Abstract
Purpose
To determine the prevalence of active convulsive epilepsy and treatment gap in two Urban slums in Enugu South East Nigeria.
Methods
A 3 phase cross-sectional descriptive study was done to survey individuals ≥15 years in 2 slums in Enugu, South East Nigeria.
Results
The prevalence of epilepsy was 6.0 (95% CI: 5.9–6.0) per 1000 (men 4.4/1000, 95% CI: 2.3–6.4, women 7.8/1000, 95% CI: 4.9–10.4), p = 0.06. The peak age of active convulsive epilepsy was 40–44 years (11.2 per 1000) with two smaller peaks at 25–29 and ≥50 years. The age and sex adjusted prevalence using WHO standard population and 2006 Nigerian census population were 5.9 per 1000 (95% CI: 4.0–7.9) and 5.4 per 1000 (95% CI: 3.4–7.4).
Conclusion
The prevalence of epilepsy is high in urban slums in Enugu. Nationwide studies should be done to find out the true prevalence in the country.
Keywords: Prevalence, Epilepsy, Urban slum, Nigeria, Africa
1. Introduction
Epilepsy is a common neurological disorder and one of the most prevalent non-communicable disorders affecting about 70 million [1] people worldwide. In the African continent, epilepsy remains a major public health problem, not only because of the impact on health but also the socio-cultural, psychological, and economic connotations. Active epilepsy is estimated to affect 4.4 million people in Sub-Saharan Africa (SSA), whilst lifetime epilepsy is estimated to affect 5.4 million with the peak in the 3rd decade of life [2]. The high prevalence of epilepsy, especially in young adults, has important consequences for both the workforce and community structures. In a recent report from South East Nigeria, the prevalence of active convulsive epilepsy was estimated to be 4.3 per 1000 (95% CI: 2.7–5.9) [3].
Despite global advances in diagnosis and treatment of epilepsy the overall estimate of treatment gap is 56/100 (95% CI: 31.1–100.0) [4]. Apart from high prevalence rate and large treatment gap, trends in SSA suggest a large proportion of symptomatic epilepsy, higher rural prevalence, low levels of awareness and high levels of stigma [1,3,5–7].
Though a number of studies provide prevalence data about epilepsy in Nigeria [3,8,9], no study has focused on special populations such as urban slum dwellers.
2. Methods
This study was carried out in Agu-Abor and Ugbodogwu slums in Enugu East Local Government Area of Enugu State, South East Nigeria. Agu-Abor has an estimated adult population of 3000–4000 while Ugbodowu has an estimated population of 5000–7000 (based on church and local records). The overall population of Enugu East Local government is 145, 905 based on 2006 census count [10]. These settlements are located at the foot of Udi hills and together occupy an area of about 2.5–5 km2 about 1–2.5 km from the city center. The nearest tertiary hospital. Agu-abor has no primary health center while Ugbodogwu has in addition to a primary school a secondary school and a primary health centers. People also access care from government health institutions within the city.
3. Study design
A 3 phase cross-sectional descriptive study was performed to survey the adult population (≥ 15 years of age). The sample size was determined using the formula. n = DZ1−α/2 2P(1 − P)/d2 (Lwanga and Lemeshow 1991) [11], where d = absolute precision = 0.00215 (for a sample size lower than 5%), P = prevalence of 4.3 per 1000 = 0.00433, D = design effect = 2, Z1−α/2 = standard normal deviate corresponding to 5% level of significance (2 sided test) = 1.96. The calculated sample size was 7116. Assuming 10% attrition, a minimum sample size of 7910 was used.
The first phase of the study was preceded by sensitization meetings in the community which included both religious and elected leaders, a 4-day training program with a review of the questionnaire, demonstration of interview technique, and back-demonstration by the interviewer. The study questionnaire was forward- and back-translated into Igbo and reviewed by bilingual individuals for accuracy. A clinic-based validation of a subset of a random sample of 50 screened individuals (25 positives, 25 negatives) was assessed by a neurologist (BAE) (blinded to screening results) who conducted a complete neurologic history, physical examination, and review of prior medical records before determining if the patient met the case definition for active convulsive epilepsy. The Igbo adapted questionnaire exhibited an overall 100% sensitivity and 96% (95% CI: 96–100) specificity (see below table). A false-positive screen occurred in an individual who had experienced repeated syncope. The positive predictive value of the instrument was 96.2% (95% CI: 91–100). Validation of the screening questionnaire (Igbo version) was carried out in Neurology clinic of the department of medicine, University of Nigeria Teaching Hospital Enugu.
Results of validation study of the Igbo version of the questionnaire.
| True positives (%) (a) |
False positives (%) (b) |
False negatives (%) (c) |
True negatives (%) (d) |
|
|---|---|---|---|---|
| Epilepsy | 25 (100) | 1 | 0 | 24 |
Diagnostic accuracy = (a + d)/N = 98%.
Sensitivity = a/(a + c) = 100%.
Specificity = d/(b + d) = 96%.
Positive predictive value = a/(a + b) = 96.2%.
Negative Predictive value = d/(c + d) = 100.
During the second phase of the study, a door-to-door survey by teams of trained research assistants was performed. In this phase the research instrument for screening neurological disorders in the community by Osuntokun et al. [9] was administered to identify persons with at least one life time episode of convulsion. Each questionnaire had a unique identification number and the three letter initials of the subjects clearly written on them during the house to house interview. Using a simple random sampling technique all consecutive consenting individuals in each of the settlements were interviewed until the minimum sample size was reached. The WHO STEPS instrument [12] was used to collect data on selected socio-demographic characteristics and lifestyle behaviors. The inhabitants of Agu-abor were surveyed over a 4-week period (August 12–September 9, 2013), while Ugbodogwu inhabitants were surveyed between November 25 and December 21, 2013. A positive history of convulsions at this stage resulted in the invitation of the individual to the clinic for the third phase of the study.
The third phase of the study was conducted at temporary clinics at the study sites. Cases identified during the second phase were interviewed by the primary investigator and a doctor not below the rank of a senior registrar in the department of medicine. The 2nd and 3rd stages ran concurrently and lasted for 6 weeks. Only subjects who had at least one lifetime episode of convulsion who reported to the clinic were included in this stage.
The exclusion criteria were refusal to participate and lack of basic knowledge of Igbo language. A minimum age of 15 years was chosen during the design of the study for three reasons: (1) to avoid recruiting students (primary and junior secondary) who were having end of year examinations at that time; (2) the anticipated difficulty in getting consent from parents; (3) the validation study was performed only in subjects within the same age group. The study of active convulsions was chosen to reduce the possibility of recall bias especially among older individuals. Non Igbos were excluded in view of the questionnaire only being validated in Igbo. Furthermore all the investigators and community health workers were Igbos.
Flow chart.
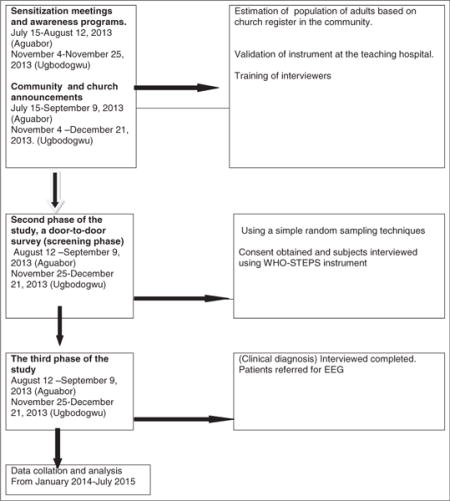
The study protocol was reviewed on behalf of State (Enugu State) Ministry of Health by the Ethics committee of the Enugu State University of Science and Technology Teaching Hospital and University of Nigeria Teaching Hospital, Enugu. All participants gave their informed consent after reading or having the consent form read for them.
To encourage subject participation free basic medical checks such as blood pressure, fasting blood glucose, urine analysis and electrocardiogram were offered to all for free at the clinic. During the third phase all cases with a history of a convulsion were reviewed.
4. Case definition
An active epilepsy case was defined as anyone on treatment for epilepsy or with a history of recurrent seizures with the most recent occurring in the past 5 years if the seizure(s) was not provoked and did not occur solely during severe febrile illness or any other acute illness such as stroke or head injury or during delivery or third trimester of pregnancy. Tobacco use was defined as the use of (any or all) cigarettes, snuff and chewing tobacco in the past 4 weeks. Alcohol use and quantity was defined as (mean quantity) the consumption of any alcoholic beverage (beer, gin, stout, local brew) in a week. The safe limit of alcohol was defined based on WHO guidelines of 21 units for men and 14 units for women per week [13]. Occupation was defined as the primary job which takes at least 50% of the working hours in a week. An artisan was defined as skilled manual laborers such as masons, mechanics, tailors, welders, metal workers and other crafts. A trader was defined as small scale businessman or businesswoman.
Medical comorbidities (hypertension, diabetes, and stroke) were defined using standard criteria or past medical history diagnosed by a qualified personnel (doctors).
5. Statistical methods
For database management and statistical analyses, we used the SPSS version 21 (IBM Corporation, New York, USA). Data were presented in tables. For continuous variables, mean values and 95% confidence intervals were calculated. Prevalence of epilepsy and lifetime convulsion in the subjects was expressed as per 1000 and percentages and the odds ratio calculated. Mean values were compared using the independent t test. Proportions were compared using McNemar’s test and Chi squared test where applicable. In all, p value of <0.05 was regarded as statistically significant. Conclusions were drawn at the level of significance. The confidence level was kept at 95%.
6. Results
6.1. Description of the study population
8259 individuals consented and were interviewed; however only 8228 (99.6%) were analyzed. This comprised of 4133 (50.2%) men and 4095 (49.8%) women, with a male to female ratio of 1:1. 306/8228 (135 males and 171 females) individuals responded positively to the screening questions on epilepsy and were clinically interviewed during the survey period with a diagnosis of active epilepsy being made in 49 (16%). The age of the respondents ranged from 15 to 95 years with a mean age of 33.0 (CI 95% CI: 32.7–33.3) years. Males were significantly older than females; males, 34.2 (95%, CI 33.75–34.61) vs females, 31.8 (95%, CI 31.4–32.2) years. p < 0.0001. The age distribution and baseline characteristics of the population are shown in Table 1.
Table 1.
Sex and age distribution with socio-demographics.
| Males N (%) |
Female N (%) |
Total N (%) |
|
|---|---|---|---|
| Age groups (years) | |||
| 15–19 | 548 (13.2) | 663 (16.2) | 1211 (14.7) |
| 20–24 | 704 (17) | 792 (19.4) | 1496 (18.2) |
| 25–29 | 619 (15) | 686 (16.8) | 1305 (15.9) |
| 30–34 | 497 (12) | 526 (12.9) | 1023 (12.4) |
| 35–39 | 414 (10) | 395 (9.7) | 809 (9.8) |
| 40–44 | 468 (11.3) | 336 (8.2) | 804 (9.8) |
| 45–49 | 260 (6.3) | 214 (5.2) | 474 (5.8) |
| ≥50 | 630 (15.2) | 476 (11.6) | 1106 (13.4) |
| Level of education | |||
| No formal education | 23 (0.6) | 40 (1) | 63 (0.8) |
| Primary | 489 (11.8) | 462 (11.3) | 951 (11.6) |
| Secondary | 2169 (52.4) | 2177 (53.7) | 4346 (52.8) |
| Tertiary | 1459 (35.2) | 1409 (34.5) | 2868 (34.9) |
| # Occupation | |||
| Students/apprentices | 1525 (36.8) | 1704 (41.7) | 3229 (39.2) |
| Traders (business) | 1033 (52) | 1299 (31.8) | 2332 (28.3) |
| † Artisans | 880 (21.3) | 313 (7.7) | 1193 (14.3) |
| Office/factory workers | 469 (11.3) | 450 (11.0) | 919 (11.2) |
| Farmers | 117 (2.8) | 135 (3.3) | 252 (3.1) |
| Unemployed | 46 (1.1) | 145 (3.5) | 191 (2.3) |
| Pensioners | 53 (1.3) | 17 (0.4) | 70 (0.9) |
| Others | 17 (0.4) | 25 (0.6) | 42 (0.5) |
| Social habits | |||
| Use tobacco* | 557 (13.5) | 169 (4.1) | 726 (8.8) |
| †† Alcohol** | 68 (1.6) | 55 (1.3) | 123 (1.5) |
| a Medical comorbidities | |||
| Head injury with LOC | 64 (1.5) | 75 (1.8) | 139 (1.7) |
| Febrile convulsion | 37 (0.9) | 44 (1.1) | 81 (1) |
| c Mental illness | 16 (0.4) | 43 (1.1) | 59 (0.7) |
| b Hypertension | 236 (5.7) | 335 (8.2) | 571 (6.9) |
| Diabetes | 163 (3.9) | 158 (3.9) | 321 (3.9) |
| Stroke | 43 (1) | 39 (1) | 82 (1) |
| Family history of convulsions | 37 (0.9) | 72 (1.8) | 109 (1.3) |
| Total | 4133 (50.2) | 4095 (49.8) | 8228 (100) |
Previous hospital diagnosis done by a doctor.
History of hypertension, use of antihypertensive.
Reciving or recived treatment from a psychiatric hospital or psychiatrist.
Occupation was defined as the primary job which takes at least 50% of the working hours in a week. (what is your primary job which accounts for more than 50% of your working time?).
Use of all forms of tobacco (do you smoke cigarette, use snuff or chew tobacco?).
Drinking more than 14 units of alcohol per week for women and 21 for men [20].
An artisan was defined as skilled manual laborers such as masons, mechanics, tailors, welders and other crafts.
Alcohol use and quantity was defined as (mean quantity) the consumption of any alcoholic beverage (beer, gin, stout, local brew) in a week.
6.2. Social habits and medical history
The sex distribution of tobacco (13.5%) and alcohol use (1.6%) is shown in Table 1. Sixty four (1.5%) reported a history of head injury with loss of consciousness. The proportion of people who had febrile seizures, stroke and mental illness are shown in Table 1. The commonest comorbidity was hypertension (5.7%).
7. Epilepsy
A total of 306 (3.7%) individuals reported a lifetime history of at least one convulsion. The distribution of lifetime prevalence of convulsions is shown in Table 2. It peaked at ≥50 years. High rates were reported among people with a, history of psychiatric treatment (mental illness) (49.2%) and in those with family history of convulsions (36.7%).
Table 2.
Distribution of epilepsy and lifetime prevalence of convulsions.
| Lifetime convulsions N (%) |
95% CI | Epilepsy N (%) |
95% CI | |
|---|---|---|---|---|
| Age groups (years) | ||||
| 15–19 | 39 (3.2) | 2.21–4.19 | 4 (3.3) | 3.21–3.34 |
| 20–24 | 40 (2.7) | 1.88–3.52 | 5 (3.3) | 3.27–3.42 |
| 25–29 | 41 (3.1) | 2.16–4.04 | 8 (6.1) | 6.01–6.25 |
| 30–34 | 28 (2.7) | 1.71–3.69 | 5 (4.9) | 4.75–5.02 |
| 35–39 | 39 (4.8) | 3.33–6.27 | 9 (11.1) | 10.87–11.38 |
| 40–44 | 28 (3.5) | 2.23–4.77 | 9 (11.2) | 10.94–11.45 |
| 45–49 | 17 (3.6) | 1.92–5.28 | 2 (4.2) | 3.95–4.49 |
| ≥50 | 74 (6.7) | 5.23–8.17 | 7 (6.3) | 6.19–6.47 |
| Social habits | ||||
| Use tobacco* | 67 (9.2) | 7.1–11.3 | 9 (12.4) | 12.10–12.70 |
| †† Alcohol** | 2 (1.6) | −0.62–3.82 | – | – |
| a Medical comorbidities | ||||
| Head injury with LOC | 29 (20.9) | 14.14–27.66 | 4 (28.8) | 26.42–31.13 |
| c Mental illness | 29 (49.2) | 36.44–61.96 | 5 (84.7) | 75.49–94.00 |
| b Hypertension | 70 (12.3) | 14.99–9.61 | 11 (19.3) | 18.79–19.74 |
| Diabetes | 31 (9.7) | 6.46–12.94 | 11 (34.3) | 33.16–35.39 |
| Stroke | 23 (28) | 37.72–18.28 | 9 (109.8)1 | 102.28–117.23 |
| Family history of convulsions | 40 (36.7) | 27.65–45.75 | 10 (91.7) | 86.56–96.93 |
| Total | 306 (3.7) | 3.29–4.11 | 49 (5.96) | 5.94–5.97 |
Previous hospital diagnosis done by a doctor.
History of hypertension, use of antihypertensive.
Receiving or received treatment from a psychiatric hospital or psychiatrist.
Use of all forms of tobacco (Do you smoke cigarette, use snuff or chew tobacco?)
Drinking more than 14 units of alcohol per week for women and 21 for men [20].
Alcohol use and quantity was defined as (mean quantity) the consumption of any alcoholic beverage (beer, gin, stout, local brew) in a week.
The prevalence of epilepsy was 6.0 (95% CI: 5.9–6.0) per 1000; similar in men (4.4/1000, 95% CI: 2.3–6.4) and women (7.8/1000, 95% CI: 4.9–10.4), p = 0.06, Table 2. The male to female ratio was 0.6:1. The age and sex gender-specific rates are shown in Table 3. The prevalence of epilepsy increased with age reaching 11.2 per 1000 at 40–44 years with two smaller peaks at 25–29 and ≥50 years. The peak age specific prevalence for men (9.7 per 1000, 95% CI: 9.2–10.1) was 35–39 years and 40–44 years for females (17.9 per 1000, 95% CI: 17.1–18.63). The age and sex adjusted prevalence using WHO standard population and Enugu East 2006 population were 5.9 per 1000 (95% CI: 4.0–7.9) and 5.4 per 1000 (95% CI: 3.4–7.4) (Table 4). The sex adjusted prevalence is shown in Table 5. The sex adjusted prevalence in women at 8.0 (95% CI: 4.6–11.7) per 1000 was almost twice that of men (3.9 (95% CI: 1.6–6.3) per 1000).
Table 3.
Age and sex distribution of epilepsy.
| Age group | Epilepsy Males (N %, 95% CI) |
Epilepsy Females (N %, 95% CI) |
p-value† |
|---|---|---|---|
| 15–19 | 2 (3.6, 3.43–3.87) | 2 (3, 2.85–3.18) | 0.848 |
| 20–24 | 3 (4.3, 4.08–4.44) | 2 (2.5, 2.40–2.65) | 0.561 |
| 25–29 | 1 (1.6, 1.49–1.74) | 7 (10.2, 9.92–1.05) | 0.072 |
| 30–34 | 2 (4, 3.77–4.27) | 3 (5.7, 5.42–5.98) | 1.000 |
| 35–39 | 4 (9.7, 9.2–10.12) | 5 (12.7, 12.10–13.21) | 0.685†† |
| 40–44 | 3 (6.4, 6.08–6.74) | 6 (17.9, 17.08–18.63) | 0.119 |
| 45–49 | 1 (3.8, 3.38–4.31) | 1 (4.7, 4.05–5.30) | 0.700 |
| ≥50 | 2 (3.2, 3.00–3.35) | 5 (10.5, 10.08–10.92) | 0.128 |
| Total | 18 (4.4, 2.3–6.4) | 31 (7.8, 4.9–10.2) | 0.057 |
McNemar’s test.
Chi squared test.
Table 4.
Age adjusted prevalence of epilepsy (%).
| Crude prev (%) | ES* weighted population | ES* expected cases | Adjusted prevalence (%) | WHO weighted population | WHO expected cases | Adjusted prevalence (%) | |
|---|---|---|---|---|---|---|---|
| <15 | – | – | – | ||||
| 15–19 | 3.6 | 973 | 3 | 3.1 (−0.4–6.6) | 697 | 2 | 2.7 (−1.1–6.8) |
| 20–24 | 4.3 | 931 | 3 | 3.2 (−0.4–6.9) | 676 | 2 | 3.0 (−1.1–7.1) |
| 25–29 | 1.6 | 723 | 4 | 5.5 (0.1–10.9) | 652 | 4 | 6.1 (0.1–12.1) |
| 30–34 | 4.3 | 531 | 3 | 5.6 (−0.7–2.0) | 626 | 3 | 4.8 (−0.6–10.2) |
| 35–39 | 9.7 | 455 | 5 | 11.0 (1.4–20.6) | 588 | 6 | 10.2 (2.1–18.3) |
| 40–44 | 6.4 | 392 | 4 | 10.2 (0.3–20.2) | 542 | 6 | 11.1 (2.3–19.9) |
| 45–49 | 3.8 | 350 | 1 | 2.9 (−2.7–8.4) | 497 | 2 | 4.0 (−1.5–9.6) |
| ≥50 | 3.2 | 861 | 5 | 5.8 (0.7–10.9) | 1796 | 11 | 6.1 (2.5–9.7) |
| Total | 6.0 | 5216 | 28 | 5.4 (3.4–7.4) | 6074 | 36 | 5.9 (4.0–7.9) |
Table 5.
Sex adjusted prevalence of Epilepsy (%).
| Males | Crude prev. (%) | ES* weighted population | ES* Expected cases | Adjusted prevalence (%) | Females Crude prev. (%) | ES weighted population | ES Expected cases | Adjusted prevalence (%) |
|---|---|---|---|---|---|---|---|---|
| 15–19 | 3.6 | 454 | 2 | 4.4 (−1.7–10.5) | 3.2 | 450 | 1 | 2.2 (−2.1–6.6) |
| 20–24 | 4.3 | 421 | 2 | 4.8 (−1.8–11.3) | 2.5 | 417 | 1 | 2.4 (−2.3–7.1) |
| 25–29 | 1.6 | 326 | 1 | 3.1 (−2.9–9.1) | 10.2 | 323 | 3 | 9.2 (−1.2–19.7) |
| 30–34 | 4.3 | 232 | 1 | 4.3 (−4.1–12.7) | 5.7 | 229 | 1 | 4.3 (−4.2–12.9) |
| 35–39 | 9.7 | 216 | 2 | 9.2 (−3.5–22.0) | 12.7 | 214 | 3 | 14.0 (−1.7–29.8) |
| 40–44 | 6.4 | 209 | 1 | 4.9 (−4.6–14.1) | 17.9 | 207 | 4 | 19.3 (0.6–38.1) |
| 45–49 | 3.8 | 213 | 1 | 4.7 (−4.5–13.9) | 4.7 | 211 | 1 | 4.7 (−4.5–14.0) |
| ≥50 | 3.2 | 722 | 2 | 2.8 (−1.1–6.6) | 10.5 | 715 | 8 | 11.1 (3.5–18.9) |
| Total | 4.4 | 2793 | 11 | 3.9 (1.6–6.3) | 7.8 | 2767 | 22 | 8.0 (4.6–11.7) |
Forty three (87.8%) were known epilepsy patients while 6 were newly diagnosed. All the previously diagnosed cases were also on treatment. Newly diagnosed cases were referred to the state hospital for follow-up. EEG was ordered for all the subjects however none attended for the investigation. Brain CT scans were not performed. Based on history alone focal onset seizures were identified in 39 (79.6%) of 49 cases, generalized onset seizures in 10 (20.4%) cases.
8. Discussion
This is a cross-sectional descriptive study of the prevalence of active epilepsy in an urban slum in South East Nigeria. Our set hypothesis was that we would see a high prevalence of epilepsy in a setting with poor public health indicators.
The age-standardized prevalence of active epilepsy in adults based on WHO standard population and Enugu East 2006 census (6/1000 (95% CI: 4.0–7.9) and 5.4/1000 (95% CI: 3.4–7.4) respectively) and the crude prevalence rate of 6.0/1000 (95% CI: 5.9–6.0), are considerably lower than the median prevalence of 15/1000 previously reported from SSA [14] but slightly higher than 4.3/1000 (95% CI: CI 2.7–5.9) reported in the same region [3]. An earlier study using the same instrument in South West Nigeria reported a prevalence rate of 5.3/1000 similar to the index study [15].
Reported prevalence rates from community based studies in SSA are variable [2,14]. Among a population of 586,607 residents in a study covering five countries the prevalence of epilepsy varied between sites and ranged from 7.8 to 14.8 per 1000, much higher than reported in the index study [16]. Hence, epilepsy in Africa is better reviewed on a regional than continental basis as risk factors vary within and between ethnic entities because of differences in cultural, religious and economic practices. The wide variation in the frequency of epilepsy in the continent could also arise from the use of different definitions of epilepsy, the nature of epilepsy studied as well as differences in the samples of population studied. Other factors may include treatment gap [2,3,17] which may bring a selective survival bias, stigmatization and urban rural drift [18,19].
The age distribution of active epilepsy in this study reveals an older peak than has been reported in Nigeria [3,8,9] and other developing countries [20–22]. Active epilepsy increased with age and peaked at 35–44 age group; a distribution which overlaps with the overall pattern in the continent where active convulsive epilepsy peaks in the 20–29 age group and the 40–49 age group [2]. This second peak corresponds to our finding and may reflect the effects of trauma, HIV, central nervous system infections and stroke. The age-specific prevalence of epilepsy identified may suggest a unique underlying cause for the high-rates of epilepsy seen in this area. A total of 306 (3.7%) individuals reported a lifetime history of at least one convulsion which may reflect the high prevalence of epilepsy risk factors. We are not aware of any study in the continent that has focused on the lifetime prevalence of convulsions.
Among the epilepsy cases identified in this survey, females predominated overall although the difference was not statistically significant. This is surprising, especially in light of more rigorous screening applied in the study. Some explanation may be plausible: women and older individuals (with or without epilepsy) are more likely to be at home during the study. Further, women are known to have a better health seeking behavior than men hence are more likely to present themselves for interview.
This finding was similar to some studies from within the continent and different from others [3,8,9,21–24]. Some studies have reported higher prevalence of epilepsy in women [24,25]. Similar to our index study, no gender differences were identified in North Africa [26] and Nigeria [9]. It has been argued that these gender differences may represent different aetiologies and gender-dependent risk factors or maybe simply an artifact of case ascertainment or competing mortality risks [23]. The reversal of the male to female trend after 24 years is similar to findings from Zambia [23]. This trend occurring after childbearing age is intriguing and may offer a clue as to the underlying etiology of epilepsy in the region. Repeated cases of eclampsia may be wrongly interpreted as epilepsy especially among previously diagnosed individuals.
In our study 109 (1.3%) of the population studied and 10 (20.4%) of individuals with epilepsy reported a family history of convulsion. In Sub-Saharan Africa, on average, a family history of epilepsy was noted in 6%–60% [14]. Consanguineous marriage is forbidden by culture in South East Nigeria however, stigmatization and marginalization of patients with epilepsy may potentially force them to intermarry thereby expanding the patient pool by promoting the genetic transmission of epilepsy.
This study provided estimates of epilepsy prevalence for an urban slum – an area with no previously available population-based epilepsy data. If our estimate is applied to all of Enugu East (total population 145,905), then close to 861 people aged 15 years and above with active epilepsy presently reside in Local government with most occurring in individuals 35–44 years. In this study non Igbo speaking individuals and children younger than 15 years were excluded. The exclusion of younger children is likely to underestimate the overall burden of epilepsy and especially lifetime prevalence of convulsions. The impact of excluding non Igbos in this study is difficult to quantify. The prevalence of epilepsy among them is not likely to be higher based on local experiences from epilepsy clinics in the city. It is important however to note that most rural to urban migration are more likely to end in slums such as areas studied.
The diagnosis of epilepsy or seizures is usually prompted by symptoms noticed by the patient or their care givers. This is particularly a problem in focal seizures and absence seizures and other rare forms of epilepsy. On many occasions a diagnosis is only made in retrospect following presentation with a generalized seizure or after many years [27,28]. In SSA, cultural and religious beliefs make this problem even more acute and such patients may be taken to the psychiatrists, herbal homes and prayer houses.
On the other hand a descriptive diagnosis of epilepsy may lead to over diagnosis with the most common differential diagnoses being dissociative seizures and syncope [29]. With the dearth of qualified specialists in SSA the overall likelihood of misdiagnosis is likely to be much higher. The combined use of EEG and clinical data allows the reclassification of some cases of clinically diagnosed generalized seizures to focal seizures evolving to a bilateral convulsive seizure [24] suggesting a possibility of even higher rates of focal seizures in the study. None of our subjects had an EEG.
The overall treatment gap of 12.2% (n = 6/49) found in our study was much lower than of 76% found in the region [3]. There is a dramatic disparity in the treatment gap in SSA countries [30] as well as between rural and urban settings. A low treatment gap of 23% has been reported in Senegal [31]. Like in Senegal where a low treatment gap was attributed to the presence of a nearby Senegalese League Against Epilepsy, the Nigerian League Against Epilepsy is based in Enugu from where it carries most of its public awareness programs. This finding therefore supports the usefulness of such organizations in epilepsy care.
A number of limitations can be highlighted with regard to this study. The instrument used could detect equally both generalized tonic clonic seizures and focal seizures evolving to convulsive seizures. Nevertheless, individuals with dyscognitive or myoclonic seziures may probably be under recognized. The use of a 2-stage interview reduced this to the barest minimum. Patients may not be aware of the nature of attacks hence our classification of seizures may not be very accurate.
Epilepsy-associated stigma may result in a reluctance to admit the disorder resulting in further underestimation of the number of prevalent cases. The number of men with epilepsy might have been underestimated because they were more likely to be out during the day. Children less than 15 years (with higher burden of epilepsy) and non Igbo speaking subjects were excluded. The analysis of antiepileptic drug use was restricted to the current therapy which were verifiable hence our estimate of treatment gap may even be lower.
9. Conclusions
This survey demonstrates that active epilepsy is common in a two Urban Slums in Enugu South East Nigeria. The prevalence of epilepsy increased with age reaching 11.2 per 1000 at 40–44 years with two smaller peaks at 25–29 and ≥50 years and was higher among individuals with medical comorbidities. Nationwide studies should be performed to find out the true prevalence in the country and the possible role of genetics in subjects with a family history of epilepsy.
Acknowledgments
The author would like to acknowledge the leaders of Agu-Abor and Ugbodogwu communities for their support, Miss Loveth Emmanuel for her help in the office and Prof. Helen Cross for proof reading and correcting the manuscript.
The project described was supported by the Medical Education Partnership Initiative in Nigeria (MEPIN) project funded by Fogarty International Center, the Office of AIDS Research, and the National Human Genome Research Institute of the National Institute of Health, the Health Resources and Services Administration (HRSA) and the Office of the U.S. Global AIDS Coordinator under Award Number R24TW008878. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Footnotes
Conflict of interest
All the authors have no conflicts of interest.
References
- 1.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a metaanalytic approach. Epilepsia. 2010;51(5):883–90. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul A, Adeloye D, George-Carey R, Kolčlć I, Grant L, Chan KY. An estimate of the prevalence of epilepsy in sub–Saharan Africa: a systematic analysis. J Glob Health. 2012;2(2):1–13. doi: 10.7189/jogh.02.020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nwani PO, Nwosu MC, Enwereji KO, Asomugha AL, Arinzechi EO, Ogunniyi AO. Epilepsy treatment gap: prevalence and associated factors in Southeast Nigeria. Acta Neurol Scand. 2013;128:83–90. doi: 10.1111/ane.12096. [DOI] [PubMed] [Google Scholar]
- 4.Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes and intervention strategies. Epilepsia. 2008;49(9):1491–503. doi: 10.1111/j.1528-1167.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pion SDS, Kaiser C, Boutros-Toni F, Cournil A, Taylor MM, Meredith SEO, et al. Epilepsy in onchocerciasis endemic areas: systematic review and meta-analysis of population-based surveys. PLoS Negl Trop Dis. 2009;3:e461. doi: 10.1371/journal.pntd.0000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezeala-Adikaibe BA, Achor JU, Onwukwe J, Ekenze OS, Onwuekwen IO, Chukwu OH, et al. Attitude and practice towards epilepsy among secondary school students in Enugu, South East Nigeria. Seizure. 2013;22:299–302. doi: 10.1016/j.seizure.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Ezeala-Adikaibe BA, Achor JU, Nwabueze AC, Agomoh AO, Chikani M, Ekenze OS, et al. Knowledge, attitude and practice of epilepsy among community residents in Enugu, South East Nigeria. Seizure. 2014;23:882–3. doi: 10.1016/j.seizure.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Longe AC, Osuntokun BO. Prevalence of neurological disorders in Udo, a rural community in Southern Nigeria. Trop Geog Med. 1989;41(1):36–40. [PubMed] [Google Scholar]
- 9.Osuntokun BO, Schoenberg BS, Nottidge VA, Adeuja A, Kale O, Adeyefa A, et al. Research instrument for measuring the prevalence of neurologic disorders in developing countries Results of a pilot study in Nigeria. Neuroepidemiology. 1982;1:143–53. [Google Scholar]
- 10.Federal Republic of Nigeria 2006 Population and Housing Census. Population distribution by age and sex (state and local government area) Nigeria: National Population Commission Abuja; Apr, 2010. [Google Scholar]
- 11.Lwanga SK, Lemeshow S. Sample size determination in health studies: a practical manual. Geneva: World Health Organization; 1991. [Google Scholar]
- 12.WHO: WHO. STEPwise approach to chronic disease risk factor surveillance (STEPS) Geneva, Switzerland: WHO; 2005. [Google Scholar]
- 13.ICAP Reports 13. International Center for Alcohol Policies; 2003. Available at: http://www.icap.org [accessed 29.04.14] [Google Scholar]
- 14.Preux PM, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in Sub-Saharan Africa. Lancet Neurol. 2005;4:21–31. doi: 10.1016/S1474-4422(04)00963-9. [DOI] [PubMed] [Google Scholar]
- 15.Osuntokun BO, Adeuja AOG, Nottidge VA, Bademosi O, Schoenberg BS, Olumide A, et al. Prevalence of the epilepsies in Nigerian Africans: a community-based study. Epilepsia. 1987;28(3):272–9. doi: 10.1111/j.1528-1157.1987.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 16.Ngugi AL, Bottomley C, Kleinschmidt I, Wagner RG, Kakooza-Mwesige A, Ae-Ngibise K, et al. For the SEEDS Group Prevalence of active convulsive epilepsy in Sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet Neurol. 2013;12:253–63. doi: 10.1016/S1474-4422(13)70003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yemadje LP, Houinato D, Quet F, Druet-Cabanac M, Preux P. Understanding the differences in prevalence of epilepsy in tropical regions. Epilepsia. 2011;52(8):1376–81. doi: 10.1111/j.1528-1167.2011.03099.x. [DOI] [PubMed] [Google Scholar]
- 18.de Boer HM, Mula M, Sander JW. The global burden and stigma of epilepsy. Epilepsy Behav. 2008;12:540–6. doi: 10.1016/j.yebeh.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Baskind R, Birbeck GL. Epilepsy-associated stigma in Sub-Saharan Africa. The social landscape of a disease. Epilepsy Behav. 2005;7:68–73. doi: 10.1016/j.yebeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Rwiza HT, Kilonzo GP, Haule J, Matuja WB, Mteza I, Mbena P, et al. Prevalence and incidence of epilepsy in Ulanga, a rural Tanzanian district: a community-based study. Epilepsia. 1992;33(6):1051–6. doi: 10.1111/j.1528-1157.1992.tb01758.x. [DOI] [PubMed] [Google Scholar]
- 21.Tekle-Haimanot R, Forsgren L, Abebe M, Gebre-Mariam A, Heijbel J, Holmgren G, et al. Clinical and electroencephalographic characteristics of epilepsy in rural Ethiopia: a community-based study. Epilepsy Res. 1990;7:230–9. doi: 10.1016/0920-1211(90)90020-v. [DOI] [PubMed] [Google Scholar]
- 22.Kaboré J, Lengani A, Drabo YJ, Melaku Z, Preux PM, Ndiaye IP, et al. Clinical aspects of seizure disorders at Ouagadougou – Burkina Faso: retrospective study of 532 cases. Afr J Neurol Sci. 1995;14:24–6. [Google Scholar]
- 23.Birbeck G, Kalichi E. A door-to-door survey to determine the prevalence of epilepsy in rural Zambia. Trop Med Int Health. 2003;5(Suppl 1):211–3. doi: 10.1046/j.1365-3156.2003.01149.x. [DOI] [PubMed] [Google Scholar]
- 24.Nicoletti A, Reggio A, Bartoloni A, Failla G, Sofia V, Bartalesi F, et al. Prevalence of epilepsy in rural Bolivia: a door-to-door survey. Neurology. 1999;53:2064–9. doi: 10.1212/wnl.53.9.2064. [DOI] [PubMed] [Google Scholar]
- 25.Garcia HH, Gilman R, Martinez M, Garcia HH, Tsang VC, Pilcher JB, et al. Cysticercosis as a major cause of epilepsy in Peru. The Cysticercosis Working Group in Peru (CWG) Lancet. 1993;341:197–200. doi: 10.1016/0140-6736(93)90064-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attia-Romdhane N, Mrabet A, Ben Hamida M. Prevalence of epilepsy in Kelibia, Tunisia. Epilepsia. 1993;34:1028–32. doi: 10.1111/j.1528-1157.1993.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 27.Hauser WA, Kurdland LT. The epidemiology of epilepsy in Rochester, Minne-sota, 1935 through 1967. Epilepsia. 1975;16:1–66. doi: 10.1111/j.1528-1157.1975.tb04721.x. [DOI] [PubMed] [Google Scholar]
- 28.Sander JW, Hart YM, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: newly diagnosed epileptic seizures in a general population. Lancet. 1990;336:1267–71. doi: 10.1016/0140-6736(90)92959-l. [DOI] [PubMed] [Google Scholar]
- 29.Smith D, Defalla BA, Chadwick DW. The misdiagnosis of epilepsy and the management of refractory epilepsy in a specialist clinic. Q J Med. 1999;92:15–23. doi: 10.1093/qjmed/92.1.15. [DOI] [PubMed] [Google Scholar]
- 30.Meyer A, Dua T, Ma J, Saxena S, Birbeck G. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ. 2010;88:260–6. doi: 10.2471/BLT.09.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndoye NF, Sow AD, Diop AG, Sessouma B, Sene-Diouf F, Boissy L, et al. Prevalence of epilepsy its treatment gap and knowledge, attitude and practice of its population in sub-urban Senegal an ILAE/IBE/WHO study. Seizure. 2005;14:106–11. doi: 10.1016/j.seizure.2004.11.003. [DOI] [PubMed] [Google Scholar]


