Abstract
The observation of an infusion reaction (IR) in a nonclinical study can cause concern among investigators and regulators in the development of biotherapeutics. Biomarkers can be informative to determine whether the reactions are immune-mediated or test-article related and if there is a potential risk to human subjects. IRs encompass a broad range of adverse events with a variety of triggers; the focus of this paper is IRs due to cytokine release syndrome or immune complex formation and the associated biomarkers. Such reactions generally do not preclude clinical development or marketing approval, because it is widely accepted that immune-mediated reactions in nonclinical species are not predictive of human outcomes. Several US approved products (from 2004 to 2016) have documented IRs in nonclinical species. This review article discusses recent examples, the biomarkers evaluated, and implications for study design and conduct.
Keywords: Infusion reaction, Cytokine release, ADA, Immune complex, Nonclinical, Biotherapeutic
Graphical abstract
Highlights
-
•
Approved biotherapeutics have produced nonclinical infusion reactions (IRs).
-
•
Nonclinical IRs after a first dose are associated with cytokine release.
-
•
Nonclinical IRs after several doses are associated with ADA.
-
•
ADA-mediated IRs may result in immune complex tissue deposition.
-
•
Diagnosing nonclinical IRs requires a weight-of-evidence approach using biomarkers.
1. Introduction
The purpose of nonclinical toxicology studies is to identify and characterize toxicology hazards that may affect clinical development. Toward that end, toxicology biomarkers—a biological endpoint that is predictive of toxicity—may be critical in the appropriate interpretation of study results 8, 9. While histopathology-based biomarkers are useful, peripheral blood biomarkers of toxicity are less invasive to obtain and easily allow for real-time monitoring over the course of a study.
The nonclinical presentation of IRs can be diverse, including but not limited to: anaphylaxis, cytokine release syndrome, and immune complex mediated hypersensitivity. Biomarkers combined with other data can aid investigators and regulators in differentiating immune-mediated toxicities that may not be relevant to humans from direct test-article toxicity that may be more likely to translate to human outcomes.
2. IRs in nonclinical studies
Nonclinical IRs (also referred to as dose reactions or infusion-related reactions [IRRs]) can occur following the first dose or multiple doses, in rodents or non-rodents, and can be dose dependent, non-dose dependent, or even bell-shaped. Infusion reactions can occur during dosing or several hours thereafter.
Nonclinical IRs are not necessarily a barrier to drug approval. Based on an examination of Pharmacology/Toxicology reviews for US approved monoclonal antibodies (mAbs) and other biotherapeutics between 2004 and 2016, of the 49 products approved during this timeframe, 15 (31%) were identified as producing nonclinical “infusion reactions”; of these 15 approved products, 12 were delayed ADA-mediated reactions, while 4 were first dose reactions (galsulfase had both first dose and delayed IRs) these are summarized in Table 1 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25.
Table 1.
Biotherapeutics approved by the FDA and EMA between 2004 and 2016, with reported nonclinical infusion reactions.a
| Productb Approval Datec |
Target/Enzyme Isotype Molecule Type |
Occurrenced Dosing |
Finding (Direct Quote from Pharmacology Review) | Cytokines/Complement Examinatione |
ADA/Immune Complex | Pretreatment/Interventionf |
|---|---|---|---|---|---|---|
| Daratumumab (Daralex) FDA 2015 EMA 2016 |
CD38 Human IgG1 kappa mAb |
1st Dose (not observed at subsequent doses) Weekly, 6 doses (5, 25 mg/kg); IV infusion |
“There was one mortality at 5 mg/kg immediately following dosing (1.5 h) in a female chimpanzee due to cytokine release response with overinflation, edema of the lungs following dosing with detectable levels of cytokines TNF-α, IL-6 and IFN-γ in the serum prior to necropsy.” “In addition to the death of the female chimpanzee in the 5 mg/kg dose group, two additional group 1 animals (at 5 mg/kg) exhibited a cytokine release response, including increased tracheal and nasal mucous production … Both Group 2 animals showed a reaction to the initial 25 mg/kg dose. Clinical signs included sneezing and mucous production was noted in A96A017 approximately 15 min (25 mL) into the dosing; this activity subsided and mucous membrane pallor was noted following administration of 44 mL of the dose (26 min).” |
Cytokines reported for animal that died. | Not reported | “conditioning dose, of 10 mg was administered 24 h prior to first infusion of high dose following cytokine response reaction in first dosing of two low dose animals” |
| Blinatumomab (Blincyto) FDA 2014 EMA 2015 |
Bi-specific CD19 CD3 N/A BiTE |
1st Dose (also at all subsequent doses) Weekly, 5 doses (0.1 μg/kg); 2-h IV infusion |
“Infusion of chimpanzees with blinatumomab was associated with T-cell activation and increases in body temperature and heart rate and decreases in blood pressure, in addition to increases in cytokines IL-2, IL-6, and INF-γ. These finding are consistent with the cytokine release syndrome observed in the clinical trial in patients …”g | Increased cytokines in vivo and in vitro (human and chimpanzee cells) | Not reportedh | Not reported |
| Siltuximab (Slyvant) FDA 2014 EMA 2014 |
IL-6 Chimeric IgG1 mAb |
1st Dose Weekly, 25 weeks (9.2, 46 mg/kg); IV infusion |
“Although not common, first-dose infusion reaction occurred in a monkey (in 1 out of 52i) and included moderate facial swelling. The swelling resolved after the infusion ended and no other instances occurred.” | Not reported | Drug interference with ADA assay proposed | Not reported |
| Reslizumab (Cinqair) FDA 2016 EMA 2016 |
IL-5 Humanized IgG4 kappa mAb |
Delayed (Doses 5–7) monthly, 7 doses (1, 5, 25 mg/kg); IV |
“Immunogenicity noted in 3 in the pivotal toxicity study … One of the animals had repeated infusion reactions consistent with hypersensitivity (salivation, emesis, recumbency within 10 min of doses 5–7).” | Not reported | ADA detected in a “few animals”; drug interference with ADA assay reported Possible ADA related vasculitis |
Not reported |
| Atezolizumab (Tecentriq) FDA 2016 EMA not yet approved |
PDL-1 Humanized IgG1 mAb |
Delayed (Day 133 and 141) Weekly; 26 weeks (5, 15, 50 mg/kg); IV infusion |
“One male in the 5 and 15 mg/kg dose groups experienced infusion-related reactions after dosing on Day 113 and 141, respectively. Clinical signs included severe hypoactivity, staggered movements, and increased heart rate (15 mg/kg male). Animals recovered after receiving glucose and sodium chloride.” | Cytokines examined in vivo; unremarkable; cytokines were not induced in vitro (human cells) | ADA detected; drug interference with ADA assay reported | Supportive care (glucose and sodium chloride) |
| Obinutuzumab (Gazyva) FDA 2013 EMA 2014 |
CD20 Humanized IgG1 mAb |
Delayed (3 died during dosing (Day 63–181); 4 died during recoveryj (Recovery Day 85–141)k Weekly; 26 weeks; IV bolus (5, 25 mg/kg), 30 min IV infusion (50 mg/kg) |
“Hypersensitivity reactions were noted at all doses … in the 26-week study and were attributed to cross-species reactivity to a foreign protein. Clinical observations included acute anaphylactic/anaphalactoid reactions (clinical signs consisted of excessive salivation, facial erythema that progressed to the arms, with evident pruritus). Microscopic findings included an increased prevalence of systemic inflammation and infiltrates consistent with immune-complex mediated hypersensitivity reactions including glomerulonephritis, and arteritis/periarteritis and serosal/adventitial inflammation in multiple tissues. These reactions led to the unscheduled deaths of 6 (possibly 7) monkeys during the 26-week study …. Immune-complex deposition in glomeruli of some animals was confirmed by detection of electron dense deposits by immunohistochemistry or transmission electron microscopy.” | Cytokines examined in vivo (results not reported); cytokines elevated in vitro (human cells)l | ADA confirmed Immune complexes detected both in tissue by IHC and TEM and as circulating complexes |
Intervention: Diphenhydramine treatment and prophylactic diphenhydra- minem |
| Ipilimumab (Yervoy) FDA 2011 EMA 2011 |
CTLA-4 Human IgG1 kappa mAb |
Delayed (Day 58) Weekly; 13 weeks; IV (10 mg/kg), Combination Studyn weeks 5–13 |
“One monkey exhibited an infusion-like reaction (signs of shock [Cyanotic, thread pulse, muffled heart sounds], requiring supportive care).” | Cytokines examined in vitro; weak induction seen under some assay conditions (human cells) | ADA response first detected on D16. ADA response exceed assay limits by D44 in the animal with reaction | Supportive care (oxygen, IV fluid, diphenhydramine, dexamethasone); drug holiday |
| Ofatumumab (Arzerra)o FDA 2009 EMA 2010 |
CD20 Human IgG1 kappa mAb |
Delayed (Onset Day 78–134) Weekly; 8 dose, followed by 5 monthly doses; (20, 100 mg/kg), 30-min IV infusion |
“Ofatumumab-treated cynomolgus monkeys only exhibited signs of infusion reactions after repeated dosing; these events resolved without treatment. No clinical observations of infusion reactions were detected in the short-term studies (i.e. 4 weeks or less of dosing). In the 7-month toxicity study, signs were observed in high-dose animals from D78 onward (the 1st monthly maintenance dose) and in low dose animals from Dl06 onward (the 2nd monthly dose). The effect manifested as transient increases in heart rate and heart force (variable range, usually beginning during the 30-minute infusion, and resolving within a few hours of the end of infusion) and occurred sporadically for particular animals. Transient trembling was observed in 3/28 animals (11%).” | Complement was investigated in a short 2-dose follow up study. The data was “difficult to interpret”. Coombs Test Positive Day 122–176 |
Reduced ADA detection compared to short term studies Immune complex data were “not informative” |
Not reported |
| Delayed (Day 148 & 162 (second cycle of dosing)) Cycled repeat dose (20, 100 mg/kg)p; 30-min IV infusion |
“An exploratory study in cynomolgus monkeys confirmed that ofatumumab treatment caused transient (15 min–4 h post-treatment) increases in serum levels of activated complement and interleukin-6 (IL-6), and neutrophil degranulation.” | Coombs Test positive D15-D267 Activated complement and interleukin-6 (IL-6), |
Not reported | Not reported | ||
| Panitumumab (Vectibix) FDA 2006 EMA 2009 |
EGFR Human IgG2 mAb |
Delayed (Day 29–141) Weekly; 13 weeks (7.5, 15, 30 mg/kg); IV bolus |
“Infusion reactions - dose-related peri-infusion emesis, lethargy, prostration, excessive salivation, pallor to skin, gums, and/or muscle spasms observed in 5 monkeys … Monkey #33F in the 15 mg/kg/dose group died of apparent anaphylactic reaction on SD-l34, shortly after completion of dosing” | Not reported | ADA detected in some animals | Pre-treated with prophylactic diphenhydramine |
| Natalizumab (Tysabri) FDA 2004 EMA 2006 |
α4- integrin Humanized IgG4 mAb |
Delayed (Day 64 and 71) Weekly; 6-month juvenile (3, 10, 30, 60 mg/kg); 30-min IV infusion |
“Infusion reaction was observed in two studies using cynomolgus monkeys and these reactions appeared to be immune mediated and correlated with high levels of anti-drug antibodies.; One female in the high dose group exhibited adverse reactions to the test article approximately 2.5–3 h following infusion on Days 64 and 71. The reactions were characterized by generalized petechial hemorrhages, severe bruising/ecchymotic hemorrhages around the face, bruising of the arms and femoral area, and on Day 71, swelling of the left side of the face.” | Complement activation observed in vivo | ADA inverse relationship to dose Development of CIC-Raji-reactive immune complexes (IC) immediately post dose @ Day 85 & 176 |
Dexamethasone and benadryl; drug holiday |
| Delayed (Day 43–155) Weekly; 6 months, adult (3, 10, 30, 60 mg/kg); 30–60 min IV infusion |
“Infusion-related incidences were observed in three animals from different dose groups, which appeared to be due to a hypersensitivity response to the study drug … In addition, minimal to moderate glomerulonephritis was observed in the kidneys of 4 animals at Week 26 and glomerulosclerosis (chronic manifestation of glomerulonephritis) was observed in 1 animal at the end of the recovery period. The glomerulonephritis findings are believed to be the result of immune complex deposition or other antibody-dependent phenomena, which is likely related to the immunogenicity of the test article in the monkey.” | Complement activation observed in vivo | ADA identified in some animals correlates with complement and immune complexes | Not reported | ||
| Rilonacept (Arcalyst) FDA 2008 EMA 2009q |
IL-1β; IL-1α Fc IgG1 Fusion protein |
Delayed Weekly; 3-weeks (5, 20, 50 mg/kg); 30 min IV infusion |
“The timing for the adverse reaction [emesis, lethargy] observed … correlated with formation of the anti product antibody after the IV administration of lL-1 Trap. Interestingly, the number animals showing such incidence were higher in the low dose group. This coincides with the observation that the increase in the quantity of the anti product antibody formation was higher in the animals at the low dose group. The intolerance of the immune complex formation in the animals might have been revealed by the clinical signs of lethargy and emesis.” | Increase in CRP; no change in complement | ADA - All animals @Day 16; large individual variation | None reported |
| Delayed 3-times/week; 26-week (15, 25, 40, 60 mg/kg); SC NOAEL not identified due to findings @ low dose |
“There were two unscheduled deaths in this study; one male (#46) from 40 mg/kg showed clinical signs of discomfort at Day 40, dosing was discontinued, the animals continue to show discomfort, therefore, sacrificed at Day 45, histopathology showed myocarditis; another male (#54) died after dosing on Day 108, histopathology showed congestion in several organs like kidney etc., this animals [sic] also showed congestion in lung due to granuloma as indicated by edema and perivascular lung blockage. Both of these animals did show high antibody titer, and the sponsor believes that the cause of deaths resulted from immune mediated hypersensitivity reaction. The reviewer agrees with sponsor analysis of data from these unscheduled deaths.” | CRP detected in individual animals; dose-dependent complement increase was observed in males only | ADA present in most but not all animals | Periodic epinephrine | ||
| Delayed Every 2nd week; 26-weeks (14 doses) (3, 10, 30, 100 mg/kg); IV infusion |
“There was an increased incidence of emesis/retching in the treatment group with the increase in the duration of the study indicating poor tolerance of the compound after IV administration.” “One female (# 43) in the 30 mg/kg dose group was observed to assume recumbent position at approximately one hour after the infusion of the 5th dosing, the animal recovered spontaneously within 7–12 mins. Another male from the high dose group become non responsive during the drug infusion of the 12th dose, the animal had dorsiflexed head position, with constricted pupils and pale gums. The animal, however, recovered within 10 mins post infusion.” |
Slight increase in CRP at high dose; no changes in complement identified | ADA present in most but not all animals | None reported | ||
| Algucosidase Alpha (Myozyme) FDA 2006 EMA 2006 |
Alpha-Glucosidase N/A ERT |
Delayed Rodent pharmacology studies (rat, mice and knockout mice); multiple dosing regimens |
“Several unscheduled animal deaths occurred during the pharmacology studies with no details regarding cause of death provided. Some of these deaths can be accounted for by hypersensitivity reactions that are a common response of rodents to the rhGAA. However, some of the deaths could not be accounted for by hypersensitivity and no data on the potential cause are provided. Due to the concern about hypersensitivity, rodents were routinely pre-treated with diphenhydraminc (DPH), usually at 5 mg/kg, 20 min prior to infusion of the rhGAA.” | Complement not detectedr | ADA detected, but not tested in all studies | Prophylactic diphenhydramine |
| Delayed Weekly (1, 10, 100 mg/kg); 4-weeks; IV NOAEL 10 mg/kg |
“Hypersensitivity response was observed in one and one[sic] male from group 4 after the third dose.” | Not reported | Not performed | Prophylactic diphenhydramine based on earlier pharmacology work | ||
| Galsulfase (Naglazyme) FDA 2005 EMA 2006 |
N-acetylgalactosamine 4-sulfatase N/A ERT |
First Dose and Delayed Weekly (1–2 mg/kg):IV NOAEL not reported |
“In the acute single dose toxicity studies, rats and dogs had swelling of the mouth, nose or paws … anaphylactic or anaphylactoid reactions …” “Trembling and coughing were often detected during infusion. A number of episodes of vomiting, defecation, and fever were also observed.” “Three types of microscopic findings were identified: 1) pulmonary perivascular inflammation, 2) interstitial pneumonia, and 3) glomerulonephropathy. Based on the present observations, we cannot rule out the possibility that they are interrelated, and may have a common immunological etiology … In addition, anti-rhASB immune complex deposition may also lead to the … pulmonary and renal pathology.” |
Complement activation observed in 2/5 | ADA detected | Not reported diphenhydramine used in subsequent fertility/EFD study |
| Evolocumab (Repatha) FDA 2015 EMA 2015 |
PCSK9 Human IgG2 |
Not reported, but interpreted to be a delayed effect. Every 2 weeks; lifetime; SC |
“Pale appearance, ataxic behavior and skin, cold to touch, could indicate an immune response to this human IgG protein [in hamsters].” | Not reported | Pharmacodynamics used as a surrogate – no significant ADA | Not reported |
| Abatacept (Orencia) FDA 2005 EMA 2007 |
CD80/86 Fc IgG1 Fusion protein |
Not reported but interpreted to be a delayed effect. Studies during which this reaction occurred were not identified. |
“In mice and dogs, when drug levels fell below immunomodulatory levels and the animals were subsequently administered an IV challenge dose of abatacept, the presence of circulating abatacept-specific antibodies was associated with clinical signs of hypersensitivity“ | Not reported | ADA detected | Not reported |
mAb = monoclonal antibody, ERT = enzyme replacement therapy.
Data from FDA Pharmacology Toxicology Reviews 10, 11, 12, 13, 14, 15, 16, 18, 19, 20,[17],21, 22, 23, 24, 25and the ofatumumab product label [59].
Infusion reactions were also reported for golimumab; however, they were only seen with a murine surrogate used for reproductive and developmental hazard identification and were not observed with golimumab itself [60].
Seen at 1st dose or delayed (following multiple doses).
Based on study report summarized in the FDA Pharmacology Review. Investigations may have taken place that are not included in the summary.
Based on study report summarized in the FDA Pharmacology Review. Interventions may have taken place that are not included in the summary.
Expected effect due to pharmacology of the drug.
Not a terminal study.
The reference 1/52, refers to all monkey studies conducted.
Recovery animals died from glomerulonephritis associated with immune complex deposition.
Six of seven mortalities were related to the hypersensitivity described in this table.
Complement cascade activated by drug pharmacology.
The intervention was successful for one animal, but for another animal, the intervention was successful at first but, over time, failed to prevent the reaction. This type of variability in response is consistent with the author's experience.
Combination study with multiple regimens Drug (8 intermittent IV doses over 88 days) + SIV vaccine (IM Days 1,2,29,30,56,58,85,85).
Additionally, hemolytic anemia attributed to monkey-specific humoral immune response was observed at 7 weeks.
Total of 4 doses. Each cycle consisted for 2 doses given 2 weeks apart; Dosing Days 1, 15, 148, 162 (only selected endpoints were reported).
No longer authorized in Europe.
When histamine was measured, moderate histamine elevation was observed after the 3rd dose and significantly elevated histamine was observed after the 8th dose. Histamine was not measured in all studies.
3. Reactions following the first dose
Dose reactions following the first dose can have different underlying mechanisms but are generally IgE-mediated (Type I hypersensitivity) or non IgE-mediated (e.g., immune activation resulting in cytokine release syndrome and/or complement activation) with more severe and uncontrolled immune activation classified by the FDA as “adverse immunostimulation” [26]. There has been intense interest in cytokine-release-syndrome and cytokine storm since the TeGenero incident (reviewed 27, 28, 29), in which life-threatening adverse events were observed in all dosed human subjects immediately following the first infusion of TGN1412, a CD28 superagonist. Examples of biotherapeutics with nonclinical first-dose reactions are daratumumab, blinatumomab, siltuximab, and galsulfase; blinatumomab activates the immune system by cross-linking and activating T-cells and therefore, the observed cytokine-release syndrome and IRs for this product are mechanism based (Table 1).
4. The anti-drug antibody response and consequences in nonclinical studies
Humanized and fully human biotherapeutics can be recognized by an animal's immune system as “non-self” and result in the generation of anti-drug antibodies (ADA) against the biotherapeutic; this is a normal and anticipated response to a foreign protein 26, 30, 31. Anti-drug antibodies are common in nonclinical studies with biologics; as acknowledged in the FDA Immunotoxicology Guidance, “Polypeptides and protein drugs … are usually immunogenic if administered to a mammalian species in which the molecule does not naturally occur” [26]. Different immunological mechanisms can lead to the generation of ADA 32, 33. The presence of ADA is often highly variable with some animals in a cohort having no ADA and others having high titers. Also, it is common to observe increased incidences of ADA, and/or increased test-article clearing at lower doses relative to higher doses, which may be due to increased immunogenicity, assay interference due to high concentrations of test article, or immune suppression. Examples of increased nonclinical immunogenicity with low doses include, but are not limited to, panitumumab, rilonacept, and atezolizumab 17, 20, 25.
Development of ADA in toxicology studies can have various consequences on study outcomes and data interpretation ∗∗2, 3, ∗∗5, 7, 31, 34, 35, 36, 37, ∗38.
-
•
No impact
-
•
Altered pharmacokinetics—increased or decreased clearance
-
•
Neutralization of pharmacological/pharmacodynamic (PD) activity—decreased modulation of target
-
•
Altered toxicology assessment—immune-mediated lesions
-
•
Infusion Reactions—clinical signs ranging from mild to severe, which may require dosing holidays, adjustments in dose delivery (e.g., bolus to infusion), veterinary intervention, and/or euthanasia.
Although a direct measurement of ADA is not required per ICHS6(R1), samples are commonly collected for direct measurement of ADA 31, 39, 40, 41, 42. The evaluation of ADA can be helpful when suspected ADA-mediated toxicity is observed. For example, immune complexes, can be deposited into tissues, causing lesions such as vasculitis, glomerulonephritis, hemorrhage, and thrombi. ADA-mediated adverse reactions can also occur with a neutralizing antibody response to an endogenous protein. For example, generation of neutralizing anti-erythropoietin antibodies has been observed in patients receiving recombinant erythropoietin, which led to pure red-cell aplasia 43, 44. In NHPs, ADAs can be against the complementarity-determining region (CDR) of a biotherapeutic, but are more frequently against the Fc-region, resulting in clearing antibodies. In humans, ADAs are more frequently against the complementarity-determining region (CDR) of a biotherapeutic, resulting in neutralizing antibodies [1].
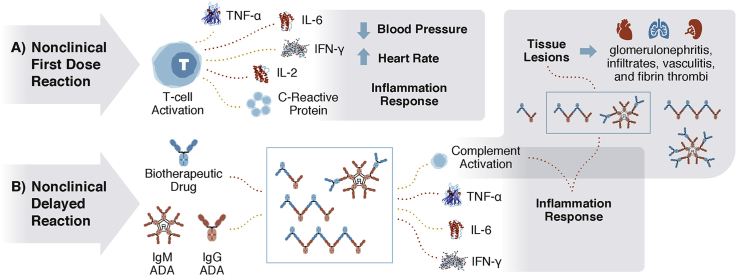
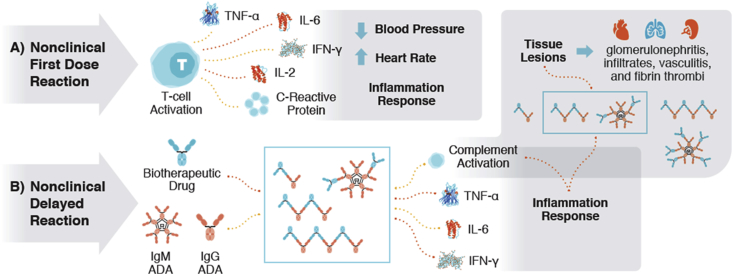
The stoichiometry of antibody:antigen (for ratios of both biotherapeutic:target and/or ADA:biotherapeutic) is known to influence the size of the immune complexes, and subsequently the severity of the ADA-mediated IRs ∗∗2, 7 (Fig. 1). Large immune complexes can be cleared by the mononuclear phagocytic system, small immune complexes can recirculate and be cleared or become mid-sized immune complexes, which have been shown to result in tissue deposition ∗∗2, 3, ∗∗4.
Fig. 1.
A) Nonclinical First Dose Reactions are a result of a biotherapeutic causing immune activation that results in cytokine release and an acute inflammatory response. B) Nonclinical Delayed Reactions are a result of anti-drug antibodies (IgG or IgM) forming and binding to the biotherapeutic (ADA + biotherapeutic = immune complex IC). In NHPs, ADAs can be against the complementarity-determining region (CDR) of a biotherapeutic, but are more frequently observed against the Fc-region, resulting in clearing antibodies [1]. Whereas in humans, ADAs are usually against the CDR. Therefore, ADA-mediated responses in nonclinical species are not considered to be predictive of those in humans [1]. The immune complexes can result in different sizes based on the ratio of excess antibody (excess ADA) or excess antigen (excess biotherapeutic). The presence of ICs can result in cytokine release and complement activation. Complement activation appears to be related to the size of the immune complex. Insoluble, large immune complexes of ADA bound to a biotherapeutic are cleared rapidly by the liver and spleen via the mononuclear phagocytic system ∗∗2, 3, ∗∗4. Conversely, smaller (dimer) soluble immune complexes are present in circulation longer, and can facilitate receptor recycling of the biotherapeutic, which can increase the size of the IC and ADA titer, or may result in class switching from IgM to IgG-ADA, which can produce a more severe IR and subsequent tissue deposition of the immune complex [3]. The most common sites for immune-complex deposition appear to be the liver, kidney, and small blood vessels, but immune complexes have also been shown to deposit in the spleen, lungs, heart, skin, and brain (choroid plexus). Lesions in these organs may include inflammation (infiltrates, vasculitis, glomerulonephritis), hemorrhage, and thrombi ∗∗2, ∗∗5, ∗6, 7.
5. Reactions following multiple doses
Nonclinical IRs following multiple doses are usually ADA-mediated and are generally observed in longer duration studies (>4 weeks) [45]. Based on approved products, severe reactions (i.e., those that require dosing to be stopped or veterinary intervention) tend to occur after 5 weeks of dosing (Table 1). Commonly, investigational new drug/clinical trial authorization (IND/CTA)-enabling Good Laboratory Practice (GLP) studies are 4 weeks in duration, which allows for the characterization of toxicology and pharmacokinetics without the complexities of managing and interpreting ADA-driven IRs on a study. If ADA or rapid clearance of the biotherapeutic (a surrogate endpoint if an ADA assay is not available) is detected in the 4-week study, these data can be used to select higher doses, slower dose rates, and/or larger group sizes for longer duration studies 15, 16. Furthermore, if IRs are observed, the ADA and/or toxicokinetic (TK) data can be used to inform dose selection and frequency.
6. Mitigating nonclinical IRs
For nonclinical management of IRs (first dose and delayed), less severe reactions (i.e., swelling, redness, increased salivation, general paleness, mild emesis) can be managed with diphenhydramine, which is routinely used to treat these reactions as is noted in 6/12 approved biotherapeutics in Table 1 with ADA-mediated IRs 14, 15, 18, 20, 21, 22. However, based on the authors' experience, severe IRs, which can involve clinical observations of convulsions, tremors, loss of consciousness, and severe emesis are unlikely to be managed by diphenhydramine alone. For first dose reactions, “split-dosing” also known as “conditioning-dosing” may also be used to mitigate IRs as was done for daratumumab [12]. For severe delayed IRs, a multi-faceted approach is typically needed, which may involve a dosing holiday to allow immune complexes to clear, a strategy used to manage ipilimumab and natalizumab nonclinical IRs ∗∗5, 14, 18. When dosing resumes, the rate of dose administration can be decreased. For example, if the route is IV bolus, administration could be modified to IV slow push, or if IV slow push, modified to 1-h infusion. In addition, pretreatment of animals with both diphenhydramine and dexamethasone or other steroids can be attempted to suppress the IR as was used to manage nonclinical IRs with ipilimumab and natalizumab 14, 18. Individual animals will likely respond differently to these different strategies, and with some animals, these strategies may not be sufficient to allow continued dosing. These approaches are consistent with those used to mitigate IRs in humans to marketed biotherapeutics 46, 47. In the context of biomarkers, being able to mitigate/suppress a nonclinical IR can add to the weight-of-evidence approach to demonstrate that a reaction was immune mediated and not a direct effect of the test article.
7. Peripheral blood biomarkers after a nonclinical IR
If an IR is observed, the collection of serum and plasma for potential future analysis of biomarkers is prudent (Table 2). If IRs are a known concern, these collections can be built into the study protocol, optimally within 2–4 h after the IR occurs. Table 2 lists examples of common biomarkers, but this is not an exhaustive list. Furthermore, while modulation of each biomarker is noted in Table 2, it is important to note that IRs manifest differently based on the biotherapeutic, route of administration, species, and the individual animal, and all biomarkers may not be observed ∗∗2, ∗6, 15. To develop a weight-of-evidence approach to demonstrate immune-mediated IRs, TK data (and/or ADA) and complement markers are key data sets. TK data can be used to demonstrate either increased test article (TA) clearance or sustained exposure to the TA if an ADA assay is not available, and complement activation can help elucidate the mechanism behind the IR. Another possible peripheral blood biomarker for immunogenicity is the measurement of circulating immune complexes (CICs), which can be measured by size-exclusion chromatography – high-pressure liquid chromatography SEC-HPLC (ADA bound to the biotherapeutic), SEC-ELISA, or by ELISA in Raji cells (complement bound to either ADA or TA) 48, 49. Even if an ADA assay is available, other biomarkers beyond ADA or TK are critical. For example, an ADA assay can be negative, even in the presence of increased clearance and immune complexes ∗6, 15. In these instances, the complement biomarkers noted in Table 2 can add to the weight of evidence supporting an IR as being immune mediated, and not a direct test-article effect ∗∗2, ∗∗5, ∗6, 15.
Table 2.
Biomarkers after a nonclinical infusion reaction.
| Biomarker | Matrix | Whole Blood vol. (mL) | Sample collection & handling considerations | Expected modulation | Timing | |
|---|---|---|---|---|---|---|
| aPharmacokinetic/Pharmacodynamic | TK | serum or plasma | 0.5–1.5 | Per protocol | bIncreased clearance/decreased TA | 2-4 h after IR |
| PD/Target | 0.5–1 | Target with decreased or no engagement | ||||
| aImmunogenicity | ADA | serum or plasma | 0.5–1 | Per protocol. For small species with TK cohorts, sample from TK, Main study, and Recovery groups |
Positive | 2-4 h after IR or prior to termination in all animals |
| CIC | serum or plasma | 0.5–1 | Method dependent 48, 49 | Positive/Increased | ||
| Complement ∗∗2, ∗∗5, ∗6, 18, 19, ∗∗51 | CH50 | serum | 0.5–1.8 | Process and freeze immediately. Minimize freeze/thaw cycles | Decreased | 2-4 h after IR |
| C3a | serum or plasma | 0.5–1.8 | Increased | |||
| C5a | Increased | |||||
| Bb | Increased | |||||
| Sc5b-9 | Increased | |||||
| Cytokines ∗∗2, ∗∗5, 10, 12, 19, ∗50, ∗∗51 | Multi-plexed assay (cytokines most commonly observed listed) IL-6, TNF-α, IFNγ, IL1-b, IL-2 |
serum or plasma | 0.5–1.0 | Process and freeze immediately. Minimize freeze/thaw cycles | Increased | 2-4 h after IR |
| Coagulation ∗∗2, ∗∗4, ∗∗5 | APTT | plasma | 1–1.8 | Samples taken by different routes or in the presence of anesthetics may impact biomarker modulation [63] | Increased | 2-4 h after IR |
| PT | Increased | |||||
| Fibrinogen | c,eDecreased or increased | |||||
| D-dimer | Increased | |||||
| Hematology ∗∗2, ∗∗5, ∗52 | Red blood cell mass (RBCs, Hgb, Hct) | plasma | 0.5–1 | Samples taken by different routes or in the presence of anesthetics may impact biomarker modulation [63] | dIncreased or Decreased | 2-4 h after IR |
| Platelets | d,eDecreased | |||||
| Neutrophils | dDecreased or Increased | |||||
| Lymphocytes | dUnchanged or Increased | |||||
| Monocytes/Macrophages | dDecreased or Increased | |||||
| Clinical Chemistry ∗∗2, ∗∗4, ∗∗5 | Albumin | serum | 1–2 | Samples taken by different routes or in the presence of anesthetics may impact biomarker modulation [63] | Decreased | 2-4 h after IR |
| Creatinine | Increased | |||||
| Triglycerides | Increased | |||||
| Total protein | Decreased | |||||
| Liver Enzymes (AST, ALT, ALP) | Unchanged or increased | |||||
| C-reactive Protein | Increased | |||||
| Total bilirubin | Increased | |||||
| aImmunohistochemistry | Human IgG (to detect biotherapeutic) | Nonclinical species Tissues | NA | Anti-human IgG that does not bind to monkey IgG ∗∗2, ∗6 | May or may not be present | Necropsy the same day as IR if no further dosing is to be attempted. Biotherapeutic or ICs may clear with time [2] |
| Nonclinical Species IgG, IgM, IgA, C3, Sc5b-9 | Albumin may be needed to discern leakage from granular deposits ∗∗2, ∗6 | Present | ||||
Not relevant to first-dose reactions.
Decreased clearance/increased TA is rare, but indicative of sustaining ADA. In a recent review of marketed biotherapeutics, no monoclonal antibodies were associated with test article sustaining ADA; only a few enzyme replacement therapies (ERTs) and protein/peptides were associated with this type of ADA [64].
Decreases in fibrinogen are commonly associated with coagulopathy; however, increases in fibrinogen are associated with inflammation ∗∗2, ∗∗5, 8.
Increases in red blood cell mass and leukocytes are associated with increased inflammation that occurs during infusion reactions. However, ADA-mediated infusion reactions where the immune complexes (ADA bound to biotherapeutic) are associated with activated complement may lead to binding of the FCγ receptors on neutrophils and/or RBCs (and platelets), which then can result in increased clearance and/or tissue deposition via phagocytosis by monocytes (i.e., resulting in subsequent decreases in circulating RBCs, platelets, neutrophils, and/or monocytes/macrophages) ∗∗2, ∗6, 7, ∗52.
The most common clinical pathology biomarkers for IRs are cytokines, complement markers, hematology, and coagulation. For cytokines, most CROs have multiplexed assays; however, IL-6 appears to be the cytokine most commonly elevated for both first dose and delayed IRs 10, 12, 19. Cytokines in general can be increased following a drug-induced, immune-mediated IR 7, ∗50. Complement can be activated by immune complexes. Therefore, complement activation markers may help demonstrate that an IR was ADA-mediated and not a direct cytokine release event 18, 19. The most common complement markers are CH50 (serum) and C3a, C5a, Sc5b-9 (plasma). CH50 is a measure of total complement. Decreases or even depletion of CH50 can occur when complement is activated and complement split products are generated ∗∗2, ∗6, ∗∗51. In this instance, the complement split products, C3a, C5a, and Sc5b-9, will increase. In the authors' experience, CH50 decreases alone can be adequate to demonstrate complement activation. For hematology, red blood cells and neutrophils play a critical role in immune complex formation, because immune complexes bind to the FCγ receptor and may be increased in animals with IRs ∗6, 7. However, following an IR in which complement is activated, RBCs and neutrophils may be phagocytized and rapidly cleared by the mononuclear phagocytic system or sequestered in tissues and thus decreased ∗∗2, ∗52. Decreases in red blood cells (RBCs), with corresponding decreases in hemoglobin (Hgb) and hematocrit (Hct), can occur immediately after an IR, resulting in subsequent increases in reticulocytes, mean corpuscular volume (MCV), and red cell distribution width (RDW). Coagulopathy is also common following an ADA-mediated IR with prolonged prothrombin time and activated partial thrombin time and/or a decrease in platelets or fibrinogen ∗∗2, ∗∗5, ∗52. Increases in fibrinogen can also indicate an inflammatory response 8, ∗38. Changes in clinical chemistry immediately following an IR may include decreased albumin and potassium and/or increased creatinine, glucose, triglycerides, and phosphorus; these changes may correlate to histopathology changes in the liver or kidney ∗∗5, 15.
8. Histology biomarkers after nonclinical IR
It is well accepted that, while immune complexes (ADA bound to a biotherapeutic) can mediate IRs in nonclinical species, these reactions are not necessarily predictive of an immune response in humans ∗∗2, ∗6. However, demonstrating that a nonclinical IR and any subsequent changes in clinical pathology and pathology are immune-mediated and not a direct test-article effect remains challenging and requires a weight-of-evidence approach. Even in the presence of substantial peripheral blood biomarker data, suggesting immune-complex formation, additional context is needed to determine definitively whether immune complex deposition or complement activation is specifically the cause of any histopathological lesions in animals with or without IRs. Distinguishing direct test-article-related lesions from immune-mediated lesions that are secondary to test-article administration generally requires immunohistochemistry evaluation (Table 2) [2]. Immune-complex formation in circulation is common, whereas tissue deposition requires a cascade of events. Although tissue deposition is considered rare, it is recognized that requests for IHC to confirm IC deposition have increased in the last decade [2]. A recent example of immune-mediated tissue deposition is described for obinutuzumab below [15]. Insoluble, large immune complexes of ADA bound to a biotherapeutic are cleared readily by the liver and spleen via the mononuclear phagocytic system ∗∗2, 3. Conversely, moderate to smaller soluble immune complexes are present in circulation longer, and can facilitate receptor recycling of the biotherapeutic, which can increase ADA titer and the severity of an IR [3] (Fig. 1). However, it is recognized that IRs can occur with low ADA titers, where the ratios of antibody (ADA) to antigen (biotherapeutic) are near molar equivalence (small soluble dimer immune complexes) ∗∗2, 3. It is also recognized that the development of an IR is dependent on the individual subject as was observed with obinutuzumab in the case study below. The most common sites for immune-complex deposition appear to occur in the liver, kidney, and small blood vessels, but immune complexes have also been shown to deposit in the spleen, lungs, heart, skin, and brain (choroid plexus). Lesions in these organs may include inflammation (infiltrates, vasculitis, glomerulonephritis), hemorrhage, and thrombi ∗∗2, ∗∗5, ∗6, 7.
9. Regulatory implications of nonclinical IRs
While ADA-mediated delayed IRs are not predictive of human incidence or severity, delayed IRs in nonclinical studies require extensive investigation and due diligence. Differentiating the underlying cause can be confounded by common background findings/tissue lesions, similar lesions in animals without IRs, or similar lesions in animals without ADA. IRs resulting in dosing holidays and unscheduled euthanasia may have an impact on the no-observed-adverse-effect level (NOAEL) in the absence of a weight-of-evidence approach. In the presence of adequate supporting data to demonstrate an immune-mediated reaction, a NOAEL or the highest non-severely toxic dose (HNSTD - for oncology products per ICHS9) could be defined by removing the animals with the IRs [53]. However, the recent Society of Toxicologic Pathology (STP) guidance on adversity does not distinguish between secondary and direct test-article or immune-mediated adverse effects [54]. Therefore, it is important that the scientific interpretation of the IRs and their impact on the overall risk versus benefit of the biotherapeutic in the context of the clinical indication is adequately described and considered in regulatory submissions. Furthermore, even in the presence of data suggesting immune-mediated IRs, regulators may still request additional information or follow-up investigative studies ∗∗5, 15, 19. While nonclinical IRs may not preclude clinical development or marketing approval, longer duration studies in a nonclinical species may be an issue if IRs render continuing a study infeasible, and/or exposure cannot be maintained in an adequate number of animals with the investigational biotherapeutic, which can invalidate a study.
With regard to first-dose infusion reactions and/or biotherapeutics that have the potential to activate the immune system, such as agonists with the potential for cytokine release syndrome, the first-in-human (FIH) starting dose should be derived from the minimally anticipated biological effect level (MABEL) 31, 53. The MABEL should consider all the nonclinical data (in vitro and in vivo), including pharmacokinetic/pharmacodynamic, pharmacology, and toxicology studies ∗55, 56. For molecules that have the potential to induce cytokine release, there are currently no standard or validated in vitro methods, and ongoing efforts to optimize an assay format have been described 28, ∗50, ∗∗51, 57.
In summary, the overall risk versus benefit of the biotherapeutic and clinical indication must be factored into determining whether nonclinical IRs support further clinical development. IRs, whether they are cytokine release-mediated or ADA-mediated, may be clinically manageable and thus need to be addressed in the clinical plan 45, 47.
10. Nonclinical IR and immune complex tissue deposition case study
Obinutuzumab is an example of a marketed product that used a weight-of-evidence approach to demonstrate that observed histopathology lesions were immune-mediated in animals with and without IRs. In a 26-week GLP study with obinutuzumab (anti-CD20 humanized IgG1 mAb), unscheduled deaths (or early decedents) across all three dose groups (6/sex/group) were reported in 7/36 treated cynomolgus monkeys between Days 63 and 230, which spanned the dosing and recovery phases of the study. There were no remarkable cytokine biomarker changes. Clinical chemistry changes were consistent with glomerulonephritis in some animals. Complement activation markers were not noted, likely because obinutuzumab (anti-CD20) is known to activate complement, and thus complement markers would not specifically inform on immune-complex mediated toxicity. Immunogenicity was evident as there was increased clearance of test article in some animals (5/36 treated animals, 0/7 early decedents), ADA-positive animals (7/36 treated animals, 1/7 early decedents), and animals positive for CICs (7/36 treated animals, 3/7 early decedents); however, these three parameters did not show a clear correlation with the early decedents or major histopathological changes (Table 3). Notably, immunogenicity was not detected in 4/7 early decedents; 2 from the dosing phase and 2 from the recovery phase (Table 3 bolded rows). Therefore, to further demonstrate immune-mediated toxicity, IHC was conducted on the kidneys of recovery animals with glomerulonephritis to detect monkey IgG, monkey IgM, and monkey C3. All three markers were increased and provided a weight-of-evidence indication that the histopathology lesions were immune-mediated, despite not detecting TA in the kidneys or being able to correlate ADA or CICs in all animals with lesions. Additionally, transmission electron microscopy (TEM) confirmed the deposition of immune complexes in tissues in two of three tested animals with glomerulonephritis [15]. Despite the weak correlation of immunogenicity with early decedents and animals with histopathological lesions, IHC conducted in recovery animal kidneys, and TEM conducted in a limited number of kidneys, the combined data supported a diagnosis of immune complex–mediated toxicity in at least 6/7 early decedents, and other animals that presented with glomerulonephritis and/or serosal/adventitial inflammation in various tissues. These findings were not considered to be a direct effect of TA administration and did not preclude further clinical development and approval.
Table 3.
Obinutuzumab early decedents and immunogenicity results.a
| Animal No. (sex) | Dose (mg/kg) | Early decedent | ADA positive | Circulating immune-complexes | Accelerated clearance after 85 and 176 days | Major finding |
|---|---|---|---|---|---|---|
| I01632 (M) | 5 | Yes | Yes | Yes | No | Early decedent Dosing Day 63. Physical signs consistent with treatment-related anaphylactoid reaction. |
| I01633 (M) | 5 | No | Yes | Yes | Yes | No major finding |
| I01634 (M) | 5 | No | Yes | NA | Yes | No major finding |
| I01657 (F) | 5 | No | Yes | NA | Yes | No major finding |
| I01659 (F) | 5 | No | Yes | No | No | No major finding |
| I01660 (F) | 5 | No | Yes | NA | Yes | No major finding |
| I01663 (F) | 25 | No | Yes | Yes | Yes | Anaphylactoid reaction; responded well to diphenhydramine pretreatment |
| I01665 (F) | 25 | Yes | Nob | Nob | Nob | Early decedent Dosing Day 149. Marked hepatocellular vacuolation. Clinical signs associated with weight loss and low/no food consumption resulting in marked hepatocellular vacuolation, renal tubular vacuolation, pancreatic acinar cell atrophy, and fat necrosis. |
| I01642 (M) | 25 | Yes | Nob | Nob | Nob | Early decedent Recovery Day 85. Glomerulonephritis, inflammation of serosa/adventitia of urinary bladder |
| I01643 (M) | 25 | Yes | No | Yes | No | Early decedent Recovery Day 230. Glomerulonephritis, inflammation of intestine (mucosa) |
| I01666 (F) | 25 | Yes | No | Yes | No | Early decedent Recovery Day 141. Glomerulonephritis, inflammation of serosa/adventitia, thyroid gland, gall bladder, and kidney interstitium |
| I01644 (M) | 50 | No | No | Yes | No | Serosal/adventitial inflammation of the kidneys, liver, GI tract and others |
| I01670 (F) | 50 | Yes | Nob | Nob | Nob | Early decedent Dosing Day 181. Inflammation of serosa/adventitia |
| I01648 (M) | 50 | No | No | Yes | No | No major finding |
| I01673 (F) | 50 | Yes | Nob | Nob | Nob | Early decedent Recovery Day 92. Glomerulonephritis, inflammation of the serosa/adventitia, pituitary gland, thyroid gland, intestine (mucosal) and kidney interstitium |
NA = Not Analyzed.
Data consolidated from the obinutuzumab pharmacology review.
Reasonably assumed negative based on information from the obinutuzumab pharmacology review.
Summary
Although animal toxicology studies are not predictive of IRs in humans, and thus do not necessarily preclude regulatory approval, observed IRs can be a potential red flag that must be described and explained to regulatory authorities using multiple biomarkers and methods beyond traditional in vivo toxicology endpoints. This weight-of-evidence approach can be used to differentiate immune-mediated effects from direct TA-related effects. Follow-up investigations are important to: (1) understand whether the finding may translate to humans—for example, CD28 agonism [58]—and (2) determine whether the nonclinical IR is associated with ADA. Such determinations are not always possible, but when the data allow for a weight-of-evidence approach, this may help investigators and regulators design or modify the clinical development program, to minimize risk while maximizing potential benefit to patients.
Acknowledgements
The authors would like to thank Dr. Gary Chellman, Dr. Marcie Wood, Ms. Deborah Proctor, Mr. Rick Nelson, and Mr. Ken Dille for their valuable contributions.
Contributor Information
Kirsten M. Mease, Email: kmease@toxstrategies.com.
Amy L. Kimzey, Email: akimzey@toxstrategies.com.
Janice A. Lansita, Email: jlansita@toxstrategies.com.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.van Meer P.J., Kooijman M., Brinks V., Gispen-de Wied C.C., Silva-Lima B., Moors E.H. Immunogenicity of mabs in non-human primates during nonclinical safety assessment. mAbs. 2013;5:810–816. doi: 10.4161/mabs.25234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko J.L., Evans M.G., Price S.A., Han B., Waine G., DeWitte M. Formation, clearance, deposition, pathogenicity, and identification of biopharmaceutical-related immune complexes: review and case studies. Toxicol Pathol. 2014;42:725–764. doi: 10.1177/0192623314526475. [DOI] [PubMed] [Google Scholar]
- 3.Krishna M., Nadler S.G. Immunogenicity to biotherapeutics - the role of anti-drug immune complexes. Front Immunol. 2016;7:21. doi: 10.3389/fimmu.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenass-Lechner F., Staack R.F., Mary J.L., Richter W.F., Winter M., Jordan G. Immunogenicity, inflammation, and lipid accumulation in cynomolgus monkeys infused with a lipidated tetranectin-apoa-I fusion protein. Toxicol Sci. 2016;150:378–389. doi: 10.1093/toxsci/kfw004. [DOI] [PubMed] [Google Scholar]
- Heyen J.R., Rojko J., Evans M., Brown T.P., Bobrowski W.F., Vitsky A. Characterization, biomarkers, and reversibility of a monoclonal antibody-induced immune complex disease in cynomolgus monkeys (Macaca Fascicularis) Toxicol Pathol. 2014;42:765–773. doi: 10.1177/0192623314522559. [DOI] [PubMed] [Google Scholar]
- Leach M.W., Rottman J.B., Hock M.B., Finco D., Rojko J.L., Beyer J.C. Immunogenicity/hypersensitivity of biologics. Toxicol Pathol. 2014;42:293–300. doi: 10.1177/0192623313510987. [DOI] [PubMed] [Google Scholar]
- 7.Chirmule N., Jawa V., Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 2012;14:296–302. doi: 10.1208/s12248-012-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campion S., Aubrecht J., Boekelheide K., Brewster D.W., Vaidya V.S., Anderson L. The current status of biomarkers for predicting toxicity. Expert Opin Drug Metab Toxicol. 2013;9:1391–1408. doi: 10.1517/17425255.2013.827170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasseville V.G., Mansfield K.G., Brees D.J. Safety biomarkers in preclinical development: translational potential. Vet Pathol. 2014;51:281–291. doi: 10.1177/0300985813505117. [DOI] [PubMed] [Google Scholar]
- 10.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2014. Blinatumomab pharmacology review. [Google Scholar]
- 11.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2016. Reslizumab pharmacology review. [Google Scholar]
- 12.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2015. Daratumumab pharmacology review. [Google Scholar]
- 13.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2014. Siltuximab pharmacology review. [Google Scholar]
- 14.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2011. Ipilimumab pharmacology review. [Google Scholar]
- 15.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2013. Obinutuzumab pharmacology review. [Google Scholar]
- 16.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2015. Evolocumab pharmacology review. [Google Scholar]
- 17.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2016. Atezolizumab pharmacology review. [Google Scholar]
- 18.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2004. Natalizumab pharmacology review. [Google Scholar]
- 19.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2009. Ofatumumab pharmacology review. [Google Scholar]
- 20.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2006. Panitumumab pharmacology review. [Google Scholar]
- 21.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2006. Algucosidase alpha pharmacology review. [Google Scholar]
- 22.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2005. Galsulfase pharmacology review. [Google Scholar]
- 23.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2008. Romiplostim pharmacology review. [Google Scholar]
- 24.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2005. Abatacept pharmacology review. [Google Scholar]
- 25.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2008. Rilonacept pharmacology review. [Google Scholar]
- 26.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2002. Immunotoxicology evaluation of investigational new drugs. [Google Scholar]
- 27.Medicines and Healthcare Products Regulatory Agency . 2006. Investigations into adverse incidents during clinical trials of TGN1412. [Google Scholar]
- 28.Stebbings R., Eastwood D., Poole S., Thorpe R. After TGN1412: recent developments in cytokine release assays. J Immunotoxicol. 2013;10:75–82. doi: 10.3109/1547691X.2012.711783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horvath C.J., Milton M.N. The Tegenero incident and the Duff report conclusions: a series of unfortunate events or an avoidable event? Toxicol Pathol. 2009;37:372–383. doi: 10.1177/0192623309332986. [DOI] [PubMed] [Google Scholar]
- 30.Sauna Z.E. 2017. Immunogenicity of protein-based therapeutics.http://www.fda.gov/BiologicsBloodVaccines/ScienceResearch/BiologicsResearchAreas/ucm246804.htm [Accessed 1 March 2017] [Google Scholar]
- 31.FDA-CDER CBER . U.S. Food and Drug Administration - Center for Drug Evaluation and Research & Center for Biologics Evaluation and Research; 2014. Immunogenicity assessment for therapeutic protein products. [Google Scholar]
- 32.Baker M.P., Reynolds H.M., Lumicisi B., Bryson C.J. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self Nonself. 2010;1:314–322. doi: 10.4161/self.1.4.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harding F.A., Stickler M.M., Razo J., DuBridge R.B. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. mAbs. 2010;2:256–265. doi: 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ICH . International conference on harmonization. 2005. Immunotoxicity studies for human pharmaceuticals S8. [Google Scholar]
- 35.Rup B., Pallardy M., Sikkema D., Albert T., Allez M., Broet P. Standardizing terms, definitions and concepts for describing and interpreting unwanted immunogenicity of biopharmaceuticals: recommendations of the innovative medicines initiative ABIRISK consortium. Clin Exp Immunol. 2015;181:385–400. doi: 10.1111/cei.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sailstad J.M., Amaravadi L., Clements-Egan A., Gorovits B., Myler H.A., Pillutla R.C. A white paper–consensus and recommendations of a global harmonization team on assessing the impact of immunogenicity on pharmacokinetic measurements. AAPS J. 2014;16:488–498. doi: 10.1208/s12248-014-9582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Innovative Medicines Initiative . 2015. ABIRISK: proposed terms and definitions for reporting immunogenicity results. Initiative IM. [Google Scholar]
- Bluemel J., Korte S., Schenck E., Weinbauer G.F., editors. The nonhuman primate in nonclinical drug development and safety assessment. Elsevier Science & Technology Books; 2015. [Google Scholar]
- 39.Pineda C., Castaneda Hernandez G., Jacobs I.A., Alvarez D.F., Carini C. Assessing the immunogenicity of biopharmaceuticals. BioDrugs. 2016;30:195–206. doi: 10.1007/s40259-016-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ICH . International Conference on Harmonization; 2011. Preclinical safety evaluation of biotechnology-derived pharmaceuticals S6(R1) [PubMed] [Google Scholar]
- 41.Song S., Yang L., Trepicchio W.L., Wyant T. Understanding the supersensitive anti-drug antibody assay: unexpected high anti-drug antibody incidence and its clinical relevance. J Immunol Res. 2016;2016:1–8. doi: 10.1155/2016/3072586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.FDA-CDER CBER CDRH . U.S. Food and Drug Administration Center for Drug Evaluation and Research & Center for Biologics Evaluation and Research & Center for Devices and Radiological Health; 2016. Assay development and validation for immunogenicity testing of therapeutic protein products guidance for industry. [Google Scholar]
- 43.Ferretti M., Casini-Raggi V., Pizarro T.T., Eisenberg S.P., Nast C.C., Cominelli F. Neutralization of endogenous Il-1 receptor antagonist exacerbates and prolongs inflammation in rabbit immune colitis. J Clin Invest. 1994;94:449–453. doi: 10.1172/JCI117345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casadevall N., Nataf J., Viron B., Kolta A., Kiladjian J.J., Martin-Dupont P. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346:469–475. doi: 10.1056/NEJMoa011931. [DOI] [PubMed] [Google Scholar]
- 45.Doessegger L., Banholzer M.L. Clinical development methodology for infusion-related reactions with monoclonal antibodies. Clin Transl Immunol. 2015;4:1–9. doi: 10.1038/cti.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenz H.J. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601–609. doi: 10.1634/theoncologist.12-5-601. [DOI] [PubMed] [Google Scholar]
- 47.Joerger M. Prevention and handling of acute allergic and infusion reactions in oncology. Ann Oncol. 2012;23(Suppl 10):313–319. doi: 10.1093/annonc/mds314. [DOI] [PubMed] [Google Scholar]
- 48.Boysen M., Schlicksupp L., Dreher I., Loebbert R., Richter M. SEC based method for size determination of immune complexes of therapeutic antibodies in animal matrix. J Immunol Res. 2016;2016:1–9. doi: 10.1155/2016/9096059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quidel Co . 2017. Microvue Cic-Raji Cell Replacement Enzyme Immunoassay Product Insert.https://www.quidel.com/immunoassays/rapid-inflammatory-autoimmune-tests/microvue-cic-raji-cell-replacement-eia-cic-c3d-eia [Accessed 24 January 2017] [Google Scholar]
- Ramani T., Auletta C.S., Weinstock D., Mounho-Zamora B., Ryan P.C., Salcedo T.W. Cytokines: the good, the bad, and the deadly. Int J Toxicol. 2015;34:355–365. doi: 10.1177/1091581815584918. [DOI] [PubMed] [Google Scholar]
- Grimaldi C., Finco D., Fort M.M., Gliddon D., Harper K., Helms W.S. Cytokine release: a workshop proceedings on the state-of-the-science, current challenges and future directions. Cytokine. 2016;85:101–108. doi: 10.1016/j.cyto.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Everds N.E., Tarrant J.M. Unexpected hematologic effects of biotherapeutics in nonclinical species and in humans. Toxicol Pathol. 2013;41:280–302. doi: 10.1177/0192623312467400. [DOI] [PubMed] [Google Scholar]
- 53.ICH . International conference on harmonization. 2009. Nonclinical evaluation for anticancer pharmaceuticals S9 step 4. [Google Scholar]
- 54.Kerlin R., Bolon B., Burkhardt J., Francke S., Greaves P., Meador V. Scientific and regulatory policy committee: recommended (“Best”) practices for determining, communicating, and using adverse effect data from nonclinical studies. Toxicol Pathol. 2016;44:147–162. doi: 10.1177/0192623315623265. [DOI] [PubMed] [Google Scholar]
- Muller P.Y., Brennan F.R. Safety assessment and dose selection for first-in-human clinical trials with immunomodulatory monoclonal antibodies. Clin Pharmacol Ther. 2009;85:247–258. doi: 10.1038/clpt.2008.273. [DOI] [PubMed] [Google Scholar]
- 56.Hansen A.R., Ricci M.S., Razer A., Le Tourneau C., McKeever K., Roskos L., Dixit R., Siu L.L., Hinrichs M.J. Choice of starting dose for biopharmaceuticals in first-in-human phase I cancer clinical trials. Oncologist. 2015;20:653–659. doi: 10.1634/theoncologist.2015-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brennan F.R., Morton L.D., Spindeldreher S., Kiessling A., Allenspach R., Hey A. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. mAbs. 2010;2:233–255. doi: 10.4161/mabs.2.3.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arzerra (ofatumumab) product label. 2014. [Google Scholar]
- 60.FDA-CDER . U.S. Food and Drug Administration Center for Drug Evaluation and Research; 2009. Golimumab pharmacology review. [Google Scholar]
- 61.FDA-CDER . 2017. Drugs@FDA: FDA Approved Drug Products.http://www.accessdata.fda.gov/scripts/cder/daf/ [Accessed 1 March 2017] [Google Scholar]
- 62.EMA . 2017. European Public Assessment Reports.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124 [Accessed 1 March 2017] [Google Scholar]
- 63.Lynch J.L., Wilcox A.L., Baxter E., Tokuyama H., Elliott G.S. Effects of two study procedures on clinical pathology data in cynomolgus monkeys: site of collection and method of restraint. Am Soc Veterinary Clin Pathology. 2012 https://www.asvcp.org/meeting/2012/posters/40.pdf [Google Scholar]
- 64.Wang Y.M., Wang J., Hon Y.Y., Zhou L., Fang L., Ahn H.Y. Evaluating and reporting the immunogenicity impacts for biological products–a clinical pharmacology perspective. AAPS J. 2016;18:395–403. doi: 10.1208/s12248-015-9857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]