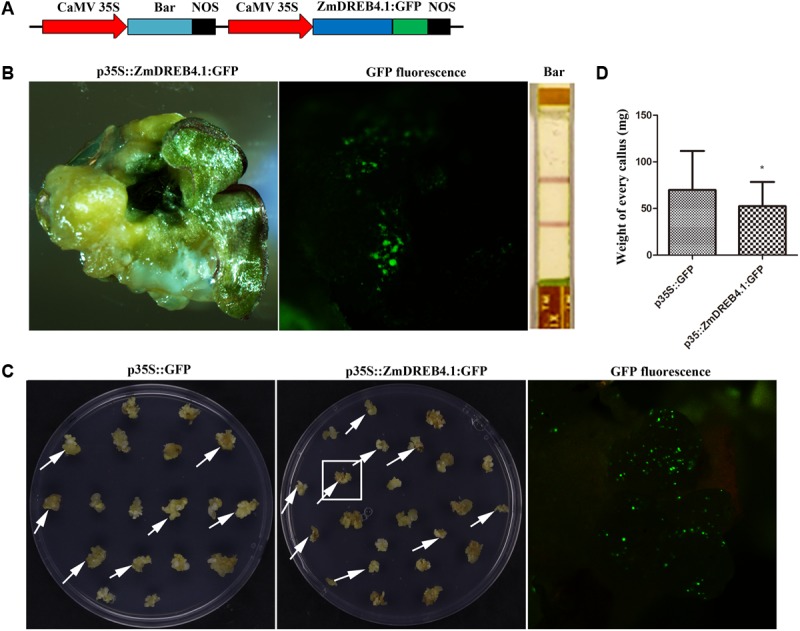
ZmDREB4.1 repressed callus growth and regeneration in maize. (A) Diagram of ZmDREB4.1 overexpression vector. ZmDREB4.1 was fused to GFP and driven by CaMV 35S promoter. Bar gene was used as a selective marker. (B) The callus transformed with p35S::ZmDREB4.1:GFP. GFP fluorescence and bar protein expression were observed in this callus. However, this callus could not regenerate seedlings. (C) The calli with transient expression of p35S::ZmDREB4.1:GFP and p35S::GFP. The GFP fluorescence was observed in the callus which is indicated by a white pane. The arrows indicate positive calli. (D) The fresh weight of positive callus in (C). The calli transformed with p35S::ZmDREB4.1:GFP were slightly lighter than those with p35S::GFP. The values represent the mean ± SD of the fresh weight from positive calli transformed with p35S::GFP and p35S::ZmDREB4.1:GFP vectors (for p35S::GFP, n = 34; for p35S::ZmDREB4.1:GFP, n = 32) and the statistically significant differences were evaluated by Student’s t-test (∗P-value < 0.05).
In combination with the phenotypes in transgenic tobacco, we predicted that inhibition of callus regeneration might result from inhibition of callus growth and proliferation by ZmDREB4.1 overexpression. Therefore, a callus-induction assay was performed to confirm this hypothesis. About 400 calli of similar size were transformed with p35S::ZmDREB4.1:GFP or p35S::GFP independently. The calli were then cultured on NB medium for 2 weeks. A fluorescence microscope was used to examine the GFP fluorescence. There were 34 and 32 positive calli transformed with p35S::ZmDREB4.1:GFP and p35S::ZmDREB4.1:GFP, respectively. The sizes of calli transformed with p35S::ZmDREB4.1:GFP were slightly smaller than those of the control (Figure 8C). The ZmDREB4.1-overexpressing callus was on average 52.39 mg, while this value was 69.84 mg for the control (Figure 8D). These results demonstrated that overexpression of ZmDREB4.1 repressed calli growth and regeneration in maize.
Discussion
A large number of DREB transcription factors have been reported to show a variety of roles in plant development and in response to abiotic stresses (Stockinger et al., 1997). In this study, we described the isolation and characterization of a DREB A-4 subgroup transcription factor from maize named ZmDREB4.1. ZmDREB4.1 exhibited relatively low basal expression (Figure 1C), which was consistent with the expression patterns of many members of the DREB subfamily (Agarwal et al., 2017). In previous studies, several DREB A-4 subgroup genes were identified from various plant species. All these genes except for DBF2 are induced by some abiotic stresses (Wilson et al., 1996; Kizis and Pagès, 2002; Wei et al., 2005; Huang and Liu, 2006; Bouaziz et al., 2013; Liu et al., 2015). Our results showed that the expression levels of ZmDREB4.1 remained stable under various abiotic or biotic stresses. This expression pattern is similar to that of DBF2, the only DREB A-4 subgroup gene reported in maize (Kizis and Pagès, 2002). These results indicated that the regulatory mechanism of ZmDREB4.1 expression differed from those of other reported DREB A-4 genes and may play different roles.
A dwarf phenotype was also observed in some transgenic plants, such as DREB1B/CBF1 and GhDREB1-overexpressing Arabidopsis and AtDREB1A-overexpressing soybean (Stockinger et al., 1997; Mok and Mok, 2001; Huang et al., 2009). In the present study, we showed that ZmDREB4.1-overexpressing tobacco exhibited a similar phenotype, including shorter hypocotyls, stems and petioles and smaller leaves (Figures 3A–C). The bioactive GA contents (GA3 and GA4) in ZmDREB4.1-overexpressing petioles were reduced compared with those of WT (Figure 7C). Quantification analysis results showed that GA2ox, GA3ox, and GA20ox genes were all up-regulated in ZmDREB4.1-overexpressing tobacco (Figure 7D). We thus proposed that reduced endogenous GA levels were caused by increased expression levels of GA2ox genes, while up-regulation of GA20ox and GA30ox genes was because of the feedback mechanism in GA homeostasis regulation. The dwarf phenotype caused by overexpression of ZmDREB4.1 could be rescued by application of exogenous GA3 (Figure 7E). Similar results were obtained in DREB1B/CBF1-overexpressing Arabidopsis and GhDREB1-overexpressing soybean (Stockinger et al., 1997; Huang et al., 2009). These results demonstrated that the dwarf phenotype of ZmDREB4.1-overexpressing tobacco might be caused by reduced contents of bioactive GAs.
In addition, our results showed that the smaller-leaf phenotype in transgenic plants could not be rescued by application of exogenous GA3. This phenomenon was also observed in AtDREB1A-overexpressing soybean and GhDREB1-overexpressing Arabidopsis (Mok and Mok, 2001; Huang et al., 2009). However, the underlying mechanisms remain unclear. The fewer number of cells and epidermal hairs (Figure 4) and the inhibition of callus induction (Figure 5) indicated that overexpression of ZmDREB4.1 severely repressed cell division in transgenic leaves. Quantitative analysis of endogenous bioactive auxin and CK showed that the IAA, ZR, DHZR, and IPA contents were severely reduced in ZmDREB4.1-overexpressing tobacco (Figure 7A). Consistent with reduced auxin and CK levels, YUCCA9, YUCCA10, and YUCCA11 (key enzymes in auxin biosynthesis) (Stepanova et al., 2011) as well as IPT3 and IPT5 (key enzymes in CK biosynthesis) (Lindner et al., 2014) were down-regulated in transgenic tobacco (Figure 7B). However, the application of exogenous auxin and CK could not rescue the smaller-leaf phenotype in OE9. We thus proposed that reduced auxin and CK levels might contribute to the inhibition of cell division but are not the main factor. In a word, our results demonstrated that the smaller-leaf phenotype resulted from inhibition of cell division. However, how ZmDREB4.1 repressed cell division in leaves requires further investigation.
The DRE element bound by DREBs was involved in response to drought, low-temperature, and high-salt stresses (Yamaguchi-Shinozaki and Shinozaki, 1994). The DREB transcription factor can activate the transcription of stress-response genes by binding to the DRE element and improve plant tolerance to various stresses (Li et al., 2014). ZmDREB4.1 is a DREB transcription factor and functions as an activator (Figures 1, 2). However, ZmDREB4.1-overexpressing tobacco exhibited reduced tolerance to dehydration stress (Supplementary Figure S6). It is reported that epidermal hairs can help plants prevent water loss (Amme et al., 2005). The reduced drought tolerance in OE9 might be due to fewer epidermal hairs. Further analysis is needed.
Conclusion
Our results demonstrated that ZmDREB4.1 is an important factor involved in the negative regulation of plant growth and development. We showed that ZmDREB4.1 belongs to the DREB A-4 subgroup and functions as a transcriptional activator. Overexpression of ZmDREB4.1 repressed calli growth and regeneration in maize. ZmDREB4.1-overexpressing tobacco displayed a dwarf phenotype with shorter hypocotyls, stems and petioles and smaller leaves. We also showed that the smaller leaves of transgenic tobacco resulted from inhibition of cell division. The shorter hypocotyls, stems, and petioles of transgenic tobacco were caused by inhibition of cell elongation because of reduced endogenous GA levels. However, how ZmDREB4.1 regulates leaf, hypocotyl, stem and petiole development in different means requires further investigation.
Author Contributions
SL and JY conceived and designed the studies. SL prepared the materials, performed the experiments, analyzed the data, and wrote the original manuscript. QZ prepared the reagents. DZ participated in genetic transformation. JY revised the manuscript thoroughly and finalized the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This work was supported by the National Natural Science Foundation of China (Grant Nos. 31571246 and 30971555).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00395/full#supplementary-material
References
- Agarwal P. K., Gupta K., Lopato S., Agarwal P. (2017). Dehydration responsive element binding transcription factors and their applications for the engineering of stress tolerance. J. Exp. Bot. 68 2135–2148. 10.1093/jxb/erx118 [DOI] [PubMed] [Google Scholar]
- Akhtar M., Jaiswal A., Taj G., Jaiswal J. P., Qureshi M. I., Singh N. K. (2012). DREB1/CBF transcription factors: their structure, function and role in abiotic stress tolerance in plants. J. Genet. 91 385–395. 10.1007/s12041-012-0201-3 [DOI] [PubMed] [Google Scholar]
- Amme S., Rutten T., Melzer M., Sonsmann G., Vissers J. P., Schlesier B., et al. (2005). A proteome approach defines protective functions of tobacco leaf trichomes. Proteomics 5 2508–2518. 10.1002/pmic.200401274 [DOI] [PubMed] [Google Scholar]
- Bouaziz D., Pirrello J., Ben Amor H., Hammami A., Charfeddine M., Dhieb A., et al. (2012). Ectopic expression of dehydration responsive element binding proteins (StDREB2) confers higher tolerance to salt stress in potato. Plant Physiol. Biochem. 60 98–108. 10.1016/j.plaphy.2012.07.029 [DOI] [PubMed] [Google Scholar]
- Bouaziz D., Pirrello J., Charfeddine M., Hammami A., Jbir R., Dhieb A., et al. (2013). Overexpression of StDREB1 transcription factor increases tolerance to salt in transgenic potato plants. Mol. Biotechnol. 54 803–817. 10.1007/s12033-012-9628-2 [DOI] [PubMed] [Google Scholar]
- Chen J., Xia X., Yin W. (2009). Expression profiling and functional characterization of a DREB2-type gene from Populus euphratica. Biochem. Biophys. Res. Commun. 378 483–487. 10.1016/j.bbrc.2008.11.071 [DOI] [PubMed] [Google Scholar]
- Chen M., Wang Q. Y., Cheng X. G., Xu Z. S., Li L. C., Ye X. G., et al. (2007). GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 353 299–305. 10.1016/j.bbrc.2006.12.027 [DOI] [PubMed] [Google Scholar]
- Chiang H. H., Hwang I., Goodman H. M. (1995). Isolation of the Arabidopsis GA4 locus. Plant Cell 7 195–201. 10.1105/tpc.7.2.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S., Dilkes B. P., Fujioka S., Takatsuto S., Sakurai A., Feldmann K. A. (1998). The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22alpha-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell 10 231–243. 10.1105/tpc.10.2.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling R. J., Kamiya Y., Seto H., Harberd N. P. (1998). Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol 117 1195–1203. 10.1104/pp.117.4.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng A., Tan W., He S., Liu W., Nan T., Li Z., et al. (2008). Monoclonal antibody-based enzyme linked immunosorbent assay for the analysis of jasmonates in plants. J. Integr. Plant Biol. 50 1046–1052. 10.1111/j.1744-7909.2008.00715.x [DOI] [PubMed] [Google Scholar]
- Dubouzet J. G., Sakuma Y., Ito Y., Kasuga M., Dubouzet E. G., Miura S., et al. (2003). OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 33 751–763. 10.1046/j.1365-313x.2003.01661.x [DOI] [PubMed] [Google Scholar]
- Elliott R. C., Betzner A. S., Huttner E., Oakes M. P., Tucker W. Q., Gerentes D., et al. (1996). AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8 155–168. 10.1105/tpc.8.2.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. R., Wang M. L., Lynch T. J., Rao S., Goodman H. M. (1998). The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10 1043–1054. 10.1105/tpc.10.6.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour S. J., Sebolt A. M., Salazar M. P., Everard J. D., Thomashow M. F. (2000). Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 124 1854–1865. 10.1104/pp.124.4.1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J., Murase K., Rieu I., Zentella R., Zhang Z. L., Powers S. J., et al. (2006). Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18 3399–3414. 10.1105/tpc.106.047415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P., Phillips A. L. (2000). Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci. 5 523–530. 10.1016/s1360-1385(00)01790-8 [DOI] [PubMed] [Google Scholar]
- Huang B., Jin L., Liu J. Y. (2008). Identification and characterization of the novel gene GhDBP2 encoding a DRE-binding protein from cotton (Gossypium hirsutum). J. Plant Physiol. 165 214–223. 10.1016/j.jplph.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Huang B., Liu J. Y. (2006). Cloning and functional analysis of the novel gene GhDBP3 encoding a DRE-binding transcription factor from Gossypium hirsutum. Biochim. Biophys. Acta 1759 263–269. 10.1016/j.bbaexp.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Huang J. G., Yang M., Liu P., Yang G. D., Wu C. A., Zheng C. C. (2009). GhDREB1 enhances abiotic stress tolerance, delays GA-mediated development and represses cytokinin signalling in transgenic Arabidopsis. Plant Cell Environ. 32 1132–1145. 10.1111/j.1365-3040.2009.01995.x [DOI] [PubMed] [Google Scholar]
- Hwang I., Chen H. C., Sheen J. (2002). Two-component signal transduction pathways in Arabidopsis. Plant Physiol. 129 500–515. 10.1104/pp.005504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku K. D., Den Boer B. G., Van Montagu M., Okamuro J. K. (1994). Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6 1211–1225. 10.1105/tpc.6.9.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitomi Y., Ito H., Hobo T., Aya K., Kitano H., Inukai Y. (2011). The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J. 67 472–484. 10.1111/j.1365-313X.2011.04610.x [DOI] [PubMed] [Google Scholar]
- Kizis D., Pagès M. (2002). Maize DRE-binding proteins DBF1 and DBF2 are involved in rab17 regulation through the drought-responsive element in an ABA-dependent pathway. Plant J. 30 679–689. 10.1046/j.1365-313x.2002.01325.x [DOI] [PubMed] [Google Scholar]
- Li C., Yue J., Wu X., Xu C., Yu J. (2014). An ABA-responsive DRE-binding protein gene from Setaria italica, SiARDP, the target gene of SiAREB, plays a critical role under drought stress. J. Exp. Bot. 65 5415–5427. 10.1093/jxb/eru302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Li X., Zhang D., Gao B., Yang H., Wang Y., et al. (2017). ScDREB8, a novel A-5 type of DREB gene in the desert moss Syntrichia caninervis, confers salt tolerance to Arabidopsis. Plant Physiol. Biochem. 120 242–251. 10.1016/j.plaphy.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Lindner A. C., Lang D., Seifert M., Podlešáková K., Novák O., Strnad M., et al. (2014). Isopentenyltransferase-1 (IPT1) knockout in Physcomitrella together with phylogenetic analyses of IPTs provide insights into evolution of plant cytokinin biosynthesis. J. Exp. Bot. 65 2533–2543. 10.1093/jxb/eru142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., et al. (1998). Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10 1391–1406. 10.2307/3870648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Q., Liu C. Y., Guo Q., Zhang M., Cao B. N., Xiang Z. H., et al. (2015). Mulberry transcription factor MnDREB4A confers tolerance to Multiple abiotic stresses in transgenic tobacco. PLoS One 10:e0145619. 10.1371/journal.pone.0145619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrnia M., Balazadeh S., Zanor M. I., Mueller-Roeber B. (2013). EBE, an AP2/ERF transcription factor highly expressed in proliferating cells, affects shoot architecture in Arabidopsis. Plant Physiol. 162 842–857. 10.1104/pp.113.214049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O., Skoog F., Von Slatzer M. H., Strong F. M. (1955). Kinetin, a cell division factor from deoxyribonucleic acid. J. Am. Chem. Soc. 77:1392 10.1021/ja01610a105 [DOI] [Google Scholar]
- Mizoi J., Shinozaki K., Yamaguchi-Shinozaki K. (2012). AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 1819 86–96. 10.1016/j.bbagrm.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Mok D. W. S., Mok M. C. (2001). Cytokinin metabolism and action. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 89–118. 10.1146/annurev.arplant.52.1.89 [DOI] [PubMed] [Google Scholar]
- Nakano T., Suzuki K., Fujimura T., Shinshi H. (2006). Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 140 411–432. 10.1104/pp.105.073783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X., Helentjaris T., Bate N. J. (2002). Maize ABI4 binds coupling element1 in abscisic acid and sugar response genes. Plant Cell 14 2565–2575. 10.1105/tpc.003400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto M. A., Floyd S. K., Fischer R. L., Goldberg R. B., Harada J. J. (2009). Effects of APETALA2 on embryo, endosperm, and seed coat development determine seed size in Arabidopsis. Sex. Plant Reprod. 22 277–289. 10.1007/s00497-009-0116-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamuro J. K., Caster B., Villarroel R., Van Montagu M., Jofuku K. D. (1997). The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 94 7076–7081. 10.1073/pnas.94.13.7076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne T., Clement J., Arnold D., Lloyd A. (1999). Heterologous myb genes distinct from GL1 enhance trichome production when overexpressed in Nicotiana tabacum. Development 126 671–682. [DOI] [PubMed] [Google Scholar]
- Qin F., Kakimoto M., Sakuma Y., Maruyama K., Osakabe Y., Tran L. S., et al. (2007). Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 50 54–69. 10.1111/j.1365-313X.2007.03034.x [DOI] [PubMed] [Google Scholar]
- Richards D. E., King K. E., Ait-Ali T., Harberd N. P. (2001). How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 67–88. 10.1146/annurev.arplant.52.1.67 [DOI] [PubMed] [Google Scholar]
- Sakuma Y., Liu Q., Dubouzet J. G., Abe H., Shinozaki K., Yamaguchi-Shinozaki K. (2002). DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 290 998–1009. 10.1006/bbrc.2001.6299 [DOI] [PubMed] [Google Scholar]
- Sakuma Y., Maruyama K., Osakabe Y., Qin F., Seki M., Shinozaki K., et al. (2006). Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 18 1292–1309. 10.1105/tpc.105.035881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif El-Yazal S. A., Seif El-Yazal M. A., Dwidar E. F., Rady M. M. (2015). Phytohormone crosstalk research: cytokinin and its crosstalk with other phytohormones. Curr. Protein Pept. Sci. 16 395–405. 10.2174/1389203716666150330141159 [DOI] [PubMed] [Google Scholar]
- Sheen J. (2002). A Transient Expression Assay Using Maize Mesophyll Protoplasts. Available at: http://genetics.mgh.harvard.edu/sheenweb/ [Google Scholar]
- Söderman E. M., Brocard I. M., Lynch T. J., Finkelstein R. R. (2000). Regulation and function of the Arabidopsis ABA-insensitive4 gene in seed and abscisic acid response signaling networks. Plant Physiol. 124 1752–1765. 10.1104/pp.124.4.1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova A. N., Yun J., Robles L. M., Novak O., He W., Guo H., et al. (2011). The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23 3961–3973. 10.1105/tpc.111.088047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger E. J., Gilmour S. J., Thomashow M. F. (1997). Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. U.S.A. 94 1035–1040. 10.1073/pnas.94.3.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M., Koornneef M., Zeevaart J. A. (1999). Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc. Natl. Acad. Sci. U.S.A. 87 7983–7987. 10.1073/pnas.87.20.7983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. G., Phillips A. L., Hedden P. (1999). Molecular cloning and functional expression of gibberellin 2- oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc. Natl. Acad. Sci. U.S.A. 96 4698–4703. 10.1073/pnas.96.8.4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L. S., Urao T., Qin F., Maruyama K., Kakimoto T., Shinozaki K., et al. (2007). Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 104 20623–20628. 10.1073/pnas.0706547105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco R., Zharkikh A., Affourtit J., Dhingra A., Cestaro A., Kalyanaraman A., et al. (2010). The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 42 833–839. 10.1038/ng.654 [DOI] [PubMed] [Google Scholar]
- Wang D., Zhao Q., Zhu D., Ao G., Yu J. (2006). Particle-bombardment-mediated co-transformation of maize with a lysine rich protein gene (sb401) from potato. Euphytica 150 75–85. 10.1007/s10681-006-9095-6 [DOI] [Google Scholar]
- Wei G., Pan Y., Lei J., Zhu Y. X. (2005). Molecular cloning, phylogenetic analysis, expressional profiling and in vitro studies of TINY2 from Arabidopsis thaliana. J. Biochem. Mol. Biol. 38 440–446. 10.5483/bmbrep.2005.38.4.440 [DOI] [PubMed] [Google Scholar]
- Wilson K., Long D., Swinburne J., Coupland G. (1996). A Dissociation insertion causes a semidominant mutation that increases expression of TINY, an Arabidopsis gene related to APETALA2. Plant Cell 8 659–671. 10.1105/tpc.8.4.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. L., Li L., Wu K., Peeters A. J., Gage D. A., Zeevaart J. A. (1995). The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: molecular cloning and functional expression. Proc. Natl. Acad. Sci. U.S.A. 92 6640–6644. 10.1073/pnas.92.14.6640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. (1994). A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6 252–264. 10.1105/tpc.6.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Chen M., Chen X., Xu Z., Guan S., Li L. C., et al. (2008). Phylogeny, gene structures, and expression patterns of the ERF gene family in soybean (Glycine max L.). J. Exp. Bot. 59 4095–4107. 10.1093/jxb/ern248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liu X., Wu L., Yu G., Wang X., Ma H. (2015). The SsDREB transcription factor from the succulent Halophyte Suaeda salsa enhances abiotic stress tolerance in transgenic tobacco. Int. J. Genomics 2015:875497. 10.1155/2015/875497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M. L., Ma J. T., Zhao Y. M., Wei Y. H., Tang Y. X., Wu Y. M. (2012). Improvement of drought and salt tolerance in Arabidopsis and Lotus corniculatus by overexpression of a novel DREB transcription factor from Populus euphratica. Gene 506 10–17. 10.1016/j.gene.2012.06.089 [DOI] [PubMed] [Google Scholar]
- Zhuang J., Deng D., Yao Q., Zhang J., Xiong F., Chen J., et al. (2010). Discovery, phylogeny and expression patterns of AP2-like genes in maize. Plant Growth Regul. 62 51–58. 10.1007/s10725-010-9484-7 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.