Abstract
Western-blot analysis and protein kinase assays identified two Ca2+-dependent protein kinases (CDPKs) of 55 to 60 kD in soluble protein extracts of embryogenic cultures of sandalwood (Santalum album L.). However, these sandalwood CDPKs (swCDPKs) were absent in plantlets regenerated from somatic embryos. swCDPKs exhibited differential expression (monitored at the level of the protein) and activity in different developmental stages. Zygotic embryos, seedlings, and endosperm showed high accumulation of swCDPK, but the enzyme was not detected in the soluble proteins of shoots and flowers. swCDPK exhibited a temporal pattern of expression in endosperm, showing high accumulation and activity in mature fruit and germinating stages; the enzyme was localized strongly in the storage bodies of the endosperm cells. The study also reports for the first time to our knowledge a post-translational inhibition/inactivation of swCDPK in zygotic embryos during seed dormancy and early stages of germination. The temporal expression of swCDPK during somatic/zygotic embryogenesis, seed maturation, and germination suggests involvement of the enzyme in these developmental processes.
Transient cytosolic influxes of Ca2+ are involved in plant responses to various environmental stimuli (Hepler and Wayne, 1985; Bush, 1995; Trewavas, 1997). These changes in the intracellular Ca2+ concentration are perceived by Ca2+-binding proteins, which in turn activate signaling cascades involving protein kinases and protein phosphatases (Poovaiah and Reddy, 1993).
A novel family of Ca2+-dependent/calmodulin (CaM)-independent protein kinases (CDPKs) was first characterized from soybean (Harmon et al., 1986), and later from a wide variety of plant species (Roberts, 1993). Unlike Ca2+/CaM-dependent protein kinases, CDPKs are activated by the direct binding of Ca2+(Harper et al., 1991). CDPKs have a C-terminal CaM-like elongation factor-hand motif enabling Ca2+ binding that leads to conformational changes and activation of the N-terminal kinase domain. They have been implicated to play a role during tuberization of potato (MacIntosh et al., 1996) and during the gibberellic acid response in barley aleurone (Ritchie and Gilroy, 1998). Expression of some CDPKs is induced by physical stress, by salt stress, by CaCl2 in mung bean (Botella et al., 1996), by osmotic stress in sorghum (Pestenacz and Erdei, 1996), and by phytohormones, methyl jasmonate, wounding, fungal elicitors, chitosan, and NaCl in tobacco leaves (Yoon et al., 1999). These observations give CDPKs credibility as key intermediates in Ca2+-mediated signaling in plants.
Little is known about signaling processes involved during embryogenesis, seed development, and germination. One of the major obstacles in understanding the cascade of events regulating zygotic embryogenesis is the relative inaccessibility of the embryos within the developing seeds. Somatic embryogenesis is an alternative system that overcomes this problem (Reinert, 1958; Steward et al., 1958). This study examines the CDPK activity and the probable involvement of CDPKs during somatic embryogenesis of sandalwood (Santalum album L.). The identification of two soluble CDPKs in embryogenic cultures of sandalwood but not in the regenerated plantlets prompted investigation into the possible differential expression of the enzyme in the course of the development of sandalwood trees. The observations show a spatio-temporal accumulation of sandalwood CDPK (swCDPK) only during somatic/zygotic embryogenesis, endosperm development, and seed germination, indicating the involvement of the enzyme in these developmental processes. This study also provides evidence for a post-translational inhibition/inactivation of swCDPK in zygotic embryos during seed dormancy and early seed germination.
MATERIALS AND METHODS
Plant Material
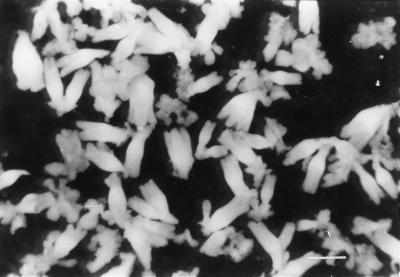
Endosperm of 4-week-old sandalwood (Santalum album L.) fruits was cultured on Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) supplemented with 1 mg L−1 each of 6-benzylaminopurine (BAP) and 2,4-dichlorophenoxyacetic acid (2,4-D) for callus initiation. The primary callus was separated from the explant and further cultured on MS medium containing 1 mg L−1 2,4-D for proliferation and embryogenic induction. The proliferated callus was dispersed in liquid MS medium and sieved through meshes of pore size 130, 80, and 50 μm sequentially to collect and enrich the embryogenic cell clumps called pro-embryogenic masses (PEMs) on the 50-μm mesh. PEMs were resuspended in growth-regulator-free liquid MS medium containing 2% (w/v) mannitol to induce embryo development. The suspension culture was agitated on an orbital shaker and incubated under diffuse light conditions of 5 μE m−2 s−1 at 26°C ± 2°C. PEMs and globular stage embryos were collected for protein analysis and immunohistochemistry by differential sieving of embryogenic cultures. Torpedo and cotyledonary stage embryos were harvested on the 21st d of culture growth in the differentiation medium (Fig. 1).
Figure 1.
Torpedo-stage somatic embryos of sandalwood harvested at d 21 of culture incubation. Scale bar = 7.5 mm.
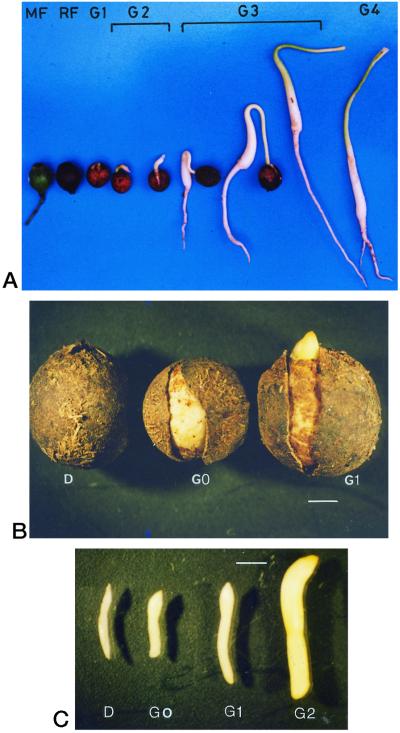
Zygotic embryos were dissected from well-developed fruits of sandalwood. Flowers, mature shoots, and fruits for endosperm were collected from sandalwood trees growing on the campus of Indian Institute of Science (Bangalore, India). Seeds were germinated in the greenhouse on a sand bed. Fruits, seeds, and seedlings were harvested at different developmental stages: mature fruit (MF), ripe fruit (RF), dormant seed (D), imbibed seed (G0), the stage of germination wherein the radicle has just emerged, breaking open the stony false seed coat (G1), the stage of germination wherein the radicle has elongated to 5 mm (G2), the stage of germination showing the hook-like configuration of the seedling hypocotyl (G3), and the stage of germination in which the seedling is erect with cotyledons exposed and approximately 5 cm in length (G4) (Fig. 2, A and B). Figure 2C shows zygotic embryos dissected from seeds of the D, G0, G1, and G2 stages. The zygotic embryo of mature fruit is comparable to the torpedo-stage somatic embryo, and the zygotic embryo of stages G0 and G1, in which the radicle has started to elongate, is comparable to the cotyledonary-stage somatic embryo.
Figure 2.
Stages of seed development and germination in sandalwood. Scale bar = 2 mm in B and C.
Chemicals
Histone III-S, Chelex 100, fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG, and all other chemicals used were purchased from Sigma Chemical (St. Louis) unless mentioned otherwise. Bovine serum albumin (BSA) fraction V was obtained from Boehringer Mannheim (Basel). Horseradish peroxidase-conjugated goat anti-rabbit IgG was purchased from Bangalore Genie (Bangalore, India). Radioactive isotope γ-32P-ATP was obtained from BRIT (Hyderabad, India).
Protein Extraction
Tissues from different developmental stages of sandalwood were frozen in liquid nitrogen and homogenized using a mortar and pestle. The homogenate was then suspended in extraction buffer (20 mm Tris[hydroxymethyl]-aminomethane [Tris], pH 7.2, 2.5 mm EDTA, and 1 mm PMSF) and held on ice for 15 min. The crude protein extracts were centrifuged at 13,650g at 4°C for 30 min. The pellet was discarded and the supernatant containing the soluble proteins was used for further experiments. Protein concentration was determined by the method of Bradford (1976) using BSA as standard.
Detection of Substrate Phosphorylation Activity in SDS-Polyacrylamide Gels
In-gel phosphorylation of histone III-S was carried out according to the method described by McCurdy and Harmon (1992). SDS-polyacrylamide gels (10%, w/v) were polymerized in the presence of 0.5 mg mL−1 histone III-S. Soluble proteins from torpedo-stage somatic embryos were incubated in Laemmli's sample buffer (Laemmli, 1970) at 70°C for 2 min. The proteins were then resolved on the gel at 45 V overnight. The gel was washed with six changes of 50 mm 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 7.2) for a period of 6 h to renature the proteins. The gel was then cut into strips, each containing the sandalwood extract. The protein kinase activity in the presence and absence of Ca2+ was detected following the method of Harmon et al. (1986). The gel strips were washed to remove unreacted γ-32P-ATP as described by Geahlen et al. (1986), except that 50 mm HEPES, pH 7.2, containing Dowex 2 × 8–50 anion-exchange resin was used with repeated changes for 24 to 48 h.
In Vitro CDPK Assays
Substrate Phosphorylation Assay
Protein kinase activity was determined by measuring the incorporation of 32P from (γ-32P) ATP into the in vitro substrate histone III-S. In a total volume of 0.15 mL, the assay mixture contained 1 mg mL−1 histone III-S, Ca2+/EGTA buffer (50 mm HEPES, pH 7.2, 10 mm MgCl2, and 0.25 mm EGTA) with or without 0.2 mm CaCl2 and 10 μg of the protein sample. The reaction was initiated with the addition of 10 nm γ-32P-ATP (5,000 nCi/pmol). Termination of the reaction, spotting of the mixture on glass fiber filters, and washing of the filters were carried out according to the method of Putnam-Evans et al. (1990). Counts were recorded on a liquid scintillation counter (LKB, Uppsala). This assay was also performed with varying concentrations of Ca2+ in the assay mixture.
Autophosphorylation Assay
Soluble protein extracts were also assayed to determine Ca2+-dependent autophosphorylation and/or endogenous substrate phosphorylation activities. The reaction was carried out in the absence of histone III-S in a reaction volume of 50 μL containing Ca2+/EGTA buffer with or without 0.2 mm CaCl2 and incubated for 20 min at room temperature. The reaction was terminated by boiling for 2 min in Laemmli's sample buffer. Proteins were resolved on a 10% (w/v) SDS-polyacrylamide gel at 45 V overnight. The gels were dried and exposed to x-ray film (Eastman-Kodak, Rochester, NY) for 24 h. This assay was repeated with various concentrations of MgCl2, CaCl2, CaM, and W7 to determine their effect on a Ca2+-dependent autophosphorylation activity in the soluble protein extracts of sandalwood.
All reagents for the assays were prepared in deionized water that had been passed through a column of Chelex 100 to chelate contaminating Ca2+. The substrate histone III-S was dialyzed extensively against deionized water before use.
Immunodetection of swCDPK Using Polyclonal Anti-Soybean CDPK
Soluble protein extracts from different developmental stages of sandalwood were resolved on a 10% (w/v) SDS-polyacrylamide gel as described by Laemmli (1970). The proteins were transferred to nitrocellulose membrane following standard conditions (Towbin et al., 1979). The blot was blocked for 1 h with rinse buffer (1× PBS, pH 7.4, and 0.05% [v/v] Tween 20) containing 1% (w/v) BSA and then incubated with polyclonal antibodies directed against the CaM-like domain of soybean CDPK (Bachmann et al., 1996) at a concentration of 15 μg mL−1 in dilution buffer (1× PBS, pH 7.4, 0.5% [v/v] Tween 20, and 1% [w/v] BSA) for 3 h. Excess antibodies were removed by washing the blot for 1 h with three changes of rinse buffer. The bound primary antibodies were detected by incubation with horseradish peroxidase conjugated to goat anti-rabbit IgG diluted to 1:1,000 in dilution buffer. The blot was washed again as described above. The bands were visualized by incubation of the blot in citrate buffer, pH 4.8, containing 3,3′-diaminobenzidine and hydrogen peroxide in dark.
Immunofluorescence
Sandalwood tissues were fixed in formaldehyde:acetic acid:70% (v/v) ethanol (5:5:90), dehydrated in an ethanol series followed by infiltration with paraffin in n-butanol, and microtome sectioned. The sections were mounted on glass slides, deparaffinated with xylene, and rehydrated. They were then incubated in rinse buffer (1× PBS and 0.05% [v/v] Tween 20) for 15 min and blocked in rinse buffer containing 1% (w/v) BSA and 10% (v/v) normal goat serum for 1 h to minimize non-specific staining. The slides were placed in a humidified chamber and sections were covered with polyclonal anti-soybean CDPK in dilution buffer (1× PBS, 0.5% [v/v] Tween 20, 1% [w/v] BSA, and 10% [v/v] normal goat serum) and incubated at 4°C overnight. Sections incubated in normal rabbit serum instead of the primary antibody represented the negative control in the experiment. All slides were then washed for 30 min with six changes of rinse buffer to remove excess primary antibodies. The sections were further incubated for 2 h in FITC-conjugated goat anti-rabbit IgG diluted in dilution buffer (1:100), washed to remove excess secondary antibodies, mounted in 90% (v/v) glycerol, and observed through a confocal microscope (TCS-MP single-photon imaging system, Leica Microsystems, Wetzlar, Germany).
RESULTS
CDPK Activity in Embryogenic Cultures of Sandalwood
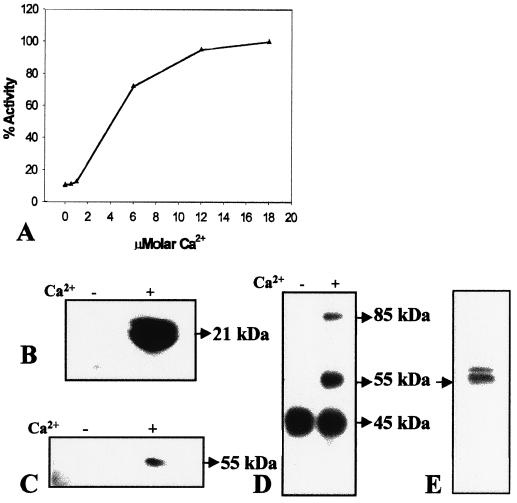
The addition of micromolar concentrations of Ca2+ to the assay buffer resulted in a 6-fold increase in the phosphorylation of the in vitro substrate histone III-S by soluble protein extracts of torpedo-stage somatic embryos (Fig. 3A). Figure 3B shows Ca2+-dependent phosphorylation of histone III-S by soluble protein extracts from torpedo-stage somatic embryos. The in-gel protein kinase assay showed a protein band at 55 kD that phosphorylated histone III-S in a Ca2+-dependent manner, indicating the CaM-independent nature of this enzyme (Fig. 3C). In vitro protein phosphorylation performed in the absence of any exogenous substrate followed by separation on a 10% (w/v) polyacrylamide gel showed Ca2+-dependent phosphorylation of proteins at the 55- and 85-kD regions (Fig. 3D). Phosphorylation of another protein of 45 kD was Ca2+ independent (Fig. 3D). Furthermore, a 55-kD protein and another protein of 55 to 60 kD cross-reacted with anti-soybean CDPK (Fig. 3E), indicating that they are isoforms of swCDPK. A subsequent experiment showed that the phosphorylated 55-kD protein is indeed the protein that cross-reacted with anti-soybean CDPK (Fig. 7, A and B).
Figure 3.
CDPK activity and protein in soluble protein extract of torpedo-stage somatic embryos. A, Effect of micromolar Ca2+ on the phosphorylation of histone III-S. One-hundred percent activity represents a specific activity of 0.48 pmol mg−1 min−1. B, Autoradiogram of an SDS-polyacrylamide gel showing the position of histone III-S phosphorylated in the presence of 0.2 mm (+) or absence (−) of Ca2+ by incubation with protein extracts (10 μg) prior to electrophoresis. C, In-gel histone III-S phosphorylation assay in which 50 μg of protein extract was loaded in each lane. Band on the autoradiogram indicates the position of histone kinase activity on the gel. D, In vitro CDPK assay in the absence of an exogenous substrate followed by separation on 10% (w/v) SDS-polyacrylamide gel. Bands at 55 and 85 kD on the autoradiogram indicate positions of proteins that are phosphorylated in presence of 0.2 mm Ca2+. E, Western-blot analysis of 50 μg of protein using polyclonal anti-soybean CDPK.
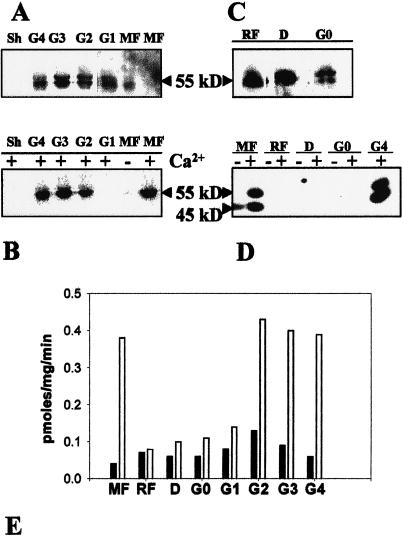
Figure 7.
swCDPK activity and protein in zygotic embryos and seedlings during seed maturation, dormancy, and germination. Proteins from zygotic embryo of MF and germination stages G1, G2, G3, and G4, and from shoot (Sh) of a 1-month-old plantlet were assayed for Ca2+-dependent phosphorylation of swCDPK protein, resolved on 10% (w/v) SDS-polyacrylamide gel, and transferred to nitrocellulose membrane. The proteins on this blot were then subjected to western-blot analysis using anti-soybean CDPK. A, Western blot; B, autophosphorylation of swCDPK. A and B represent the same blot. C, Western-blot analysis of proteins from zygotic embryos of stages RF, D, and G0. D, Autoradiogram of SDS-polyacrylamide gel depicting the autophosphorylation activity of 55-kD swCDPK in proteins of zygotic embryo of MF and G4, but not in zygotic embryo of stages RF, D, and G0. E, Graph representing Ca2+-dependent histone III-S phosphorylation activity in proteins of zygotic embryo/seedling from MF, RF, D, and germination stages G0, G1, G2, G3, and G4. The mean value of five replicates has been plotted. Black bars, 0.0 mm CaCl2; white bars, 0.2 mm CaCl2.
Characterization of swCDPK Phosphorylation
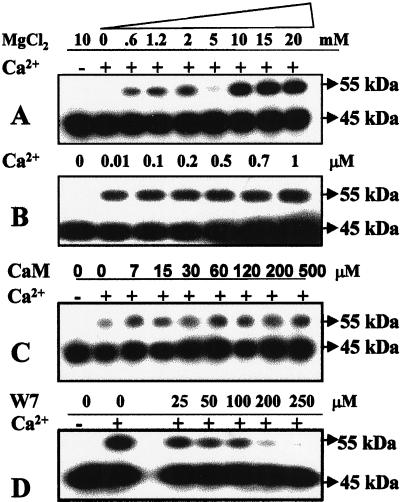
In vitro assays performed in the absence of exogenous substrate showed maximal phosphorylation of swCDPK with millimolar MgCl2 (Fig. 4A) and nanomolar Ca2+ (Fig. 4B) concentrations, indicating the activator role of Ca2+ for this activity. Phosphorylation of swCDPK remained unaffected by increasing concentrations of CaM in the assay buffer (Fig. 4C) and was inhibited by the CaM antagonist W7 (Fig. 4D). These observations indicate that the phosphorylation of the 55-kD protein is brought about by a CDPK, and since this 55-kD protein strongly cross-reacted with anti-soybean CDPK, the phosphorylation of swCDPK in all probability is an autophosphorylation event.
Figure 4.
Effect of Mg2+, Ca2+, CaM, and W7 on the in vitro phosphorylation of swCDPK in the soluble protein extracts of torpedo-stage somatic embryos (20 μg of protein assayed in the absence of exogenous substrate prior to electrophoresis). Assays were performed with various concentrations of MgCl2 (A), Ca2+ (B), CaM (C), and CaM antagonist W7 (D). Experiments B, C, and D were performed in the presence of 10 mm Mg2+, and experiments A, C, and D were performed in the absence or presence of 0.2 mm Ca2+.
Detection of swCDPK in Different Stages of Somatic Embryogenesis
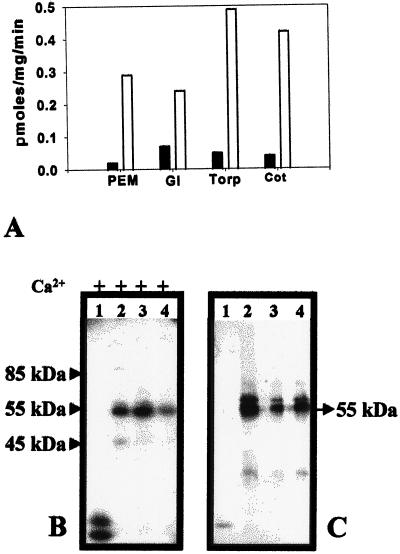
Strong Ca2+-dependent substrate phosphorylation activity was detected in PEMs, globular, torpedo, and cotyledonary stage somatic embryos, with activity being the highest at the torpedo stage (Fig. 5A; Table I). In vitro assays performed in the absence of exogenous substrate followed by separation on 10% (w/v) SDS-polyacrylamide gel resulted in an intensely labeled 55-kD protein band and a faint protein band between 55 and 60 kD in the soluble proteins of PEMs, globular, and torpedo/cotyledonary stages of somatic embryogenesis (Fig. 5B; Table I). However, these phosphorylated bands were not detected in extracts of plantlets regenerated from somatic embryos (Fig. 5B), even after prolonged exposure of the gel to the x-ray film (data not shown). The two phosphorylated 55- to 60-kD proteins (Fig. 5B) cross-reacted strongly with anti-soybean CDPK in all somatic embryogenic stages (Fig. 5C; Table I). swCDPK was not immunodetected in the extracts of plantlets (Fig. 5C).
Figure 5.
swCDPK activity and protein in various stages of somatic embryogenesis. A, Ca2+-dependent phosphorylation of the in vitro substrate histone III-S by soluble proteins of somatic embryogenic stages of sandalwood, PEMs, globular (Gl), torpedo (Torp), and cotyledonary (Cot) stages. The mean value of five replicates has been plotted. Black bars, 0.0 mm CaCl2; white bars, 0.2 mm CaCl2. B, Autoradiogram of 10% (w/v) SDS-polyacrylamide gel showing proteins (10 μg) assayed for in vitro phosphorylation of swCDPK protein in the absence of exogenous substrate prior to electrophoresis. Phosphorylation of the 45-kD protein was observed from the torpedo stage onwards. C, Western-blot analysis of 50-μg protein extracts using polyclonal anti-soybean CDPK. The samples were: lanes 1, regenerated plantlet; lanes 2, torpedo/cotyledonary stage somatic embryos; lanes 3, globular-stage somatic embryos; and lanes 4, PEMs.
Table I.
Summary of the temporal accumulation and activity of swCDPK during developmental stages of sandalwood
| Tissue
|
Embryogenic Cultures
|
Sh | Fl | Endosperm
|
Zygotic Embryo/Seedlings
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stages | PEM | Gl | Torp | Cot | MF | D | G0 | G1 | G2 | G3 | MF | RF | D | G0 | G1 | G2 | G3 | G4 | ||
| Western analysis | + | + | + | + | − | − | + | − | + | +* | +* | + | + | + | + | + | + | + | + | + |
| Immunohistochemistry | NT | +* | + | +* | NT | NT | NT | NT | NT | +* | + | +* | +* | NT | NT | NT | +* | +* | NT | NT |
| Ca2+ dep. auto-phosphorylation | + | + | + | + | − | − | +* | −* | NT | NT | NT | +* | + | − | − | − | − | + | + | + |
| Ca2+ dep. histone phosphorylation (% activity) | 59 | 38 | 100 | 86 | 11 | 12 | 72 | 5 | 18 | 45 | 59 | 84 | 77 | 5 | 9 | 12 | 14 | 90 | 93 | 81 |
Sh, Shoot; Fl, flower; Gl, globular stage; Torp, torpedo stage; Cot, cotyledonary stage. The percentage Ca2+-dependent histone III-S phosphorylation activity has been calculated after subtracting the Ca2+-independent histone III-S phosphorylation in the respective stages. One-hundred percent activity represents a specific activity of 0.44 pmoles mg−1 min−1. NT, Not tested; *, data not shown.
Detection of swCDPK in Other Developmental Stages of Sandalwood
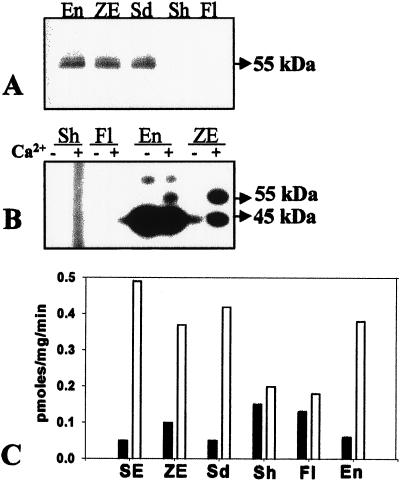
Western-blot analysis of soluble proteins of sandalwood showed the presence of swCDPK in the zygotic embryos, seedlings, and endosperm, but not in shoots and flowers of the mature tree (Fig. 6A). Figure 6B shows the in vitro Ca2+-dependent phosphorylation of swCDPK in the extracts of zygotic embryos and endosperm. However, this activity was not detected in shoots and flowers (Fig. 6B), even with prolonged exposure of the gel to x-ray film. swCDPK was not detected in shoots and flowers, even with rapid extraction of proteins in extraction buffer containing 1 mm PMSF, 25 μg/mL aprotinin, and 10 μg/mL leupeptin (data not shown), thereby ruling out the possibility of proteolysis of the enzyme during the extraction procedure. The Ca2+-dependent phosphorylation activity of the in vitro substrate histone III-S was low in mature shoots and flowers compared with that in the endosperm, zygotic embryos, and germinating seedlings (Fig. 6C). These results (Table I) indicate a temporal pattern of expression of swCDPK during certain developmental processes as embryo/endosperm development and seed germination.
Figure 6.
CDPK activity and protein in various developmental stages of sandalwood. A, Western-blot analysis of soluble proteins of flowers (Fl), shoots (Sh), seedling (Sd), endosperm (En), and zygotic embryo (ZE). B, In vitro Ca2+-dependent phosphorylation assay performed in the absence of exogenous substrate followed by electrophoresis, autoradiogram shows phosphorylated swCDPK in zygotic embryos (ZE) and endosperm (En), but not in shoots (Sh) and flowers (Fl). C, Ca2+-dependent phosphorylation of histone III-S by protein extracts of somatic embryo (SE), zygotic embryo (ZE), seedling (Sd), shoot (Sh), flower (Fl), and endosperm (En). The mean value of five replicates has been plotted.
Differential swCDPK Activity in Zygotic Embryos/Seedlings during Dormancy and Seed Germination
Anti-soybean CDPK cross-reacted with the 55- to 60-kD swCDPK bands in zygotic embryos of MF and in embryos/seedlings of stages G1 to G4 (Fig. 7A). swCDPK was, however, not immunodetected from shoots of a 1-month-old sandalwood plant (Fig. 7A). Phosphorylation of swCDPKs was observed in all extracts in which the enzyme was immunodetected except in the one from zygotic embryos of stage G1 (Fig. 7B). swCDPK was also immunodetected in extracts of embryos from ripe fruit (RF), dormant seed (D), and imbibed seed (G0) (Fig. 7C). However, protein extracts of these stages did not show Ca2+-dependent phosphorylation of swCDPK (Fig. 7D). Ca2+-dependent phosphorylation of the in vitro substrate histone III-S was also negligible during dormancy and the early germination stage G0, and increased as germination progressed (Fig. 7E). These observations (Table I) indicate a probable post-translational inhibition/inactivation of both substrate phosphorylation and autophosphorylation activities of swCDPK in the zygotic embryo during the onset of dormancy, dormancy, and early germination stages of the seed.
Temporal Expression and Activity of swCDPK in the Endosperm
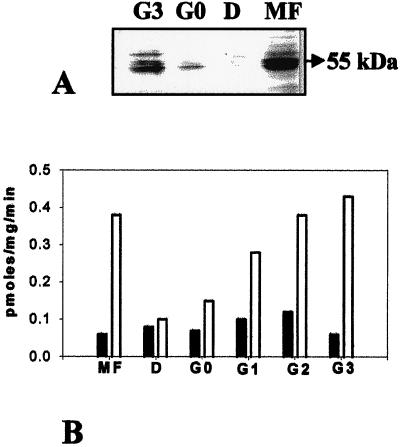
Accumulation of swCDPK and its activity in the endosperm during seed development and germination was examined. Anti-soybean CDPK strongly cross-reacted with the 55- to 60-kD swCDPKs in endosperm extracts of mature and stage G3 fruit (Fig. 8A). swCDPKs were not immunodetected in endosperm of stage D and very faintly at stage G0 (Fig. 8A). Ca2+-dependent in vitro substrate phosphorylation activity was high in the endosperm of mature fruit and stages G2 and G3 (Fig. 8B). A 10-fold reduction in Ca2+-dependent substrate phosphorylation activity was observed in the endosperm of dormant seeds compared with that in the endosperm of G3 (Fig. 8B). Corroboratively, Ca2+-dependent phosphorylation of swCDPK protein was detected in the endosperm of MF and stage G3, but not in the dormant (D) stage (data not shown). The observations (Table I) indicate that although swCDPK is clearly expressed during endosperm maturation, it is absent or undetectable during seed dormancy and re-accumulates with progressive seed germination.
Figure 8.
swCDPK activity and protein in the endosperm. A, Western-blot analysis of proteins of endosperm of MF, D, and germination stages G0 and G3 using anti-soybean CDPK. B, Ca2+-dependent phosphorylation of histone III-S by proteins of endosperm of MF, D, G0, and stages G1, G2, and G3 of germination. Black bars, 0.0 mm CaCl2; white bars, 0.2 mm CaCl2.
Immunofluorescent Localization of swCDPK in Sandalwood
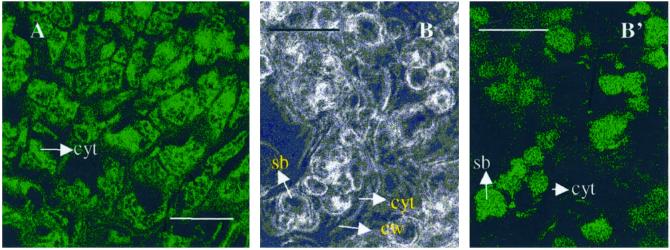
The cells of sandalwood embryos showed strong immunofluorescent localization of swCDPK in their cytoplasm (Fig. 9A). The localization was uniformly distributed in all cells of globular-stage somatic embryos (data not shown). A similar pattern of localization was observed in the torpedo-stage somatic embryos and mature zygotic embryos, with the enzyme being localized in the parenchyma cells and in the vascular procambial strands (data not shown). Intense swCDPK localization was also observed in the endosperm cells and confined to the storage bodies present in these cells (Fig. 9, B and B′). Negative controls wherein the sections were incubated with normal rabbit sera showed no FITC staining (data not shown).
Figure 9.
Immunofluorescence localization of swCDPK. A, Cytoplasmic localization of swCDPK in cells of embryos. B, Bright-field image showing cells of endosperm of the G2 stage of germination; B′, immunofluorescence localization of swCDPK in storage bodies of the endosperm cells. cyt, Cytoplasm; cw, cell wall; sb; storage bodies. Scale bars = 30 μm.
All observations pertaining to the temporal accumulation and activity of swCDPK have been summarized in Table I.
DISCUSSION
This study identifies two immunological homologs of soybean CDPK of 55 to 60 kD in the soluble proteins of all stages of sandalwood somatic embryogenesis (Fig. 5; Table I). In-gel Ca2+-dependent histone III-S phosphorylation and in vitro Ca2+-dependent phosphorylation of the 55- to 60-kD protein bands confirmed that they are CDPKs (Figs. 3C, 5, and 7B). The submicromolar levels of Ca2+ required for maximal phosphorylation of swCDPK protein in the in vitro assays indicate the activator role of Ca2+ for this phosphorylation. Furthermore, the failure of CaM to enhance phosphorylation of swCDPK (Fig. 4C) and its inhibition by W7 (Fig. 4D) indicates that this is an activity of a CDPK. The data suggest that the phosphorylation of swCDPK protein is the autophosphorylation activity of the enzyme. The present study shows that the Ca2+-dependent autophosphorylation (Fig. 4B) and Ca2+-dependent substrate phosphorylation activities (Fig. 3A) of swCDPK occur at different ranges of Ca2+ concentrations. A similar observation has been made with recombinant soybean CDPK (Lee et al., 1998). The role of autophosphorylation in the regulation of swCDPK, however, remains an enigma.
Although another prominent phosphorylated band was observed at 45 kD in the autophosphorylation assays, the activity was independent of Ca2+. This unidentified phosphorylated band (Fig. 3D) was detected in protein extracts of only some tissue types (Fig. 6B) and only in the later stages of embryogenesis (Fig. 5B) and germination in sandalwood. A lesser amount of protein was assayed in the experiment shown in Figure 5B compared with that in other autophosphorylation assays. This variation in the experimental conditions might have brought about a decrease in the intensity of the phosphorylated 45-kD band in torpedo/cotyledonary embryo extracts, as seen in Figure 5B, lane 2, compared with its intense phosphorylation in other experiments (Figs. 3D and 4).
The absence of swCDPK in the soluble protein extracts of regenerated plantlets was the first indication that the enzyme might show a temporal pattern of expression during sandalwood development. While the data in this paper indicate that neither CDPK protein nor activity is present in shoots and flowers of mature sandalwood trees, both have been reported in mature organs of some plant species (Hong et al., 1996; Yoon et al., 1999). This suggests that CDPKs that are detectable by these antibodies (Bachmann et al., 1996) and that can phosphorylate histone III-S are not present in the shoots and flowers of sandalwood. The predominant accumulation and activity of the 55- to 60-kD swCDPKs only in zygotic embryos, endosperm, and seedlings (Fig. 6; Table I) indicates that they are isoforms specific to these tissues, and are involved in embryogenesis, seed development, and germination. Distribution of Arabidopsis mRNAs encoding isoforms atCDPK6, atCDPK9, and atCDPK19 has been shown to differ in leaf, root, stem, and flowers (Hong et al., 1996). This further demonstrates that different isoforms of CDPK differ in their temporal and spatial distribution to accomplish their diverse physiological roles. The similarity in localization and distribution of swCDPK observed in both somatic and zygotic embryos (data not shown) indicates that a parallel could be drawn with respect to the probable role played by swCDPK during somatic and zygotic embryogenesis and germination.
swCDPK is present not only in the embryo but also in the endosperm of seeds during some stages of development. The accumulation of swCDPK protein and activities (Fig. 8, A and B; Table I) only in the endosperm of mature fruit and seeds during advanced stages of germination suggests a role for the enzyme in the endosperm during seed maturation and germination. Regulation of swCDPK in the endosperm appears to be via a temporal pattern of expression during development, dormancy, and germination of the seed.
The cytoplasmic localization of the enzyme in the cells of embryos supports the observation that swCDPK is present as a soluble protein in juvenile tissues of sandalwood. However, immunofluorescent staining localizes swCDPK in the storage bodies rather than in the cytoplasm of the endosperm cells. These spatial differences in swCDPK localization in different cell types may be an important criterion in the enzyme's physiological role in different tissues and developmental processes. Expression of a CDPK in developing rice seed has been previously reported (Kawasaki et al., 1993), but our data are the first (to our knowledge) showing that CDPK protein and activity are present in the endosperm and associated with storage bodies. In leaves, CDPK is induced by Suc (Iwata et al., 1998) and is also known to phosphorylate and regulate enzymes involved in carbon metabolism, such as Suc synthase (Huber et al., 1996) and Suc-phosphate synthase (McMichael et al., 1995), suggesting the involvement of CDPKs in the regulation of starch synthesis or breakdown. The presence of high levels of swCDPK in the endosperm cells during germination and its localization in the storage bodies suggests that swCDPK in this tissue could be involved in the mobilization of nutrients during germination.
Accumulation of swCDPK was high in the zygotic embryos of mature fruit, dormant seeds, early germination stages, and seedlings (Table I). Interestingly, Ca2+-dependent autophosphorylation and Ca2+-dependent substrate phosphorylation activities of swCDPK were absent/negligible, respectively, in zygotic embryos of ripe fruit, dormant seed, and stages G0 and G1 of germination (Table I). The addition of metavanadate and NaF in the assay mixture did not restore swCDPK activity in these stages (data not shown), suggesting that phosphatases may not be involved in the inhibition mechanism. However, inactive swCDPKs were not detected during the germination of somatic embryos into plantlets, probably due to the absence of dormancy prior to germination of somatic embryos. Polya et al. (1990) have reported the inhibition of wheat germ CDPKs by basic polypeptides such as histone H4, protamine, and synthetic amino acid homopolymers. Camoni et al. (1998) have demonstrated an in vitro activation of an Arabidopsis CDPK by 14-3-3 proteins. The presence of a basic protein inhibitor(s) or the absence of a small protein activator(s) in embryos during seed dormancy and early germination could be responsible for the inhibition or inactivation, respectively, of swCDPK in these stages. Further experimental evidence suggests the presence of inhibiting factor(s) in protein extracts of embryos belonging to these stages (V.S. Anil and K.S. Rao, unpublished data). Knowledge of the exact mechanism of this inhibition/inactivation would facilitate a better understanding of the regulation and role of CDPK during early plant development.
ACKNOWLEDGMENTS
We thank Prof. S.K. Podder for valuable advice and critical reading of the manuscript and Prof. C. Jayabaskaran for helpful suggestions. We thank Dr. P. Sarala for assistance with confocal microscopy.
Footnotes
This research was supported by a grant from the Department of Science and Technology, Government of India. Experiments were carried out at the Department of Biochemistry, Indian Institute of Science, Bangalore–560 012, India. Confocal microscopy was carried out in the facility supported by the Biotechnology Department, Government of India.
LITERATURE CITED
- Bachmann M, Shiraishi N, Campbell WH, Yoo B-C, Harmon AC, Huber SC. Identification of Ser-543 as the major regulatory phosphorylation site in spinach leaf nitrate reductase. Plant Cell. 1996;8:505–517. doi: 10.1105/tpc.8.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella JR, Arteca JM, Somodevilla M, Arteca RN. Calcium-dependent protein kinase gene expression in response to physical and chemical stimuli in mungbean (Vigna radiata) Plant Mol Biol. 1996;30:1129–1137. doi: 10.1007/BF00019547. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of micrograms of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Camoni L, Harper JF, Palmgren GM. 14-3-3 proteins activate a plant calcium-dependent protein kinase (CDPK) FEBS Lett. 1998;430:381–384. doi: 10.1016/s0014-5793(98)00696-6. [DOI] [PubMed] [Google Scholar]
- Geahlen LR, Anostario M, Jr, Low PS, Harrison LM. Detection of protein kinase activity in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1986;153:151–158. doi: 10.1016/0003-2697(86)90074-6. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Putnam-Evans C, Cormier MJ. Calcium dependent but calmodulin independent protein kinase from soybean. Plant Physiol. 1986;83:830–837. doi: 10.1104/pp.83.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Sussman MR, Schaller GE, Putnam-Evans C, Charbonneau H, Harmon AC. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991;252:951–954. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- Hepler PK, Wayne RO. Calcium and plant development. Annu Rev Plant Physiol. 1985;36:397–439. [Google Scholar]
- Hong Y, Takano M, Liu C-M, Gasch A, Chye M-L, Chua N-H. Expression of three members of the calcium-dependent protein kinase gene family in Arabidopsis thaliana. Plant Mol Biol. 1996;30:1259–1275. doi: 10.1007/BF00019557. [DOI] [PubMed] [Google Scholar]
- Huber SC, Huber JL, Liao PC, Gage DA, McMichael RW, Jr, Chourey PS, Hannah LC, Koch K. Phosphorylation of serine-15 of maize leaf sucrose synthase: occurrence in vivo and possible regulatory significance. Plant Physiol. 1996;112:793–802. doi: 10.1104/pp.112.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Kuriyama M, Nakakita M, Kojima H, Ohto M, Nakamura K. Characterization of a calcium-dependent protein kinase of tobacco leaf that is associated with the plasma membrane and is inducible by sucrose. Plant Cell Physiol. 1998;39:1176–1183. doi: 10.1093/oxfordjournals.pcp.a029318. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Hayashida N, Baba T, Shinozaki K, Shimada H. The gene encoding a calcium-dependent protein kinase located near the sbe1 gene encoding starch branching enzyme-I is specifically expressed in developing rice seeds. Gene. 1993;129:183–189. doi: 10.1016/0378-1119(93)90267-7. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee JY, Yoo BC, Harmon AC. Kinetic and calcium-binding properties of three calcium-dependent protein kinase isoenzymes from soybean. Biochemistry. 1998;37:6801–6809. doi: 10.1021/bi980062q. [DOI] [PubMed] [Google Scholar]
- MacIntosh GC, Ulloa RM, Raices M, Tellez-Inon MT. Changes in calcium-dependent protein kinase activity in in vitro tuberization in potato. Plant Physiol. 1996;112:1541–1550. doi: 10.1104/pp.112.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy DW, Harmon AC. Calcium-dependent protein kinase in the green alga Chara. Planta. 1992;188:54–61. doi: 10.1007/BF00198939. [DOI] [PubMed] [Google Scholar]
- McMichael RW, Jr, Bachmann M, Huber SC. Spinach leaf sucrose-phosphate synthase and nitrate reductase are phosphorylated/inactivated by multiple protein kinases in vitro. Plant Physiol. 1995;108:1077–1082. doi: 10.1104/pp.108.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog G. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Pestenacz A, Erdei L. Calcium-dependent protein kinase in maize and sorghum induced by polyethylene glycol. Physiol Plant. 1996;97:360–364. [Google Scholar]
- Polya GM, Nott R, Klucis E, Minichiello J, Chandra S. Inhibition of plant calcium-dependent protein kinases by basic polypeptides. Biochim Biophys Acta. 1990;1037:259–262. doi: 10.1016/0167-4838(90)90177-h. [DOI] [PubMed] [Google Scholar]
- Poovaiah BW, Reddy ASN. Calcium and signal transduction in plants. Crit Rev Plant Sci. 1993;12:185–211. doi: 10.1080/07352689309701901. [DOI] [PubMed] [Google Scholar]
- Putnam-Evans CL, Harmon AC, Cormier MJ. Purification and characterization of a novel calcium-dependent protein kinase from soybean. Biochemistry. 1990;29:2488–2495. doi: 10.1021/bi00462a008. [DOI] [PubMed] [Google Scholar]
- Reinert J. Morphogenes und ihre kontrolle an gewebekuturen aus carotten. Naturwissenschaften. 1958;45:344–345. [Google Scholar]
- Ritchie S, Gilroy S. Calcium-dependent protein phosphorylation may mediate the gibberellic acid response in barley aleurone. Plant Physiol. 1998;116:765–776. doi: 10.1104/pp.116.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM. Protein kinases with calmodulin-like domains: novel targets of calcium signals in plants. Curr Opin Cell Biol. 1993;5:242–246. doi: 10.1016/0955-0674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Steward FC, Mapes MO, Smith J. Growth and organized development of cultured cells: II. Organization in cultures grown from freely suspended cells. Am J Bot. 1958;45:705–708. [Google Scholar]
- Towbin H, Staehelin T, Gordon T. Electrophoretic transfer of proteins from polyacrylamide gel to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas JA. Signal perception and transduction: the origin of the phenotype. Plant Cell. 1997;9:1181–1195. doi: 10.1105/tpc.9.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon GM, Cho HS, Jung Ha H, Liu JR, Lee HP. Characterization of NtCDPK1, a calcium-dependent protein kinase gene in Nicotiana tabacum, and the activity of its encoded protein. Plant Mol Biol. 1999;39:991–1001. doi: 10.1023/a:1006170512542. [DOI] [PubMed] [Google Scholar]