Abstract
As nanoparticles exhibit unique properties attractive for vaccine development, they have been progressively implemented as antigen delivery platforms and immune potentiators. Recently, cell membrane-coated nanoparticles have provided a novel approach for intercepting and neutralizing bacterial toxins by leveraging their natural affinity to cellular membranes. Such toxin–nanoparticle assemblies, termed nanotoxoids, allow rapid loading of different types of toxins and have been investigated for their ability to effectively confer protection against bacterial infection. This review will cover the current progress in antibacterial vaccine nanoformulations and highlight the nanotoxoid platform as a novel class of nanoparticulate vaccine. We aim to provide insights into the potential of nanotoxoids as a platform that is facile to implement and can be broadly applied to help address the rising threat of super pathogens.
Keywords: antibiotic-resistant bacteria, biomimetic nanoparticles, nanotoxoids, immunoengineering, vaccine development
Table of Content Graphic
INTRODUCTION
Antibiotic resistance has become an undeniable burden on global health as we move further into the twenty-first century. It is predicted that drug-resistant infections could lead to an annual mortality rate of 10 million people by 2050 and a cumulative cost of up to 100 trillion US dollars on the world’s economy.1, 2 These unsettling projections have necessitated the exploration of new and more effective ways to manage bacterial infection. One area that has received a significant amount of attention is vaccine development. A number of licensed prophylactic vaccines have proven useful in helping to reduce the need for antibiotics, which can tremendously cut healthcare costs,3 as well as hinder the prevalence of drug-resistant bacterial strains.4 However, safe and efficacious vaccines are still unavailable against many pressing infections caused by pathogenic bacteria, including Streptococcus pyogenes, Staphylococcus aureus, Helicobacter pylori, Chlamydia, Shigella, Escherichia coli, and many others.5–8
Conventional antibacterial vaccines are derived from attenuated bacteria, killed bacteria, bacterial subunit antigens, or inactivated bacterial toxins. These platforms have been employed to successfully prevent millions of deaths worldwide every year,9 although some limitations still persist that can affect their widespread applicability.10, 11 Live attenuated vaccines, although they show high immunogenic potency, can present a safety risk, especially for immunocompromised individuals. In contrast, inactivated bacteria have reduced virulence, but are often less immunogenic and cannot effectively elicit protective immunity against infections. Similarly, inactivated subunit antigen and toxoid vaccines may also suffer from suboptimal antigenicity. To overcome the challenges faced by existing formulations, novel antibacterial vaccine strategies are being developed with the goal of enhancing both safety and efficacy.
NANOTECHNOLOGY IN VACCINE DEVELOPMENT
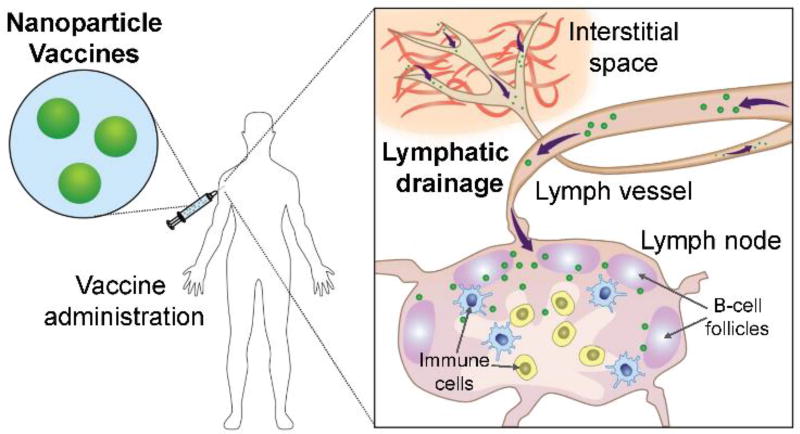
In recent decades, nanotechnology has progressively influenced the field of medicine and biomedical science.12 A number of nanoparticle-based drug delivery systems and vaccines have been explored for the treatment and prevention of infectious diseases.13–16 Inspired by previous successes, the use of nanotechnology in vaccine research has been steadily increasing,17 owing to the unique advantages offered by nanoparticles.17–21 In general, nanoparticulates are prone to cellular uptake. This property allows the nanoparticle-associated antigens to be efficiently taken up and processed by antigen presenting cells. As delivery systems for antigens and/or adjuvants, nanoparticles can be designed with varying physical and material properties, all of which can be fine-tuned to positively impact vaccine efficacy.17, 22, 23 For instance, to take advantage of lymphatic drainage for improved lymph node delivery (Figure 1), particle sizes between 20 and 200 nm are typically used, with the efficiency increasing as nanoparticle size decreases.24 The optimal size window for lymphatic transport may vary depending on the material properties of the nanocarrier.24–26
Figure 1.
Schematic of nanoparticle-based vaccine delivery and transport. Upon administration via the commonly employed subcutaneous route of delivery, the small size of the nanoparticles enables efficient drainage and transport to the lymph nodes. Here, the nanoparticles can deliver their payloads to activate the resident immune cells (i.e. dendritic cells, T cells, and B cells) and promote immunity.
Vaccine antigens are typically loaded into nanoparticles by encapsulation or surface functionalization.17 Immunostimulatory molecules can also be integrated into the nanoformulations, and co-delivery of antigen and adjuvants together has been shown to improve immune responses. In addition, surface functionalization with targeting moieties specific to certain organs, tissues, or cell populations can help to enhance nanoparticle accumulation and immunogenicity.27, 28 Nanoparticles can be designed for prolonged release of encapsulated antigens,29, 30 or they can offer environmental protection and enhanced retention of payloads, enabling local delivery to the gastrointestinal tract and mucosal surfaces.31, 32 Conjugation of antigens onto nanoparticle surfaces mimics the natural presentation of antigens by pathogens such as viruses and microbes.33 In this manner, the repetitive display of immunogens has been increasingly implemented using a variety of nanostructures,34–37 as this enables enhanced recognition by the immune system, which can lead to enhanced immune stimulation.38
NANOPARTICLE-BASED ANTIBACTERIAL VACCINES
In response to the antibiotic resistance crisis, an increasing number of nanovaccine formulations against bacterial infection have been reported. Specifically, the focus has been on tackling high profile drug-resistant infections with no available licensed vaccine. Various nanoparticle-based antibacterial vaccines have been designed taking into consideration the most appropriate antigens, specific bacterial pathology, and the physiological barriers that need be overcome. Collectively, those antibacterial nanovaccines can be classified into two main categories: subunit vaccines and toxoid vaccines.
Subunit vaccine nanoparticles
Subunit vaccines contain only parts of a pathogen. They can be based off isolated proteins, synthetic peptides, or polysaccharides, each of which can elicit a specific protective immune response. Peptides and polysaccharides are typically weak immunogens and often require adjuvants for immune activation. As peptides are T cell-dependent antigens, an association of peptide epitopes with immunostimulatory nanoparticles has been shown to be sufficient in raising immune responses.39 Meanwhile, carbohydrate-based antigens are T cell-independent and generally cannot generate long-term immunity on their own. As such, they are usually conjugated with a protein carrier to facilitate T cell-dependent immunity.40 Various nanoformulations have been investigated as vehicles to deliver polysaccharide-protein conjugates in order to improve vaccine potency.41–43 A recombinant antigenic molecule composed of two Helicobacter pylori antigen epitopes and two adjuvant motifs was developed to enhance Toll-like receptor (TLR)-5 activation.44 Oral vaccination using this formulation encapsulated in acid-resistant nanoparticles induced high antigen-specific antibody titers and completely protected against Helicobacter pylori in 43% of immunized mice.45 Another subunit encapsulating formulation took a biomimetic route for targeted delivery. Hollow vault nanoparticles, surface engineered with staphylococcal protein A,46 were employed to deliver Chlamydia muridarum major outer membrane protein via intranasal immunization and effectively reduced bacteria burden from chlamydial genital infection.47
Beyond single peptides and polysaccharides, a class of material that has gained increasing attention as a collective source of bacterial surface antigens are bacterial outer membrane vesicles (OMVs). OMVs are composed of proteins and glycans in a profile resembling the source bacteria and are also inherently immunostimulatory.48, 49 The vesicles have been shown to promote protective immune responses and have been used clinically as a meningococcal vaccine.50 To extend the efficacy of OMV vaccination even further, a bacterial mimicking nanoparticle platform was developed by coating OMVs onto gold nanoparticles (Figure 2).26 The solid nanoparticle backbone offered homogeneous size tunability and enhanced the stability of the vesicles, while the direct use of OMVs allowed for the faithful delivery of major immune determinants, such as pathogen associated-molecular patterns. This enabled the OMV-coated nanoparticles to effectively activate dendritic cells and enhanced antibacterial immune responses to a level that outperformed a control formulation consisting of OMVs alone.
Figure 2.
Membrane-coated nanoparticles for antibacterial vaccination. (A) Schematic illustration of bacterial membrane-coated nanoparticles for modulating antibacterial immunity. Outer membrane vesicles (OMVs) are collected from source bacteria and coated onto gold nanoparticles (AuNPs) to form bacterial membrane-coated AuNPs (BM-AuNPs). When used for vaccination, BM-AuNPs can elicit specific immunity against the source bacteria. (B) A representative electron microscopy image showing the core-shell structure of the BM-AuNPs negatively stained with uranyl acetate (scale bar, 50 nm). Inset: a zoomed-in view of a single BM-AuNP (scale bar, 10 nm). (C) Size intensity distribution of OMVs and AuNPs, before and after coating with bacterial membrane, as measured by dynamic light scattering. (D) BM-AuNPs elicit strong anti-E. coli IgG titers 21 days after vaccination. (E, F) BM-AuNPs induce pronounced bacterium-specific T cell activation in vivo. On day 21 after vaccination, splenocytes were collected and stimulated with E. coli bacteria. After 72 hours of culture, the levels of (E) IFN-γ and (F) IL-17 were quantified by ELISA. Reprinted with permission from ref 26. Copyright 2015 American Chemical Society.
Toxoid vaccine nanoparticles
Toxoids, or inactivated forms of live bacterial toxins, are among the most common of antivirulence vaccines. By immunizing against bacteria virulence factors, the body generates defensive measures against bacterial mechanisms of attack, thus decreasing their invasiveness. Currently, bacterial toxoids that are clinically used include vaccines against tetanus and diphtheria.51 Toxoids can also be exploited as protein carriers to enhance the immunogenicity of antigenic polysaccharides.52 The fabrication of conventional toxoid vaccines generally involves chemical or heat treatment to eliminate the harmful effects of the toxin in its native form.53, 54 However, such inactivation treatments are often disruptive to the tertiary structure of the toxins and can compromise vaccine efficacy.55 Alternatively, recent efforts have turned towards generating non-toxic, mutated toxins with minimal alteration in antigenic configuration to help overcome the tradeoff that usually exists between safety and immunogenicity.56
The role of nanoparticles in toxoid vaccine formulation has generally been for the enhancement of antigen delivery to target tissues and cell types. For transcutaneous immunization, nano-sized aggregates of tetanus toxoid and polymeric micelles have shown the ability to increase the induction of antibody titers compared to free toxoid alone.57 The adjuvanticity of the micelle–toxoid aggregates was postulated to be due to enhanced absorption through the skin, as demonstrated by increased uptake of the toxoid through the epidermis and dermis. Several platforms have also been designed for mucosal delivery of toxoid vaccines.58, 59 A formulation consisting of cationic nano-sized hydrogels encapsulating non-toxic fragments of Clostridium botulinum type-A neurotoxin (Figure 3) has demonstrated prolonged retention in the nasal cavity, where the released antigen was effectively taken up by mucosal dendritic cells.58 Consequently, strong serum IgG and secretory IgA antibody responses against the botulinum neurotoxin were induced. In addition, oral administration of tetanus toxoid loaded in bilosomes, bile salt stabilized vesicles, has effectively protected the cargo from the hostile environment in the gastrointestinal tract and significantly induced both systemic and mucosal immunity.60 Taken collectively, formulating toxoids into nanoparticulate forms has proven to be effective at enhancing immunostimulation.57–61
Figure 3.
Self-assembled nanogels for toxoid delivery. (A) Schematic of a nano-sized hydrogel (cCHP nanogel) generated from cationic cholesteryl-bearing pullulan. (B) Quantitative study with [111In]-labelled Clostridium botulinum type-A neurotoxin subunit antigen Hc (BoHc/A) demonstrated that the antigen was retained in the nasal tissues significantly more when formulated using the cCHP nanogel. (C) Antibody-forming cells (AFCs) were effectively induced and recruited in the lamina propria and paranasal sinuses of the nasal mucosa 1 week after final immunization with cCHP–BoHc/A. (D) Strong C. botulinum type-A neurotoxin (BoNT/A)-specific IgA antibody responses were observed in nasal washes collected from mice intranasally immunized with cCHP–BoHc/A. (E) Strong BoNT/A-specific serum IgG antibody responses were induced by intranasal immunization with cCHP–BoHc/A. Reprinted with permission from ref 58. Copyright 2010 Springer Nature.
BIOMIMETIC NANOPARTICLE-BASED TOXOID VACCINES
Recently, there has been significant interest in biomimetic nanocarriers, including an emerging cell membrane-coated nanoparticle platform.62–68 The nanoparticles have been leveraged for their biointerfacing capabilities, which enables them to detain pore forming toxins, the largest class of virulent factors present in almost all bacterial pathogens, in a manner that is independent of their molecular structures.69 Red blood cell (RBC) membrane-coated nanoparticles can be fabricated from a combination of poly(lactic-co-glycolic acid) (PLGA) polymeric nanoparticle cores and biological membrane vesicles derived from erythrocytes. The two components are independently prepared and subsequently assembled together by introducing mechanical disruption or ultrasonic forces to facilitate membrane fusion.66, 70 This robust top-down approach of cell membrane translocation results in nanoparticles with a core-shell structure that displays RBC membrane proteins in their proper orientations.71 The nanoparticles can be used as “nanosponges” that naturally attract and provide an anchoring substrate to membrane-targeted toxins, as demonstrated with a variety of pore-forming toxins, including α-hemolysin from Staphylococcus aureus, streptolysin-O from Streptococcus pyogenes, and melittin from bee venom.72 Nanosponges can efficiently neutralize the membrane-damaging activity of pore-forming toxins and prevent the hemolysis of erythrocytes, whereas RBC membrane vesicles do not exhibit any protective capacity. This highlights the role of the inner polymeric core, which acts to stabilize the RBC membrane shell and prevents its fusion with healthy cells. Given their complete lack of toxicity, nanosponge–toxin complexes, or “nanotoxoids”, have been explored as a novel vaccine platform for generating antibacterial immunity (Figure 4).73
Figure 4.
Schematic of nanotoxoid vaccination for protection against MRSA skin infection. Under normal conditions, MRSA bacteria secret toxins to facilitate their colonization, causing skin lesions and systemic invasion. After vaccination using the nanotoxoid formulation, which is fabricated by neutralizing MRSA-secreted toxins with cell membrane-coated nanosponges, anti-toxin antibody production is stimulated. This immunity can prevent necrotic lesion formation at the bacterial challenge site, as well as restrict the systemic invasion of the bacteria.
Single-toxin nanotoxoids
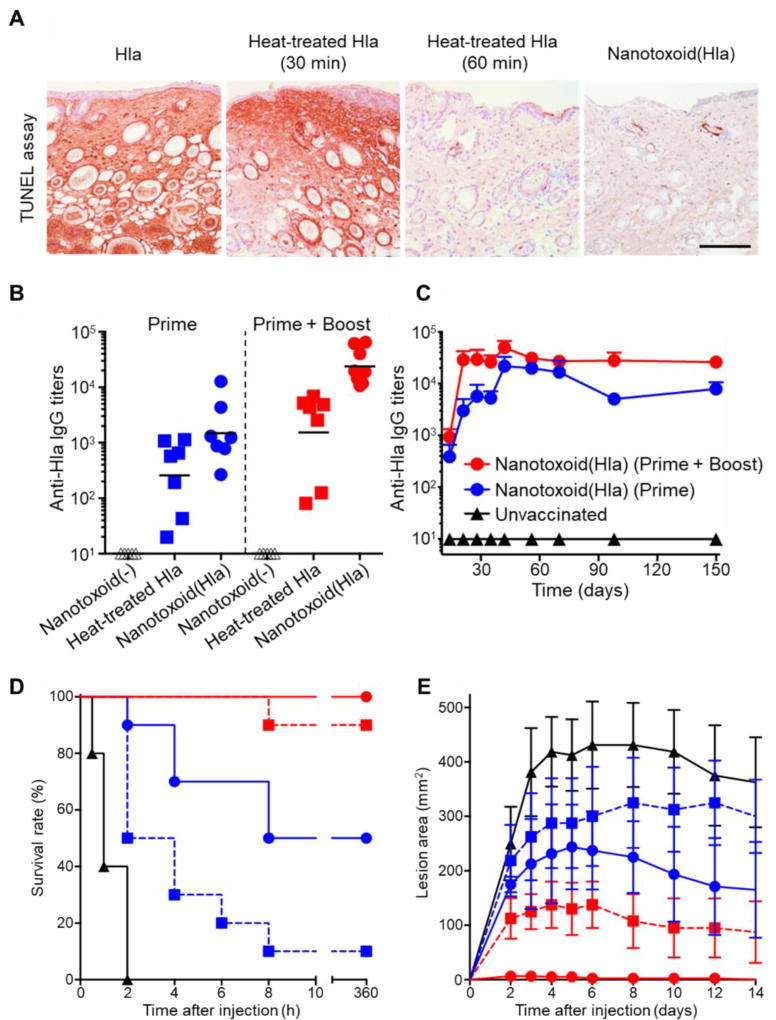
Using staphylococcal α-hemolysin as a model toxin, nanotoxoids were prepared by simply mixing the toxin with a predetermined amount of nanosponges (Figure 5).73 Each nanotoxoid was shown to load approximately 40 toxin molecules, and the complex was confirmed to be a stable assembly, showing no release of toxin over the span of 2 days. In terms of safety, nanotoxoids did not inflict skin lesions after subcutaneous injection in mice, nor did they induce apoptosis on dendritic cells in culture. In comparison, α-hemolysin alone required 60 minutes of heat inactivation at 70 °C to achieve a comparable safety profile. When administered to mice, the nanotoxoid vaccine triggered germinal center formation in the draining lymph nodes, suggesting potent B cell activation,74 and stimulated significantly higher titer production with increased avidity as compared to heat-denatured toxin. With higher humoral immune responses, mice immunized with nanotoxoids were protected against both subcutaneous and systemic challenges with large, bolus doses of α-hemolysin, outperforming mice vaccinated with the heat-inactivated toxin.
Figure 5.
Single-toxin nanotoxoid against staphylococcal α-hemolysin. (A) Nanoparticles detaining α-hemolysin (Hla), termed nanotoxoid(Hla), do not induce apoptosis when injected into the superficial dorsal skin of mice, while 60 minutes of treatment is necessary to achieve the same effect by heat denaturation at 70 °C (scale bar, 400 mm). (B) When used to vaccinate mice, nanotoxoid(Hla) induces higher anti-Hla titers than heat-treated Hla on a prime only or prime plus boost schedule. (C) The antibody titers elicited by the nanotoxoid(Hla) are long lasting, remaining stable for up to 150 days. (D) Nanotoxoid(Hla) protects against a bolus dose of Hla administered intravenously. (E) Nanotoxoid(Hla) prevents the formation of skin lesions when mice are subcutaneously challenged with Hla. Reprinted with permission from ref 73. Copyright 2013 Springer Nature.
In a follow-up study, the potency of the nanotoxoid formulation was further evaluated for its ability to protect against live methicillin-resistant Staphylococcus aureus (MRSA) skin infection in a murine model.74 After bacterial challenge, mice receiving prophylactic immunization with nanotoxoids showed significant reduction in the formation of necrotic lesions. Remarkably, immunized mice exhibited bacteria count reductions of several orders of magnitude in major organs, including the heart, lungs, kidneys, and spleen. The study confirmed that vaccination against a major virulence factor of MRSA using a nanotoxoid formulation could help to stem systemic invasion of the bacteria, ultimately lessening the severity of infection.
Nanotoxoids present a number of attractive features as nanoparticle-based vaccines. In contrast to classically inactivated toxins, nanotoxoids are formed by loading toxins in their native form onto their natural substrates, a process that neutralizes virulence while conserving antigenic determinants. Given the high responses generated against α-hemolysin, this nanoparticle-based detainment strategy can likely be generalized to a wide range of toxins. Although virulence factors from different organisms75 have varying molecular structures, most of them must interact with cellular membranes in order to carry out their function.69 Cell membrane-based nanosponges therefore represent an action mechanism-targeted sequestration platform with broad applicability.72, 76 For vaccine platforms, another aspect to consider is the long-term immunocompatibility of the carrier itself, which is a challenging issue that is hard to overcome for some traditional vaccination schemes.77 With the biocompatible nature of RBC membranes and PLGA polymers, no significant acute or long-term immune responses have been observed against the nanoparticulate vector when vaccinating using nanotoxoids, despite the strong anti-toxin responses induced.78 This ultimately enables flexibility in the design of dosing schedules, allowing for repeated administration in order to further boost immunity.
Multi-toxin nanotoxoid
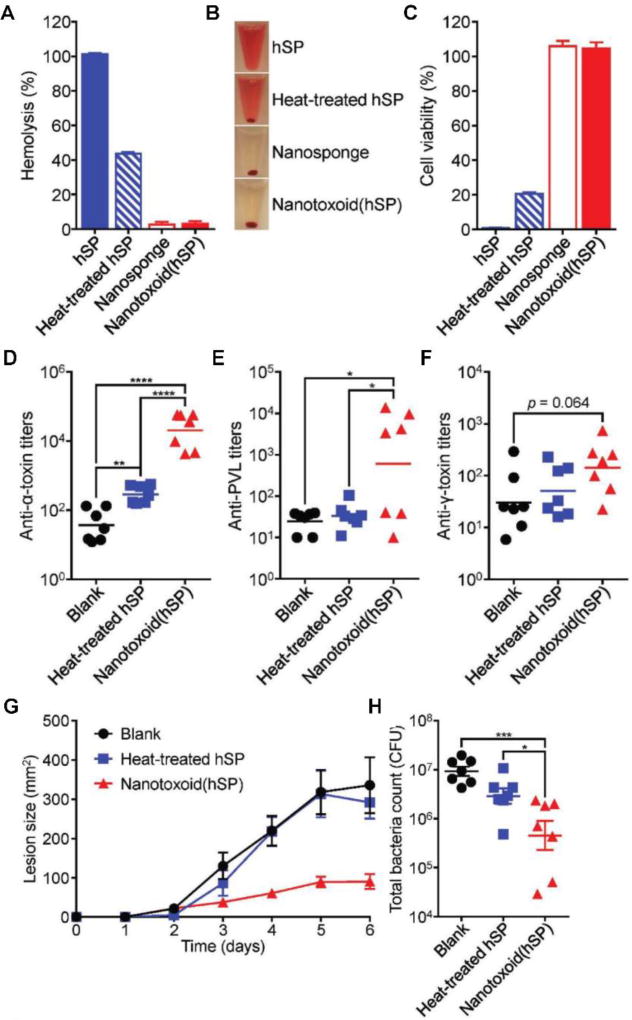
The robust ability of cell membrane-coated nanosponges to detain toxins and promote high immunogenicity in an adjuvant-free manner has further inspired a new approach for the on-demand generation of multi-antigen vaccine formulations (Figure 6).79 To accomplish this, RBC nanosponges were incubated with a crude protein fraction derived from the supernatant of MRSA culture, enabling the nanoparticles to capture any membrane-attacking toxins present in situ. It was confirmed that multiple virulent proteins, including α-hemolysin, γ-hemolysin, and Panton–Valentine leucocidin, could be concurrently absorbed. Additionally, with their ability to capture and neutralize toxins, the nanoparticles were able to completely eliminate the hemolytic and cytotoxic properties of the MRSA proteins. In contrast, despite 4 hours of boiling, heat-treated MRSA proteins still retained almost half of their hemolytic activity and exhibited significant cytotoxicity. The fact that nanoparticle-based toxin detainment could neutralize even heat-insensitive toxins attests to the versatility of the approach. Using the detained toxins as a multi-antigenic nanotoxoid formulation, it was demonstrated that antibody titers could be generated against all three of the aforementioned toxins. This offered significant immune protection against live bacteria in a MRSA skin infection model, helping to greatly reduce both lesion formulation and bacterial load.
Figure 6.
Multi-toxin nanotoxoids against MRSA infection. (A, B) When incubating a hemolytic secreted protein (hSP) fraction from MRSA culture with RBC nanosponges, the resulting nanotoxoid(hSP) formulation does not induce hemolysis, as demonstrated by both (A) hemolysis quantification and (B) visual observation. (C) The nanotoxoid(hSP) formulation does not affect bone marrow-derived dendritic cell viability after 24 hours of incubation. (D–F) When administered into mice on days 0, 7, and 14, nanotoxoid(hSP) can elicit antibody titers against known toxins, including (D) α-hemolysin, (E) Panton–Valentine leucocidin, and (F) γ-hemolysin, when measured on day 21. (G) Nanotoxoid(hSP) vaccination greatly reduces the formation of skin lesions upon subcutaneous challenge with MRSA. (H) After intravenous challenge with bacteria, mice vaccinated with nanotoxoid(hSP) exhibit significantly reduced bacterial loads. Reprinted with permission from ref 79. Copyright 2017 WILEY-VCH.
Many pathogenic bacteria can simultaneously produce multiple toxins and virulence factors.80, 81 The supernatant from bacterial cultures would seem to be an attractive source of multi-antigenic material for the generation of toxoid vaccines, yet this approach has rarely been studied,82 which is likely due to the intrinsic toxicity of such preparations. Additionally, the presence of irrelevant proteins may serve to dilute immune focus. The on-demand nanotoxoid strategy is able to address these challenge, demonstrating affinity for virulent proteins while concurrently neutralizing their activity. By preparing the formulation using crude bacterial protein preparations of unknown composition, such an approach also does not require prior knowledge on the specific structure and function of individual toxin components. This could ultimately be of great advantage, as the process can easily be adapted for different bacterial species and strains regardless of their toxin secretion profiles.80, 81
CONCLUSIONS
Vaccination is considered one of the most cost-effective antibacterial interventions and has the potential to greatly aid in the battle against antibiotic resistance. As vaccines work by preventing the occurrence of bacterial infections, their use can drastically reduce our dependence on antibiotics, helping to preserve the effectiveness of current drugs while limiting the spread of resistance. Along these lines, the application of nanotechnology towards vaccine design has offered useful solutions for addressing some of the limitations facing traditional vaccine formulations. Nanocarriers can protect encapsulated antigens from hostile environments, prolong release over time, respond to environmental cues, and preferentially target tissues and cells of interest. Taking advantage of these properties, both subunit and toxoid vaccines against bacteria have been augmented through formulation into nanoparticulates. Recently, the emergence of the cell membrane-coated nanoparticle platform has enabled the detainment and neutralization of bacterial toxins for the fabrication of nanotoxoid complexes. Single-toxin nanotoxoids have demonstrated the ability to effectively confer immune protection against bolus toxin challenge, as well as live bacterial infections. Furthermore, multiple-toxin nanotoxoids can be formulated in situ against bacterial pathogens with unknown toxin secretion profiles. This facile approach for toxoid vaccine preparation has the potential to be applicable towards a wide range of different bacterial infections.
Although development is still in the early stages, nanotoxoids have proven to be an exciting technology worth future investigation. By leveraging the unique interactions between bacterial virulence factors and specific types of cell membranes, a countless number of nanotoxoid formulations can be explored for vaccine development. These toxin–nanoparticle complexes can further be integrated with other innovative, noninvasive delivery systems for enhanced local immune responses and improved patient compliance.83 For instance, vaccination via the skin can be facilitated by microneedles,84–86 hydrogel patches,87 or skin-penetrating solution,88 whereas oral administration of intestinal patches could assist delivery to the intestinal mucosa.89 In terms of the nanoparticle core, the cell-membrane coating technique has been used successfully with a wide variety of nanomaterials,90–92 offering extensive options for improving immunogenicity. To advance nanotoxoids, along with other nanoparticulate vaccines, from their current exploratory phase to clinical translation, the cost of robust large-scale production under good manufacturing practices must be considered. Fortunately, a variety of industrial-scale manufacturing processes have been developed for nanoformulations,93, 94 making cost-effective commercialization well within reach. In all, this unique toxin detainment approach has brought a new dimension to the established field of toxoid vaccines, and it is anticipated that continued development on these next-generation antibacterial vaccines will ultimately enable more effective ways to manage the threat of antibiotic-resistant bacteria.
Acknowledgments
This work was supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Number HDTRA1-14-1-0064 and by the National Institutes of Health under Award Number R01EY025947.
References
- 1.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017;45:D566–573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piao JG, Wang L, Gao F, You YZ, Xiong Y, Yang L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8:10414–10425. doi: 10.1021/nn503779d. [DOI] [PubMed] [Google Scholar]
- 3.Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008;86:140–146. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, Thomas AR, Harrison LH, Bennett NM, Farley MM, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 5.Ganda IS, Zhong Q, Hali M, Albuquerque RLC, Padilha FF, da Rocha SRP, Whittum-Hudson JA. Dendrimer-conjugated peptide vaccine enhances clearance of Chlamydia trachomatis genital infection. Int. J. Pharm. 2017;527:79–91. doi: 10.1016/j.ijpharm.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camacho AI, Irache JM, de Souza J, Sanchez-Gomez S, Gamazo C. Nanoparticle-based vaccine for mucosal protection against Shigella flexneri in mice. Vaccine. 2013;31:3288–3294. doi: 10.1016/j.vaccine.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N. Engl. J. Med. 2012;366:454–461. doi: 10.1056/NEJMra1108296. [DOI] [PubMed] [Google Scholar]
- 8.Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 2012;10:243–254. doi: 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- 9.Plotkin SA. Vaccines, vaccination, and vaccinology. J. Infect. Dis. 2003;187:1349–1359. doi: 10.1086/374419. [DOI] [PubMed] [Google Scholar]
- 10.Lin IY, Van TT, Smooker PM. Live-attenuated bacterial vectors: Tools for vaccine and therapeutic agent delivery. Vaccines (Basel) 2015;3:940–972. doi: 10.3390/vaccines3040940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbosa T, Barral-Netto M. Challenges in the research and development of new human vaccines. Braz. J. Med. Biol. Res. 2013;46:103–108. doi: 10.1590/1414-431X20131873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emerich DF, Thanos CG. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003;3:655–663. doi: 10.1517/14712598.3.4.655. [DOI] [PubMed] [Google Scholar]
- 13.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 14.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev. Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 15.Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine. 2011;29:9563–9572. doi: 10.1016/j.vaccine.2011.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couvreur P, Vauthier C. Nanotechnology: Intelligent design to treat complex disease. Pharm. Res. 2006;23:1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg AP. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 18.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JD, Morton LD, Ulery BD. Nanoparticles as synthetic vaccines. Curr. Opin. Biotechnol. 2015;34:217–224. doi: 10.1016/j.copbio.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Fang RH, Zhang L. Nanoparticle-based modulation of the immune system. Annu. Rev. Chem. Biomol. Eng. 2016;7:305–326. doi: 10.1146/annurev-chembioeng-080615-034446. [DOI] [PubMed] [Google Scholar]
- 21.Fang RH, Kroll AV, Zhang LF. Nanoparticle-based manipulation of antigen-presenting cells for cancer immunotherapy. Small. 2015;11:5483–5496. doi: 10.1002/smll.201501284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013;3:13. doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamo T, Poland GA. Nanovaccinology: The next generation of vaccines meets 21st century materials science and engineering. Vaccine. 2012;30:6609–6611. doi: 10.1016/j.vaccine.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 25.Oussoren C, Zuidema J, Crommelin DJ, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid composition and lipid dose. Biochim. Biophys. Acta. 1997;1328:261–272. doi: 10.1016/s0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 26.Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P, Zhang Q, Hu CM, Zhang L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15:1403–1409. doi: 10.1021/nl504798g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du J, Zhang YS, Hobson D, Hydbring P. Nanoparticles for immune system targeting. Drug Discov. Today. 2017;22:1295–1301. doi: 10.1016/j.drudis.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Zeng BJ, Chuan YP, O'Sullivan B, Caminschi I, Lahoud MH, Thomas R, Middelberg AP. Receptor-specific delivery of protein antigen to dendritic cells by a nanoemulsion formed using top-down non-covalent click self-assembly. Small. 2013;9:3736–3742. doi: 10.1002/smll.201300078. [DOI] [PubMed] [Google Scholar]
- 29.Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, Fahmy TM. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33:4957–4964. doi: 10.1016/j.biomaterials.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva AL, Rosalia RA, Sazak A, Carstens MG, Ossendorp F, Oostendorp J, Jiskoot W. Optimization of encapsulation of a synthetic long peptide in PLGA nanoparticles: Low-burst release is crucial for efficient CD8+ T cell activation. Eur. J. Pharm. Biopharm. 2013;83:338–345. doi: 10.1016/j.ejpb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Marasini N, Skwarczynski M, Toth I. Oral delivery of nanoparticle-based vaccines. Expert Rev. Vaccines. 2014;13:1361–1376. doi: 10.1586/14760584.2014.936852. [DOI] [PubMed] [Google Scholar]
- 32.Marasini N, Skwarczynski M, Toth I. Intranasal delivery of nanoparticle-based vaccines. Ther. Deliv. 2017;8:151–167. doi: 10.4155/tde-2016-0068. [DOI] [PubMed] [Google Scholar]
- 33.Little SR. Reorienting our view of particle-based adjuvants for subunit vaccines. Proc. Natl. Acad. Sci. U. S. A. 2012;109:999–1000. doi: 10.1073/pnas.1120993109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc. Natl. Acad. Sci. U. S. A. 2010;107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elamanchili P, Lutsiak CM, Hamdy S, Diwan M, Samuel J. "Pathogen-mimicking" nanoparticles for vaccine delivery to dendritic cells. J. Immunother. 2007;30:378–395. doi: 10.1097/CJI.0b013e31802cf3e3. [DOI] [PubMed] [Google Scholar]
- 36.Nam J, Son S, Moon JJ. Adjuvant-loaded spiky gold nanoparticles for activation of innate immune cells. Cell. Mol. Bioeng. 2017;10:341–355. doi: 10.1007/s12195-017-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowling DJ, Scott EA, Scheid A, Bergelson I, Joshi S, Pietrasanta C, Brightman S, Sanchez-Schmitz G, Van Haren SD, Ninkovic J, et al. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 2017;5:1339–1350. doi: 10.1016/j.jaci.2016.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinkernagel RM. On natural and artificial vaccinations. Annu. Rev. Immunol. 2003;21:515–546. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]
- 39.Azmi F, Ahmad Fuaad AA, Skwarczynski M, Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum. Vaccin. Immunother. 2014;10:778–796. doi: 10.4161/hv.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldblatt D. Conjugate vaccines. Clin. Exp. Immunol. 2000;119:1–3. doi: 10.1046/j.1365-2249.2000.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calderon-Gonzalez R, Teran-Navarro H, Frande-Cabanes E, Ferrandez-Fernandez E, Freire J, Penades S, Marradi M, Garcia I, Gomez-Roman J, Yanez-Diaz S, et al. Pregnancy vaccination with gold glyco-nanoparticles carrying Listeria monocytogenes peptides protects against listeriosis and brain- and cutaneous-associated morbidities. Nanomaterials (Basel) 2016;6:151. doi: 10.3390/nano6080151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gregory AE, Judy BM, Qazi O, Blumentritt CA, Brown KA, Shaw AM, Torres AG, Titball RW. A gold nanoparticle-linked glycoconjugate vaccine against Burkholderia mallei. Nanomedicine. 2015;11:447–456. doi: 10.1016/j.nano.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrade GR, New RR, Sant'Anna OA, Williams NA, Alves RC, Pimenta DC, Vigerelli H, Melo BS, Rocha LB, Piazza RM, et al. A universal polysaccharide conjugated vaccine against O111 E. coli. Hum. Vaccin. Immunother. 2014;10:2864–2874. doi: 10.4161/21645515.2014.972145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song H, Lv X, Yang J, Liu W, Yang H, Xi T, Xing Y. A novel chimeric flagellum fused with the multi-epitope vaccine CTB-UE prevents Helicobacter pylori-induced gastric cancer in a BALB/c mouse model. Appl. Microbiol. Biotechnol. 2015;99:9495–9502. doi: 10.1007/s00253-015-6705-z. [DOI] [PubMed] [Google Scholar]
- 45.Tan Z, Liu W, Liu H, Li C, Zhang Y, Meng X, Tang T, Xi T, Xing Y. Oral Helicobacter pylori vaccine-encapsulated acid-resistant HP55/PLGA nanoparticles promote immune protection. Eur. J. Pharm. Biopharm. 2017;111:33–43. doi: 10.1016/j.ejpb.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Kickhoefer VA, Han M, Raval-Fernandes S, Poderycki MJ, Moniz RJ, Vaccari D, Silvestry M, Stewart PL, Kelly KA, Rome LH. Targeting vault nanoparticles to specific cell surface receptors. ACS Nano. 2009;3:27–36. doi: 10.1021/nn800638x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Champion CI, Kickhoefer VA, Liu G, Moniz RJ, Freed AS, Bergmann LL, Vaccari D, Raval-Fernandes S, Chan AM, Rome LH, et al. A vault nanoparticle vaccine induces protective mucosal immunity. PLoS One. 2009;4:e5409. doi: 10.1371/journal.pone.0005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015;10:1689–1706. doi: 10.1002/biot.201400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerritzen MJH, Martens DE, Wijffels RH, van der Pol L, Stork M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 2017;35:565–574. doi: 10.1016/j.biotechadv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Holst J, Oster P, Arnold R, Tatley MV, Naess LM, Aaberge IS, Galloway Y, McNicholas A, O'Hallahan J, Rosenqvist E, et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): Lessons from past programs and implications for the future. Hum. Vaccin. Immunother. 2013;9:1241–1253. doi: 10.4161/hv.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendoza N, Ravanfar P, Satyaprakash A, Pillai S, Creed R. Existing antibacterial vaccines. Dermatol. Ther. 2009;22:129–142. doi: 10.1111/j.1529-8019.2009.01225.x. [DOI] [PubMed] [Google Scholar]
- 52.Gupta RK, Siber GR. Adjuvants for human vaccines—Current status, problems and future prospects. Vaccine. 1995;13:1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 53.Cryz SJ, Jr, Furer E, Germanier R. Effect of chemical and heat inactivation on the antigenicity and immunogenicity of Vibrio cholerae. Infect. Immun. 1982;38:21–26. doi: 10.1128/iai.38.1.21-26.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metz B, Kersten GF, Hoogerhout P, Brugghe HF, Timmermans HA, de Jong A, Meiring H, ten Hove J, Hennink WE, Crommelin DJ, et al. Identification of formaldehyde-induced modifications in proteins: Reactions with model peptides. J. Biol. Chem. 2004;279:6235–6243. doi: 10.1074/jbc.M310752200. [DOI] [PubMed] [Google Scholar]
- 55.Jones RG, Liu Y, Rigsby P, Sesardic D. An improved method for development of toxoid vaccines and antitoxins. J. Immunol. Methods. 2008;337:42–48. doi: 10.1016/j.jim.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Donald RG, Flint M, Kalyan N, Johnson E, Witko SE, Kotash C, Zhao P, Megati S, Yurgelonis I, Lee PK, et al. A novel approach to generate a recombinant toxoid vaccine against Clostridium difficile. Microbiology. 2013;159:1254–1266. doi: 10.1099/mic.0.066712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saydam M, Cheng WP, Palmer N, Tierney R, Francis R, MacLellan-Gibson K, Khan A, Mawas F. Nano-sized Soluplus® polymeric micelles enhance the induction of tetanus toxin neutralising antibody response following transcutaneous immunisation with tetanus toxoid. Vaccine. 2017;35:2489–2495. doi: 10.1016/j.vaccine.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Nochi T, Yuki Y, Takahashi H, Sawada S, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010;9:572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 59.Pirouzmand H, Khameneh B, Tafaghodi M. Immunoadjuvant potential of cross-linked dextran microspheres mixed with chitosan nanospheres encapsulated with tetanus toxoid. Pharm. Biol. 2017;55:212–217. doi: 10.1080/13880209.2016.1257032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mann JF, Scales HE, Shakir E, Alexander J, Carter KC, Mullen AB, Ferro VA. Oral delivery of tetanus toxoid using vesicles containing bile salts (bilosomes) induces significant systemic and mucosal immunity. Methods. 2006;38:90–95. doi: 10.1016/j.ymeth.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Nait Mohamed FA, Laraba-Djebari F. Development and characterization of a new carrier for vaccine delivery based on calcium-alginate nanoparticles: Safe immunoprotective approach against scorpion envenoming. Vaccine. 2016;34:2692–2699. doi: 10.1016/j.vaccine.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 62.Dehaini D, Fang RH, Zhang L. Biomimetic strategies for targeted nanoparticle delivery. Bioeng. Transl. Med. 2016;1:30–46. doi: 10.1002/btm2.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang RH, Jiang Y, Fang JC, Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83. doi: 10.1016/j.biomaterials.2017.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu CM, Fang RH, Zhang L. Erythrocyte-inspired delivery systems. Adv. Healthc. Mater. 2012;1:537–547. doi: 10.1002/adhm.201200138. [DOI] [PubMed] [Google Scholar]
- 65.Fang RH, Hu CMJ, Zhang LF. Nanoparticles disguised as red blood cells to evade the immune system. Expert Opin. Biol. Ther. 2012;12:385–389. doi: 10.1517/14712598.2012.661710. [DOI] [PubMed] [Google Scholar]
- 66.Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, O'Connor DE, Zhang L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang RH, Luk BT, Hu CMJ, Zhang LF. Engineered nanoparticles mimicking cell membranes for toxin neutralization. Adv. Drug Deliver. Rev. 2015;90:69–80. doi: 10.1016/j.addr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Copp JA, Fang RH, Luk BT, Hu CMJ, Gao WW, Zhang K, Zhang LF. Clearance of pathological antibodies using biomimetic nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2014;111:13481–13486. doi: 10.1073/pnas.1412420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luk BT, Hu CM, Fang RH, Dehaini D, Carpenter C, Gao W, Zhang L. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale. 2014;6:2730–2737. doi: 10.1039/c3nr06371b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu CM, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013;8:336–340. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu CM, Fang RH, Luk BT, Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 2013;8:933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang F, Fang RH, Luk BT, Hu CJ, Thamphiwatana S, Dehaini D, Angsantikul P, Kroll AV, Pang Z, Gao W, et al. Nanoparticle-based antivirulence vaccine for the management of methicillin-resistant Staphylococcus aureus skin infection. Adv. Funct. Mater. 2016;26:1628–1635. doi: 10.1002/adfm.201505231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Los FC, Randis TM, Aroian RV, Ratner AJ. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013;77:173–207. doi: 10.1128/MMBR.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Escajadillo T, Olson J, Luk BT, Zhang L, Nizet V. A red blood cell membrane-camouflaged nanoparticle counteracts streptolysin O-mediated virulence phenotypes of invasive group A streptococcus. Front. Pharmacol. 2017;8:477. doi: 10.3389/fphar.2017.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schutze MP, Leclerc C, Jolivet M, Audibert F, Chedid L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J. Immunol. 1985;135:2319–2322. [PubMed] [Google Scholar]
- 78.Luk BT, Fang RH, Hu CM, Copp JA, Thamphiwatana S, Dehaini D, Gao W, Zhang K, Li S, Zhang L. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics. 2016;6:1004–1011. doi: 10.7150/thno.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei X, Gao J, Wang F, Ying M, Angsantikul P, Kroll AV, Zhou J, Gao W, Lu W, Fang RH, et al. In situ capture of bacterial toxins for antivirulence vaccination. Adv. Mater. 2017;29:1701644. doi: 10.1002/adma.201701644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008;46(Suppl 5):S350–359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barnett TC, Cole JN, Rivera-Hernandez T, Henningham A, Paton JC, Nizet V, Walker MJ. Streptococcal toxins: Role in pathogenesis and disease. Cell. Microbiol. 2015;17:1721–1741. doi: 10.1111/cmi.12531. [DOI] [PubMed] [Google Scholar]
- 82.Shewen PE, Wilkie BN. Vaccination of calves with leukotoxic culture supernatant from Pasteurella haemolytica. Can. J. Vet. Res. 1988;52:30–36. [PMC free article] [PubMed] [Google Scholar]
- 83.Schulz C, Efinger K, Schwarz W, Mauer UM. Experiences with cement leakage after balloon kyphoplasty. Orthopade. 2012;41:881–888. doi: 10.1007/s00132-012-1964-1. [DOI] [PubMed] [Google Scholar]
- 84.de Groot AM, Du G, Monkare J, Platteel ACM, Broere F, Bouwstra JA, Sijts A. Hollow microneedle-mediated intradermal delivery of model vaccine antigen-loaded PLGA nanoparticles elicits protective T cell-mediated immunity to an intracellular bacterium. J. Control. Release. 2017;266:27–35. doi: 10.1016/j.jconrel.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 85.DeMuth PC, Moon JJ, Suh H, Hammond PT, Irvine DJ. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano. 2012;6:8041–8051. doi: 10.1021/nn302639r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du G, Hathout RM, Nasr M, Nejadnik MR, Tu J, Koning RI, Koster AJ, Slutter B, Kros A, Jiskoot W, et al. Intradermal vaccination with hollow microneedles: A comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J. Control. Release. 2017;266:109–118. doi: 10.1016/j.jconrel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 87.Ishii Y, Nakae T, Sakamoto F, Matsuo K, Matsuo K, Quan YS, Kamiyama F, Fujita T, Yamamoto A, Nakagawa S, et al. A transcutaneous vaccination system using a hydrogel patch for viral and bacterial infection. J. Control. Release. 2008;131:113–120. doi: 10.1016/j.jconrel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Desai P, Patlolla RR, Singh M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010;27:247–259. doi: 10.3109/09687688.2010.522203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Banerjee A, Mitragotri S. Intestinal patch systems for oral drug delivery. Curr. Opin. Pharmacol. 2017;36:58–65. doi: 10.1016/j.coph.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Li LL, Xu JH, Qi GB, Zhao X, Yu F, Wang H. Core-shell supramolecular gelatin nanoparticles for adaptive and "on-demand" antibiotic delivery. ACS Nano. 2014;8:4975–4983. doi: 10.1021/nn501040h. [DOI] [PubMed] [Google Scholar]
- 91.Zhang J, Gao W, Fang RH, Dong A, Zhang L. Synthesis of nanogels via cell membrane-templated polymerization. Small. 2015;11:4309–4313. doi: 10.1002/smll.201500987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xuan M, Shao J, Dai L, He Q, Li J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv. Healthc. Mater. 2015;4:1645–1652. doi: 10.1002/adhm.201500129. [DOI] [PubMed] [Google Scholar]
- 93.Paliwal R, Babu RJ, Palakurthi S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech. 2014;15:1527–1534. doi: 10.1208/s12249-014-0177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perry JL, Herlihy KP, Napier ME, Desimone JM. PRINT: A novel platform toward shape and size specific nanoparticle theranostics. Acc. Chem. Res. 2011;44:990–998. doi: 10.1021/ar2000315. [DOI] [PMC free article] [PubMed] [Google Scholar]