Abstract
Objectives:
To determine the mortality rates and predictors among patients hospitalized with active tuberculosis (TB) at King Abdulaziz University Hospital (KAUH) in Jeddah.
Methods:
A retrospective study was performed on 291 active TB patients hospitalized in KAUH, Jeddah, Saudi Arabia from December 2011 to December 2016. Medical records were collected and evaluated using a dedicated data extraction sheet. The records included demographics, clinical, radiological, laboratory, and drug resistance data.
Results:
Of the 291 patients, 168 had pulmonary TB, 39 had extrapulmonary TB, and 84 had both pulmonary and extrapulmonary TB. After a mean hospital stay of 1.74 months, 85.9% were successfully treated and discharged. However, 14% died in the hospital after a mean stay of 1.87 months. Predictors of inpatient TB mortality were older age, congestive heart failure, renal failure, diabetes mellitus, chronic lung disease, hepatitis B virus infection, bilateral pulmonary TB, miliary TB, pleural effusion, and leukopenia. In particular, a logistic regression model revealed a mortality probability of 90% in patients older than 65 with congestive heart failure and bilateral lung involvement. However, drug resistance did not significantly affect the mortality rate.
Conclusions:
The inpatient TB mortality rate was lower than mortality rates described previously. Nevertheless, early recognition, appropriate treatments, and education for patients and caregivers concerning treatment, efficient medical management, and effective preventive measures can further reduce mortality.
Tuberculosis (TB) remains one of the world’s deadliest diseases, and TB patients display a high mortality rate. It is estimated that almost one-third of the world’s population suffers from this disease.1 In 2013, the global incidence of TB was 9 million people, and 1.5 million died from TB. This increased to 10.4 million reported cases in 2015 and 1.8 million deaths, making TB one of the 10 most prevalent causes of deaths worldwide.2 Several obstacles to controlling the global spread of TB have been reported. These include poor health system strategies, malnutrition, diabetes, smoking, delayed diagnoses, delayed treatments (especially in poor countries), and the lack of research regarding the incidence of TB and factors contributing to its high mortality rates.3,4
The main factors considered for TB epidemiology are incidence rates and mortality. Therefore, knowing the death rates of TB and risk factors associated with mortality is necessary for devising effective interventions that lower death rates.5 A striking difference between other bacterial infections and TB is that only 5% to 10% of infected TB patients actually develop the active form of the disease, and the incubation period ranges from weeks to years.6 This worsens the prognosis of TB because the delay likely increases the mortality rate. The emergence of multi-drug-resistant strains of Mycobacterium tuberculosis (MDR-TB) is another reason for the global surge in TB-related mortality.7
Many studies have been conducted on the mortality rates of patients with TB. However, these results have been mostly reported in low-income, developing countries and in regions with a high prevalence of human immunodeficiency virus (HIV).8,9 Several factors have been predicted to be associated with deaths resulting from active TB. These include a positive sputum smear result in patients in the late stages of the disease.10 Few studies have reported mortality among patients hospitalized with active TB. Inaccurate death certification reports and imperfect surveys have added to the unreliability of existing data.4 As a result, research on the mortality rates of TB-affected hospitalized populations is needed.
Saudi Arabia, the third-largest Middle East country with a population composed of both Saudi nationals and non-Saudi nationals, continues to have a moderate TB burden. Tuberculosis remains a major health problem in Saudi Arabia, especially the extra-pulmonary form of the disease.11 Despite all efforts by the Saudi government, the treatment success rate for TB in this country is 62%. This is well below the ideal benchmark set by the World Health Organization (WHO), which is considered to be a treatment success rate of at least 85%.2 Furthermore, there is lack of data pertaining to the mortality rates of patients hospitalized with active TB.
Accurate identification of the predictors and quantification of the mortality rates caused by an active TB infection will help in devising effective measures to control the spread of the disease. This will also help lower the mortality rate. In this study, an attempt has been made to assess the mortality rate and mortality predictors among patients hospitalized with active TB in King Abdulaziz University Hospital in Jeddah, Saudi Arabia.
Methods
A retrospective study was performed on 291 TB patients hospitalized with active TB in King Abdulaziz University Hospital (a tertiary care hospital with 738 beds) in Jeddah, Saudi Arabia over a 5-year period from December 2011 to December 2016. All patients with a confirmed TB diagnosis based on microbiological (positive TB culture) or histopathological features (visualization of necrotizing granulomatous inflammation) consistent with TB with good response to anti-TB treatments were included. The patients’ demographics, comorbidities, radiological, laboratory, and microbiological data were collected using a dedicated data extraction sheet. Information about drug susceptibilities and side effects were also collected. All data remained classified and were utilized only for the purpose of this research.
Before conducting the research, ethical approval was obtained from the Research Committee of the Unit of Biomedical Ethics at the Faculty of Medicine of King Abdulaziz University, which is part of the Ministry of Higher Education in the Kingdom of Saudi Arabia. The study adhered to the guidelines of the Declaration of Helsinki.
Statistical analyses were performed using Statistical Package of Social Science (SPSS) Version 16 (Chicago, IL, USA). The quantitative data were presented as the means and standard deviations. The Student’s t-test was used to test significance for the quantitative data. The qualitative data were presented as numbers and percentages. The chi-square test was used to test significance for the qualitative data. Logistic regression models were developed to determine the significant predictors that might help predict mortality among the patients studied. Significance was considered to be P-values less than 0.05.
Results
A total of 291 patients were hospitalized for TB in King Abdulaziz University Hospital over a 5-year period (December 2011 to December 2016). Of these, 250 were successfully treated and discharged after a mean stay of 1.74 months. However, 41 patients died in the hospital after a mean stay of 1.87 months (Table 1). Among these, 168 had pulmonary TB, 39 had extrapulmonary TB, and 84 had mixed pulmonary and extrapulmonary TB. Of the 89 Saudi nationals hospitalized, 12 died in the hospital and 77 were discharged. Meanwhile, 29 died and 173 were discharged among the 202 non-Saudi nationals.
Table 1.
Demographic data for hospitalized tuberculosis (TB) patients.
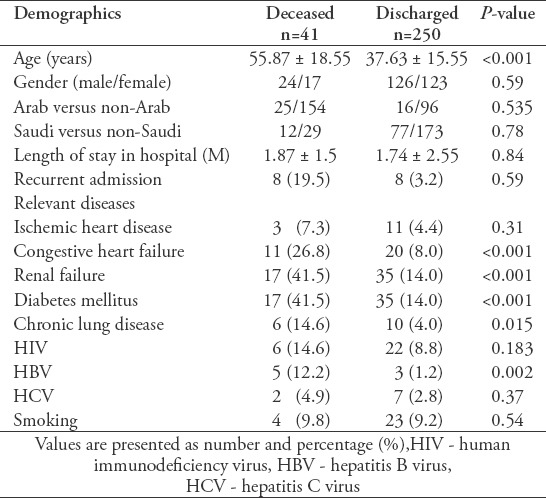
Medical comorbidities for TB patients (such as congestive heart failure, renal failure, diabetes mellitus, chronic lung disease, and hepatitis B virus [HBV] infection) were found to be significantly higher in the deceased group (Table 1). Documented clinical causes of death for those patients were obtained from their medical records. Respiratory failure occurred in 7 patients (17%) and septic shock-related multi-organ failure occurred in 13 patients (32%). These were believed to be either directly or indirectly related to TB. The remaining 21 patients (51%) died from general deterioration resulting from aging or cardiovascular, neurological, or renal conditions.
The deceased patients were significantly older (55.87 years) than those in the discharged group (37.63 years) (Table 1). Although the number of patients older than 65 was comparatively smaller than the younger patients, the mortality was higher. Among the dead patients, 8.7% were younger than 65 and 51.4% were older than 65 (Table 2). Tuberculosis-independent risk factors for mortality, such as HBV infection, were more common in the elderly group compared to the younger group. Significant differences in the levels of basal hemoglobin, discharged hemoglobin, and discharged albumin were observed between the younger and elderly groups (Table 2).
Table 2.
Relationship between patient age and medical and laboratory data.
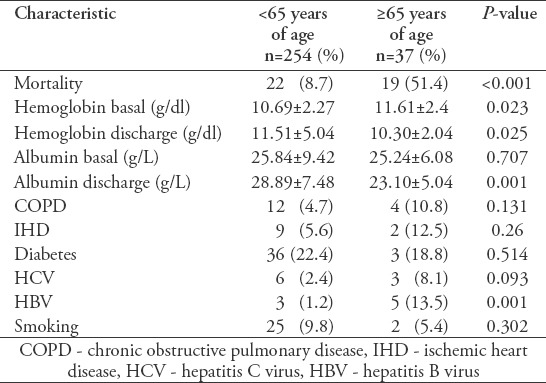
Table 3 shows the radiographic data and laboratory analyses. The presence of bilateral pulmonary TB (PTB), miliary TB, and a pleural effusion were found to be significantly more frequent in the deceased group than in the discharged group. However, there were no significant differences between the unilateral pulmonary TB and extrapulmonary TB groups. Data for the laboratory analyses showed significant differences in numbers of basal white blood cells (WBCs), the levels of basal albumin, and the presence of hypoalbuminemia between the 2 groups.
Table 3.
Clinical and laboratory data for tuberculosis (TB) patients.
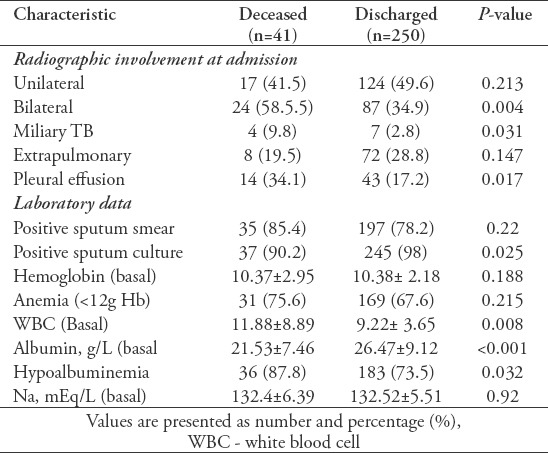
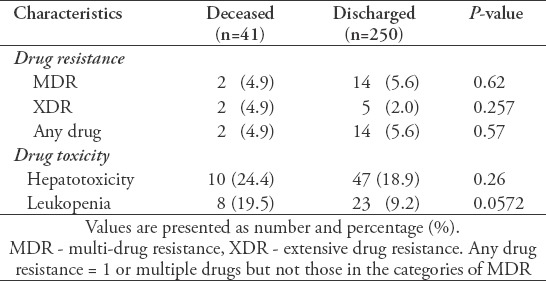
Table 4 shows the results of the anti-TB treatment. No significant differences were found with respect to the prevalence of MDR-TB, extensive drug-resistant (XDR) TB, or any drug resistance between the deceased and discharged groups. However, the occurrence of leukopenia was significantly higher in the deceased group than in the discharged group. Furthermore, there was no significant difference in hepatotoxicity among the 2 groups.
Table 4.
Effects of tuberculosis (TB) drug treatment.
In this study, numerous clinical features were found to be predictors of mortality based upon a univariate analysis, including old age (>65 years), congestive heart failure, renal failure, diabetes mellitus, chronic lung disease, and HBV. Similarly, laboratory parameters, such as basal WBC, basal albumin, hypoalbuminemia, and treatment-induced leukopenia, were found to be risk factors for mortality in TB patients. Furthermore, the results indicated that bilateral pulmonary TB, miliary TB, and pleural effusions were strong predictors for mortality. However, several mortality-related characteristics were not found to be predictors of death, including nationality, length of hospital stay, smoking, ischemic heart disease (IHD), HIV, hepatitis C virus (HCV), unilateral pulmonary TB, positive sputum smear, basal hemoglobin, and anemia.
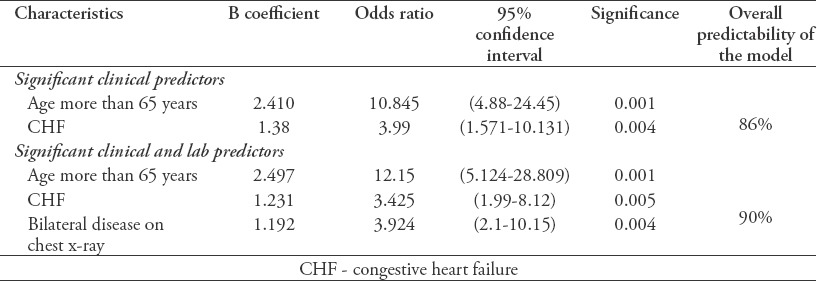
A logistic regression was performed to determine the most significant predictors of mortality for these clinical variables using a univariate analysis (which included age, congestive heart failure, renal failure, and diabetes). The logistic model for clinical variables showed that being older than 65 years of age and having congestive heart failure (CHF) were significant predictors, yielding a mortality prediction accuracy of 86%. Similarly, logistic regression was also performed to ascertain the most significant risk factors for death based upon clinical and laboratory variables (age, CHF, renal failure, diabetes, chest x-ray, sputum culture). Being older than 65 years of age, having CHF, and displaying bilateral disease in a chest x-ray were significant predictors, yielding a mortality prediction accuracy of 90% (Table 5).
Table 5.
Logistic regression models determining predictors of mortality among tuberculosis (TB) patients.
Discussion
In an effort to elucidate predictors associated with inpatient TB mortality at King Abdulaziz University Hospital, a retrospective study was carried out on 291 patients hospitalized for active TB over a period of 5 years. This study showed that mortality predictors among TB patients included older age, CHF, renal failure, diabetes mellitus, chronic lung disease, HBV infection, bilateral lung involvement, miliary TB, and pleural effusion. Furthermore, numerous laboratory variables, such as leukocytosis on admission and hypoalbuminemia, were significant predictors of mortality in TB patients.
The inpatient TB mortality rate in this study was 14%. This mortality rate is slightly higher than the mortality rate reported in Taiwan (12.3%).5 However, it is lower in comparison to China (18.9%),12 Korea (30.4%),13 the Philippines (37.5%),14 and Pakistan (42.5%).15 The differences in mortality rates between these countries may be due to the difference in the patient populations studied, which could be influenced by ethnic backgrounds and comorbidities. It is important to note that TB mortality rates from long-term studies of ambulatory patients rather than hospitalized patients are generally lower. The overall long-term mortality rate of ambulatory TB patients was 0.14% in Poland and 6% in Saudi Arabia.16,17 This discrepancy in mortality rates indicates that the highest mortality in TB patients occurs during the early phase of the disease or during hospitalization.
Although TB affects all age groups, it mostly affects elderly people.2 Age plays an important role in TB-related mortality.18 Numerous studies have reported older age as a predictor of TB mortality.12,15-17 In this study, a mortality rate of 51.4% was observed in patients older than 65, while a rate of 8.7% was observed in patients younger than 65. This higher mortality rate in older patients may be due to a lowered immune response. Furthermore, it is more common for elderly people to have multiple comorbidities, such as diabetes mellitus and renal disease, which may alter their immune status and delay diagnosis (especially in the case of CHF, which can mimic the symptoms of TB).
Clinical causes of death in this study were attributed to TB in 17% of patients and to associated comorbidities in 51% of patients. These findings are similar to those of Lin et al,5 who found that most patients (82.7%) died from non-TB-related causes. Furthermore, other studies showed 30% to 50% of patients died from non-TB-related causes.15,19 This fact highlights the effect of comorbidities on a TB patient’s outcomes. Similarly, studies have shown that comorbidities, such as CHF, diabetes mellitus, renal failure, malignancy, and liver disease, are independent factors and significant predictors of TB mortality.5,15,19
Leukocytosis and hypoalbuminemia upon admission were found to be significant predictors of mortality. This may indicate that deceased patients had a suppressed immune system as a result of malnourishment and were therefore sicker upon initial presentation. Similar findings were also reported in another study.13 Various radiographic features were also found to be significant predictors of mortality, such as bilateral lung involvement, pleural effusion, and miliary TB. These findings may result from diagnostic ambiguity upon presentation, which may lead to a delay in diagnosis and treatment initiation. This is especially important in the presence of other comorbidities, such as CHF or renal failure.
Multi-drug resistance is a risk factor for TB-related mortality.2 According to the WHO, there were 250,000 deaths worldwide in 2015 from MDR/RR-TB. Most of these patients were from Asian countries, including India, China, and the Russian Federation.2 Another study has reported a 21% mortality rate for MDR-TB.20 In contrast, drug resistance was not found to be associated with mortality in this study. This finding may be explained by the small sample size of patients with MDR-TB in this study. A similar result was reported by other researchers.21
People with HIV have a greater risk of developing TB because of their suppressed immune system. According to the WHO, HIV-positive people are 20-30 times more likely to develop active TB.2 An HIV infection is a significant risk factor for TB mortality.22 Studies have linked TB-HIV coinfection to higher mortality rates, and a TB-HIV coinfection is generally considered an important predictor of TB mortality.23,24 This may be because of the higher prevalence of MDR among TB-HIV coinfected patients. Surprisingly, the present study does not show any correlation between TB-HIV coinfection and mortality. This outcome may be the result of the small number of patients with TB-HIV coinfection in the present study. It may also be attributed to the fact that the study exclusively examined inpatient TB mortality rather than longer term mortality. These findings may also be elucidated by the low TB-HIV mortality rate (0.06 per 100,000 people) in Saudi Arabia in comparison to the highest TB-HIV mortality rate (133 per 100,000 people) in South Africa.2
Study limitations
The findings of this study should be interpreted in view of its limitations. First, it is a retrospective, single-center study, which may not represent national mortality rates among hospitalized TB patients. Second, for TB patient mortality, multiple factors contributing to the cause of death may occur at the same time. Therefore, the cause of mortality may not be determined with precision, especially in the absence of an autopsy. Third, we could not ascertain whether the delay in diagnosis impacted mortality rates, especially among patients with comorbidities.
In order to more fully understand the mechanisms underpinning TB mortality rates, future studies should focus on different health care centers in all provinces of Saudi Arabia. A longer study duration should also be employed, and comorbidities should be more closely examined as a predictor of mortality.
In conclusion, old age (>65 years) and medical comorbidities (such as CHF, renal failure, diabetes mellitus, chronic lung disease, and HBV infection) are significantly correlated with greater rates of inpatient TB mortality. Mortality happens primarily in high-risk groups (elderly patients with comorbidities), which can be recognized at the beginning of treatment. The strongest predictors of mortality are old age (>65 years), CHF, and bilateral lung involvement. Early recognition, correct treatments, proper medical management of comorbidities, and prevention policies may help reduce TB mortality rates.
Acknowledgment
We acknowledge Dar Research and Knowledge, Jeddah, Saudi Arabia for providing professional editing services to improve the writing in our manuscript. We also acknowledge the help by American Manuscript Editors for providing English language editing of this manuscript
Footnotes
References
- 1.Centers for Disease Control and Prevention. Tuberculosis: data and statistics-United States. [[cited 2016, Accessed 2017 January 9]]. Available at: https://www.cdc.gov/tb/statistics/default.htm .
- 2.World Health Organization. Global tuberculosis report 2016. [[Accessed 2017 January 9]]. Available at: http://www.afro.who.int/sites/default/files/2017-05/9789241565394-eng.pdf .
- 3.Lienhardt C, Glaziou P, Uplekar M, Lonnroth K, Getahun H, Raviglione M. Global tuberculosis control: lessons learnt and future prospects. Nat Rev Microbiol. 2012;10:407–416. doi: 10.1038/nrmicro2797. [DOI] [PubMed] [Google Scholar]
- 4.Glaziou P, Falzon D, Floyd K, Raviglione M. Global epidemiology of tuberculosis. Semin Respir Crit Care Med. 2013;34:3–16. doi: 10.1055/s-0032-1333467. [DOI] [PubMed] [Google Scholar]
- 5.Lin CH, Lin CJ, Kuo YW, Wang JY, Hsu CL, Chen JM, et al. Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis. 2014;14:5. doi: 10.1186/1471-2334-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, et al. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med. 2014;2:321–338. doi: 10.1016/S2213-2600(14)70031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez M, Bartholomay P, Arakaki-Sanchez D, Enarson D, Bissell K, Barreira D, et al. Outcomes of TB treatment by HIV status in National Recording Systems in Brazil 2003-2008. PLoS One. 2012;7:e33129. doi: 10.1371/journal.pone.0033129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Huang F, Chen W, Du X, Zhou MG, Hu J, et al. Estimates of tuberculosis mortality rates in China using the disease surveillance point system 2004-2010. Biomed Environ Sci. 2012;25:483–488. doi: 10.3967/0895-3988.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Duro RP, Dias PF, Ferreira AA, Xerinda SM, Lima Alves CL, Sarmento AC, et al. Severe tuberculosis requiring intensive care: A descriptive analysis. Crit Care Res Pract. 2017;2017:1–9. doi: 10.1155/2017/9535463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Memish ZA, Bamgboye EA, Abuljadayel N, Smadi H, Abouzeid MS, Al Hakeem RF. Incidence of and risk factors associated with pulmonary and extra-pulmonary tuberculosis in Saudi Arabia (2010-2011) PLoS One. 2014;9:e95654. doi: 10.1371/journal.pone.0095654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lui G, Wong RYK, Li F, Lee MKP, Lai RWM, Li TCM, et al. High mortality in adults hospitalized for active tuberculosis in a low HIV prevalence setting. PLoS One. 2014;9:e92077. doi: 10.1371/journal.pone.0092077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CW, Kim SH, Lee SN, Lee SJ, Lee MK, Lee JH, et al. Risk factors related with mortality in patient with pulmonary tuberculosis. Tuberc Respir Dis (Seoul) 2012;73:38–47. doi: 10.4046/trd.2012.73.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimazaki T, Marte SD, Saludar NR, Dimaano EM, Salva EP, Ariyoshi K, et al. Risk factors for death among hospitalised tuberculosis patients in poor urban areas in Manila, The Philippines. Int J Tuberc Lung Dis. 2013;17:1420–1426. doi: 10.5588/ijtld.12.0848. [DOI] [PubMed] [Google Scholar]
- 15.Haque G, Kumar A, Saifuddin F, Ismail S, Rizvi N, Ghazal S, et al. Prognostic factors in tuberculosis related mortalities in hospitalized patients. Tuberc Res Treat. 2014;2014:624671. doi: 10.1155/2014/624671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abouzeid MS, Al Hakeem RF, Memish ZA. Mortality among tuberculosis patients in Saudi Arabia (2001-2010) Ann Saudi Med. 2013;33:247–252. doi: 10.5144/0256-4947.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korzeniewska-Koseła M. Tuberculosis in Poland in 2014. Przegl Epidemiol. 2016;70:261–272. [PubMed] [Google Scholar]
- 18.Heunis JC, Kigozi NG, Chikobvu P, Botha S, van Rensburg HD. Risk factors for mortality in TB patients: a 10-year electronic record review in a South African province. BMC Public Health. 2017;17:38. doi: 10.1186/s12889-016-3972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walpola HC, Siskind V, Patel AM, Konstantinos A, Derhy P. Tuberculosis-related deaths in Queensland, Australia 1989-1998: characteristics and risk factors. Int J Tuberc Lung Dis. 2003;7:742–750. [PubMed] [Google Scholar]
- 20.Salinas JL, Armstrong LR, Silk BJ, Haddad MB, Cegielski JP. Factors associated with all-cause mortality among patients with multidrug-resistant tuberculosis-United States 1993-2013. Clin Infect Dis. 2017;65:1924–1926. doi: 10.1093/cid/cix667. [DOI] [PubMed] [Google Scholar]
- 21.El Sahly HM, Teeter LD, Pawlak RR, Musser JM, Graviss EA. Drug-resistant tuberculosis: a disease of target population in Houston, Texas. J Infect. 2006;53:5–11. doi: 10.1016/j.jinf.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Field N, Lim MS, Murray J, Dowdeswell RJ, Glynn JR, Sonnenberg P. Timing, rates, and causes of death in a large South African tuberculosis programme. BMC Infect Dis. 2014;14:3858. doi: 10.1186/s12879-014-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sileshi B, Deyessa N, Girma B, Melese M, Suarez P. Predictors of mortality among TB-HIV co-infected patients being treated for tuberculosis in Northwest Ethiopia: a retrospective cohort study. BMC Infect Dis. 2013;13:297. doi: 10.1186/1471-2334-13-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mabunda TE, Ramalivhana NJ, Dambisya YM. Mortality associated with tuberculosis/HIV co-infection among patients on TB treatment in the Limpopo province, South Africa. Afr Health Sci. 2014;14:849–854. doi: 10.4314/ahs.v14i4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]


