Abstract
Purpose
Lung cancer has the highest mortality rate among all types of cancer in the United States. National Lung Screening Trial (NLST) demonstrated low dose CT (LDCT) for lung cancer screening decreases both lung cancer related mortality and all-cause mortality. Currently, the only CMS approved lung cancer screening registry is the Lung Cancer Screening Registry (LCSR) administered by the American College of Radiology (ACR). We aim to assess access to lung cancer screening services as estimated by the number and distribution of screening facilities participating in the LCSR, by state, and to evaluate state-level covariates that correlate with access.
Method
The ACR LCSR list of participating lung cancer screening facilities was used as a proxy for the availability of lung cancer screening facilities in each state. Additionally, we normalized the number of facilities by state by the number of screening-eligible individuals using BRFSS data. State-level demographics were obtained from the 2015 BRFSS: poverty level, insured population, unemployed, Black and Latino. We obtained state-specific lung cancer incidence and death rates, number of active physician per 100,000 and Medicare expenditure per capita. We performed linear regression models to examine the influence of these state-level covariates on state-level screening facility number using Stata 11. QGIS, an open source geographic information system, was used to map the distribution of lung cancer screening facilities and to estimate the nearest neighbor index, a measure of facility clustering within each state.
Result
As of 11/18/2016, 2,423 facilities participated in the LCSR. When adjusted by the rate of screening-eligible individuals per 100,000, median population-normalized facility number was 15.7 (interquartile rang (IQR) 25%,75% 10.7,19.3). There was a positive independent effect (coefficient=12.87, 95% CI= 10.93–14.8) between state-level number of screening facility and rate of screen-eligible individuals rate per 100,000. There were no significant correlations between numbers of facility and lung cancer outcomes, state demographic characteristics, or physician supply and Medicare expenditure. In most states, facilities are clustered rather than dispersed with a median nearest neighbor index of 0.65 (IQR25%,75% 0.51,0.81).
Conclusion
Facility number correlated with the rate of screening-eligible individuals per 100,000, a measure of the at-risk population. Alignment of screening facility number and distribution with other clinically relevant epidemiologic factors remains a public health opportunity.
Introduction
Lung cancer, the leading cause of cancer mortality(1), has a high incidence rate and causes a significant portion of new cancer cases (2). Despite well-published data on lung cancer hazards, late stage diagnosis limits effective treatment options (2)(1). Diagnostic delay highlights the importance of lung cancer screening as an effective measure to reduce the mortality and treatment cost of lung cancer. National Lung Screening Trial (NLST) demonstrated low dose CT (LDCT) for lung cancer screening decreases both lung cancer related mortality by 20% and all-cause mortality by 6.7% compared to radiography (chest x ray) screening method(3)(4). This mortality benefit underscores the importance of rapid diffusion of lung cancer screening to practice and eliminating barriers to its implementation. The United States Preventive Services Task Force (USPSTF) recommends that adults 55-80 who are asymptomatic, current smoker or have quit smoking within the last 15 year, tobacco smoking history of at least 30 pack years receive annual LDCT (Grade B)(5). With this recommendation, non-grandfathered commercial insurers must cover LDCT in this population without any out of pocket cost requirement to reduce financial barriers to access effective preventive services(6).
Currently, the only CMS approved lung cancer screening registry is the Lung Cancer Screening Registry (LCSR) administered by the American College of Radiology (ACR)(7). LCSR registers practices that provide LDCT and collects standardized information regarding those individuals who receive lung cancer screening in these registered practices. The LCSR also facilitates reimbursement of LDCT services in Medicare patients for these registered providers (8). While the some of the financial barriers to screening have been reduced, geographic access to services including proximity of at-risk populations to screening facilities represent a potential barrier. We aim to assess the relationship between the availability of lung cancer screening facilities and the size of the at-risk population as well as state-level clinically relevant epidemiologic and demographic variables.
Methods
Data sources
Primary outcome
The publicly available list of screening facilities participating in the LCSR was accessed on November 18th, 2016 from ACR(9). Facility number by state, the primary outcome, was used as a proxy for the availability of lung cancer screening facilities in each state. Additionally, we normalized the number of facilities by state by the number of screening-eligible individuals defined below.
Covariates of the primary outcome
We used population-based data from the 2015 Behavioral Risk Factor Surveillance System (BRFSS) to estimate the lung cancer screening eligible population. The BRFSS, which is described in detail elsewhere(10)(11)is an annual random telephone survey that uses stratified sampling methods for data collection and provides estimates that are representative of U.S. non-institutionalized residents aged ≥18 years. BRFSS produces a large dataset of information regarding health, health risk behaviors, and health service utilization, which for many states is the only source of data available to policymakers(12).
USPSTF recommends lung cancer screening for 55 to 80 years old current or former smokers who have quit smoking in less than 15 years with a 30 pack-year history. We defined current smokers as smoking every day or some days and former smokers as those who have stopped smoking within the past 10 years. The BRFSS response choices precluded assessment of pack-year history and those who have quit within the past 15 years. The BRFSS sampling yielded state-specific proportions of screen-eligible individuals, rather than the absolute number of at-risk individuals. We chose 79 years as the upper age limit to be able to estimate the absolute number of screen-eligible individuals using U.S. Census data, which report population data at 5-year intervals from 55-79 years. We then expressed this as the rate of screen-eligible per 100,000 individuals 55-79 years.
Age-adjusted lung cancer death and incidence per 100,000 persons representing lung cancer related outcomes were obtained from the U.S. Cancer Working Group(13). Smoking prevalence in 2015 was obtained from Centers for Disease Control and Prevention (CDC)(14).
The following state-level demographic data were obtained from the 2015 BRFSS: proportions of those below the poverty level, insured, unemployed, Black and Latino. We defined the poverty level as an annual household income level less than $25,000. Insured individuals are those who report any kind of health care coverage including commercial insurance or noncommercial plans such as Medicare or Medicaid. Individuals were considered unemployed if they report having been unemployed for any period of time.
Physician supply, defined as the number of active physicians per 100,000 individuals were obtained from 2015 State Physician Workforce Data Book(15), which provide state specific data about active physicians and physicians in training. Medicare standardized risk-adjusted per capita costs were obtained from the Centers for Medicare and Medicaid Services Geographic Variation Public Use Files(16).
All correlates were estimated at the state-level.
Analysis
The location and distribution of screening facilities by state were graphically represented using QGIS 2.18, open-source geographic information system (GIS) application that provides data viewing and analysis(17). We calculated the nearest neighbor index (NNI) within each state to investigate state-specific facility location clustering and dispersion patterns. If the index is less than 1, the pattern exhibits clustering while an index great than 1 indicates dispersion. The magnitude in either direction indicates the degree of clustering or dispersal.
We use BRFSS sampling weights and stratum indicators to adjust for the complex sampling design. We first used descriptive statistics to describe the sample’s characteristics. Continuous data were summarized using means and standard deviations; categorical data were summarized using weighted percentages. We performed multivariable linear regression models to further examine the influence of these covariates on state-level screening facility number. We specifically controlled for the screening eligible population in the model. We chose to use non-normalized screening facility number rather than facility number normalized to screening-eligible population to be able to explicitly estimate the independent effect of the rate of screening-eligible individuals per 100,000 compared to other covariates. All statistical analyses were performed using Stata 11 (StataCorp LLC, College Station, TX).
Results
As of 11/18/2016, 2,423 facilities participated in the LCSR (Table 1), with a median number of 32 facilities per state (IQR25%–75% = 13–76) with highest number in the Florida (n=198) and the lowest number in the District of Colombia and Montana (n=3). When adjusted by the rate of screening-eligible individuals per 100,000, median population-normalized facility number was 15.7 (IQR25%–75%= 10.7–19.3). Figure 1 graphically represents the location and distribution of lung cancer screening facilities by state-specific rates of screen-eligible individuals. Figure 2 depicts screening facility location and distribution by NNI. 71% of states had significant facility clustering while 12% demonstrated significant dispersal. Of these states with dispersed facilities, 60% were western states (Utah, Wyoming and Montana).
Table 1.
Lung screening service availability, clinically relevant epidemiologic and demographic factors, by state.
| State | Facility number |
Rate of screening eligibles per 100k |
Population- normalized facility number1 |
Lung cancer incidence rate2 |
Lung cancer death rate2 |
Poverty (%) |
Insured (%) |
Unemployed (%) |
Black (%) |
Latino (%) |
Physician supply3 |
Medicare expenditure4 |
Nearest neighbour index |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alabama | 32 | 2.98 | 10.73 | 66.7 | 54.7 | 34.48 | 86.66 | 19.33 | 25.33 | 3.4 | 206 | $9,887.66 | 0.435 | |
| Alaska | 6 | 0.35 | 16.98 | 54.7 | 47.3 | 21.73 | 86.36 | 11.81 | 5 | 6.36 | 255.6 | $8,213.33 | 0.63 | |
| Arizona | 22 | 3.66 | 5.99 | 48.4 | 35.3 | 33 | 85.5 | 11.59 | 4.54 | 26.57 | 234 | $9,464.51 | 0.255 | |
| Arkansas | 12 | 2.01 | 5.94 | 78.7 | 60.4 | 36.26 | 86.81 | 17.58 | 14.28 | 5.71 | 198.1 | $9,307.84 | 0.575 | |
| California | 97 | 12.74 | 7.60 | 42.6 | 32.1 | 29.79 | 88.12 | 11.95 | 6.93 | 34.69 | 262.5 | $8,531.57 | 0.365 | |
| Colorado | 49 | 2.54 | 19.26 | 42.2 | 29.9 | 21.21 | 88.62 | 9.58 | 4.25 | 17.96 | 273.2 | $9,157.91 | 0.425 | |
| Connecticut | 38 | 1.69 | 22.39 | 62.3 | 37.7 | 22.72 | 92.03 | 12.38 | 10.61 | 13.27 | 337.8 | $9,004.67 | 0.86 | |
| Delaware | 13 | 0.63 | 20.48 | 69.1 | 47.6 | 22.75 | 90 | 11.66 | 20.33 | 7.33 | 266.8 | $9,560.28 | 0.71 | |
| DC | 3 | 0.32 | 9.17 | 55.3 | 42.7 | 25.9 | 90.9 | 18.63 | 44.54 | 8.63 | 849.3 | $8,247.27 | 3.365 | |
| Florida | 198 | 12.61 | 15.69 | 58.8 | 42.6 | 31.08 | 82.91 | 13.81 | 15.52 | 22.67 | 257.2 | $10,298.46 | 0.225 | |
| Georgia | 65 | 5.52 | 11.76 | 64 | 46.4 | 32.13 | 82.14 | 14.28 | 30.19 | 8.11 | 220.9 | $9,370.97 | 0.685 | |
| Hawaii | 10 | 0.57 | 17.41 | 49.3 | 32.3 | 22.91 | 91.11 | 8.66 | 0.93 | 10.44 | 296.5 | $6,535.37 | 0.555 | |
| Idaho | 16 | 0.70 | 22.72 | 46.9 | 36.2 | 26.92 | 85.71 | 9.18 | 0.69 | 10 | 189.6 | $9,189.77 | 0.655 | |
| Illinois | 76 | 7.28 | 10.42 | 63.2 | 45.9 | 26.01 | 90.37 | 10.63 | 14.43 | 14.68 | 271.5 | $9,614.56 | 0.665 | |
| Indiana | 67 | 4 | 16.74 | 71.6 | 54.3 | 28.84 | 88.05 | 12.93 | 8.95 | 5.47 | 222.6 | $9,431.58 | 0.815 | |
| Iowa | 39 | 1.69 | 23.00 | 62 | 46.8 | 21.87 | 92.7 | 7.81 | 2.91 | 4.68 | 211 | $9,073.08 | 0.875 | |
| Kansas | 32 | 1.54 | 20.66 | 61.8 | 44.2 | 25.58 | 86.2 | 9.77 | 5.97 | 9.42 | 214.2 | $9,913.07 | 0.735 | |
| Kentucky | 80 | 3.15 | 25.32 | 93.4 | 69.5 | 28.97 | 92.64 | 17.64 | 8.08 | 2.5 | 225.1 | $9,258.16 | 0.575 | |
| Louisiana | 30 | 3.05 | 9.82 | 68 | 53.8 | 34.3 | 83.8 | 16.9 | 30.98 | 3.23 | 240.9 | $10,300.84 | 0.525 | |
| Maine | 19 | 0.85 | 22.22 | 74.8 | 55.1 | 28.26 | 90.69 | 12.55 | 0.97 | 13.25 | 313.8 | $8,491.39 | 0.545 | |
| Maryland | 54 | 2.94 | 18.33 | 56.6 | 41.1 | 20 | 90.37 | 11.22 | 28.87 | 8.55 | 370.6 | $9,535.89 | 0.55 | |
| Massachusetts | 47 | 3.04 | 15.42 | 62.6 | 41.6 | 22.11 | 93.51 | 12.03 | 6.94 | 9.72 | 432.4 | $9,001.54 | 0.86 | |
| Michigan | 93 | 6.32 | 14.70 | 62.4 | 47.9 | 26.79 | 89.28 | 13.31 | 13.63 | 4.22 | 271.9 | $9,458.63 | 0.65 | |
| Minnesota | 59 | 2.84 | 20.73 | 56.6 | 39 | 19.65 | 93.45 | 7.73 | 5.17 | 4.1 | 282.9 | $8,411.85 | 0.846 | |
| Mississippi | 21 | 1.82 | 11.50 | 75.2 | 58.6 | 42.39 | 82.22 | 21.11 | 37.77 | 1.4 | 184.7 | $10,267.82 | 0.77 | |
| Missouri | 43 | 4.03 | 10.65 | 73.7 | 54.8 | 26.73 | 87.7 | 12.83 | 11.22 | 3.68 | 260.4 | $9,427.52 | 0.535 | |
| Montana | 3 | 0.66 | 4.54 | 58 | 37.6 | 26.87 | 87.5 | 10.62 | 0.5 | 3.43 | 229.5 | $8,490.20 | 3.735 | |
| Nebraska | 30 | 0.92 | 32.50 | 60.1 | 42.8 | 23.72 | 87.71 | 8.77 | 4.56 | 8.42 | 226 | $9,781.99 | 0.89 | |
| Nevada | 16 | 1.55 | 10.29 | 60.1 | 45.5 | 29.41 | 85.22 | 12.5 | 8.86 | 23.86 | 197.4 | $9,554.72 | 0.085 | |
| New Hampshire | 11 | 0.70 | 15.51 | 64.8 | 42.1 | 18.53 | 90.69 | 10.69 | 1.13 | 2 | 300.3 | $8,954.63 | 1.19 | |
| New Jersey | 85 | 4.19 | 20.28 | 57.5 | 38.6 | 22.76 | 88.12 | 11.51 | 13.3 | 18.34 | 290.1 | $9,467.34 | 0.525 | |
| New Mexico | 9 | 1.27 | 7.08 | 39.6 | 30.5 | 36.92 | 88.88 | 13.96 | 2.38 | 44.44 | 235.3 | $7,983.15 | 1.21 | |
| New York | 115 | 9.91 | 11.60 | 59.5 | 39.6 | 29.56 | 89.53 | 13.36 | 16.26 | 17.39 | 353.8 | $8,597.49 | 0.385 | |
| North Carolina | 91 | 6.08 | 14.95 | 68.5 | 49.1 | 32.43 | 84.19 | 14.83 | 21.61 | 7.41 | 244 | $9,075.11 | 0.515 | |
| North Dakota | 7 | 0.37 | 18.63 | 56.4 | 41 | 20 | 91.3 | 7.82 | 2.43 | 2.69 | 237 | $9,419.42 | 7.395 | |
| Ohio | 124 | 7.48 | 16.56 | 67.4 | 51.3 | 27.85 | 91.08 | 12.25 | 11.97 | 2.75 | 279.8 | $9,394.39 | 0.655 | |
| Oklahoma | 21 | 2.62 | 8.00 | 68.7 | 56.8 | 30.76 | 85.59 | 15.25 | 7.45 | 8.22 | 201.8 | $9,961.87 | 0.8 | |
| Oregon | 22 | 2.36 | 9.30 | 55.6 | 42.2 | 27.64 | 90.47 | 12.69 | 2.22 | 10.31 | 291.3 | $7,906.94 | 0.726 | |
| Pennsylvania | 133 | 7.82 | 16.99 | 64.3 | 45.1 | 23.19 | 91.31 | 11.16 | 10.42 | 5.7 | 306.4 | $9,246.61 | 0.655 | |
| Rhode Island | 18 | 0.51 | 34.94 | 69.9 | 50.5 | 25.31 | 91.17 | 14.7 | 5.58 | 12.05 | 346.5 | $8,524.63 | 0.9 | |
| South Carolina | 36 | 2.97 | 12.10 | 64.4 | 49.3 | 32.23 | 85.52 | 15.78 | 26.31 | 4.21 | 223.1 | $9,733.01 | 0.475 | |
| South Dakota | 13 | 0.48 | 26.57 | 59.4 | 40.9 | 22.8 | 92.3 | 8.07 | 1.07 | 2.61 | 231.4 | $9,284.06 | 0.595 | |
| Tennessee | 80 | 4.38 | 18.24 | 74.1 | 56.4 | 33.66 | 86.27 | 17.15 | 16.17 | 3.03 | 247.1 | $9,580.76 | 0.385 | |
| Texas | 173 | 11.02 | 15.69 | 52.7 | 38.7 | 31.57 | 76.04 | 12.34 | 12.34 | 34.93 | 213.3 | $10,156.71 | 0.335 | |
| Utah | 10 | 0.65 | 15.32 | 26.1 | 18.7 | 19.04 | 87.95 | 6.62 | 1.09 | 12.04 | 207.5 | $9,779.76 | 1.445 | |
| Vermont | 6 | 0.36 | 16.36 | 59.1 | 42.5 | 22.5 | 95 | 10.5 | 0.55 | 1.1 | 337.7 | $8,238.74 | 1.526 | |
| Virginia | 80 | 4.28 | 18.65 | 58.2 | 43.3 | 23.39 | 88.84 | 10 | 19.23 | 8.07 | 255.9 | $9,089.89 | 0.425 | |
| Washington | 48 | 3.48 | 13.77 | 55 | 39.9 | 21.52 | 89.59 | 10.85 | 3.52 | 9.95 | 268.7 | $8,332.85 | 0.545 | |
| West Virginia | 15 | 1.39 | 10.78 | 79.1 | 56.9 | 32.72 | 91.52 | 18.64 | 3.72 | 1.08 | 246.7 | $8,825.58 | 0.75 | |
| Wisconsin | 52 | 3.25 | 15.99 | 59 | 42.5 | 22.27 | 92.69 | 9.55 | 5.61 | 5.28 | 254.9 | $8,572.58 | 0.65 | |
| Wyoming | 4 | 0.31 | 12.70 | 38.7 | 35.9 | 21.57 | 83.33 | 10 | 0.94 | 8.8 | 196.7 | $9,230.26 | 2.245 | |
Number of facilities/screening-eligible rate.
Age-adjusted rates per 100,000.
Number of active physicians per 100,000.
Medicare standardized risk-adjusted per capita costs.
Statistically significant, p<0.01.
Statistically significant, p<0.05.
Figure 1.
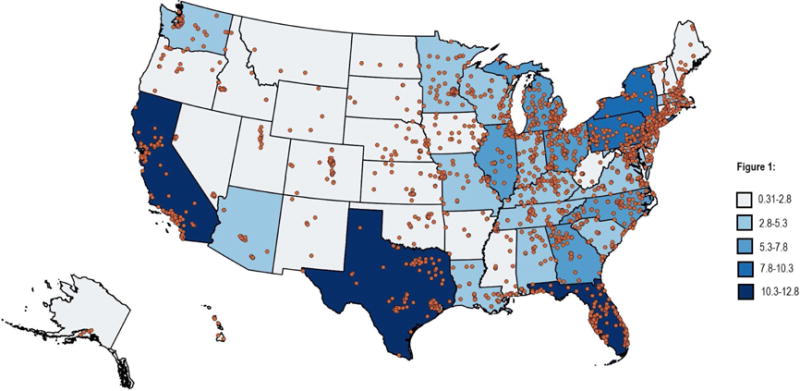
Distribution of lung cancer screening facilities and the rate of lung cancer screening-eligible per 100,000 individuals by state.
Figure 2.
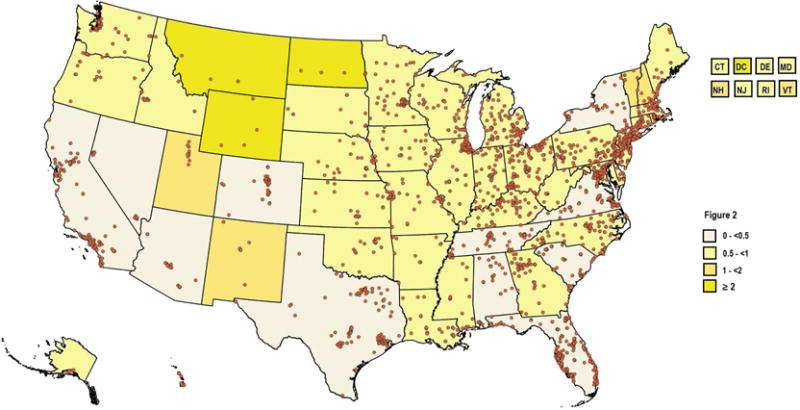
Distribution of lung cancer screening facilities and state-specific nearest neighbor index (NNI), a measure of site clustering or dispersal. If the index is less than 1, the pattern exhibits clustering while an index great than 1 indicates dispersion.
When evaluating the independent effects of state-level covariates on availability of lung cancer screening services, we demonstrated a positive independent effect (coefficient=12.87, 95% CI= 10.93–14.8) between state-level number of screening facilities and the rate of screen-eligible individuals per 100,000 (Table 2). There were no significant correlations between facility number and state-specific lung cancer death rates, smoking prevalence, demographic characteristics, physician supply nor Medicare expenditure, although a trend toward a positive correlation between lung cancer incidence rates (coefficient=1.25, 95%CI= −0.15 – 2.65, p=0.08) and facility number and a trend toward a negative correlation between the proportion of the population in poverty (coefficient= −2.14, 95%CI= −4.48–0.19, p=0.07).
Table 2.
Correlates of screening site availability and state-specific clinically relevant epidemiologic and demographic characteristics.
| Facility number | Coefficient | 95% Confidence Interval |
P value |
|---|---|---|---|
| Rate eligible per 100k | 12.871 | 10.939 – 14.803 | <0.01 |
| Lung incidence | 1.255 | −0.150 – 2.658 | 0.08 |
| Lung death | −0.741 | −2.827 – 1.345 | 0.48 |
| Physician supply | −0.006 | −0.083 – 0.096 | 0.89 |
| Poverty | −2.144 | −4.481 – 0.192 | 0.07 |
| Insured | −1.349 | −3.618 – 0.920 | 0.24 |
| Unemployed | −0.176 | −4.211 – 3.858 | 0.93 |
| Black | 0.044 | −0.821 – 0.909 | 0.92 |
| Latino | 0.212 | −0.726 – 1.152 | 0.65 |
| Medicare expenditure | 0.003 | −.006 – 0.013 | 0.46 |
| Smoking prevalence | 0.667 | −1.402 – 2.737 | 0.52 |
Discussion
We demonstrated state-level variability in lung cancer screening facility number when normalized to the rate of screening-eligible individuals as well as variability in facility clustering. Appropriately, the number of lung cancer screening facilities correlated with the estimated number of individuals eligible for screening by state; however, the frequency of screening facilities was not associated with lung cancer incidence and death rates, smoking prevalence, or other state-level demographic variables.
Equitable and efficient use of proven preventive services requires reduction of a host of barriers to access. The Affordable Care Act (ACA) expanded the number of insured individuals to decrease the lack of insurance coverage as a barrier to care. Further, cost of care even for those insured limits use of these services, therefore, the ACA concomitantly eliminated out of pocket cost sharing for high value preventive services for those with commercial insurance. With regard to lung cancer screening, Medicare has similarly extended coverage with no cost sharing as long as patients participate in an approved registry. Having eliminated cost to the patient and lack of reimbursement to the practice as barriers to screening, access to services may still be limited by geographic availability. For example, western states demonstrated significant lung screening service dispersal likely mirroring a metropolitan area or population distribution that should reduce geographic limitations on access; however, individuals who live in more remote locations may have to travel farther to access these screening services compared to those who live more centrally. Conversely, states with highly clustered services but a large geographic area may similarly restrict geographic access, such as California or Texas, due to travel distance or duration. Conducting these analyses is beyond the scope of the current study but represent key areas for future work.
That the availability of screening services correlates strongly with the number of screening-eligible individuals, for whom practice reimbursement is assured, may suggest response to market forces and local demand. Nonetheless, opportunity to align lung screening availability with relevant clinical variables such as state-level lung cancer deaths or other population risk factors such as smoking prevalence remains as a public health consideration. Smoking trends continue to shift from cities, where most facilities are located, to rural areas(17)(18), increasing the challenge of optimizing screening facility distribution. Changes in smoking prevalence raise the need for further investigation on how should resources be allocated geographically(19). From analogous studies of breast cancer screening, geographic access increases use of screening mammography and potentially decreases stage at diagnosis(20)(21).
Having geographic access to the facility alone insufficiently guarantees access to screening. Full implementation of lung cancer screening requires increasing screening capacity per facility, particularly in areas with currently constrained care delivery. Smieliauskas estimated that lung cancer screening implementation would increase imaging procedures by 4% across the U.S., representing a significant workforce issue and likely to result in disparities to access lung cancer screening (22). Manpower constraints can limit temporal service availability with office closures and limited hours. We demonstrated no correlation between physician supply as measured by the total number of active physicians per 100,000 individuals and the number of screening facilities. The LCSR gives the physical location of screening facilities, not the hours that service is provided, another measure of access. While the physical structures exist to render service, the low supply of radiologists in some states may represent a limiting factor.
A key limitation of this brief report is the inability to precisely define the distribution of the population considered at risk, particularly of those with the risk profile specified by the USPSTF and Medicare as eligible for LDCT coverage, as the BRFSS has insufficient data to define those with at least a 30 pack year smoking history or those who have recently quit within the last 15 years. We likely underestimated the number of screening-eligible individuals which, however, would further bias toward the observed correlation between facility availability and the rate of screening-eligible. As with all self-reported data, BRFSS responses are subject to recall bias, similar to the bias encountered when clinically implementing a lung screening program and ascertaining smoking status. Nevertheless, we derived the most representative state population estimates of screening-eligible individuals by using the BRFSS. Using the BRFSS to estimate the screening-eligible population precludes evaluation of county level contributors of geographic access and limits analyses to state-specific variables. This latter focus may underestimate the geographic availability of screening services particularly for those who reside in border communities straddling more than one state. Similarly, it is beyond the scope of our analyses to measure travel distances and durations to the nearest screening facility, particularly for individuals who live in these border communities. However, we are able to describe the relationship of state-specific variables. The LCSR itself presents only the geographic location of screening sites, not an estimate of relative use nor the potential clinical impact of actual use among screen-eligible individuals. However, we used the LSCR as a source of screening service availability. It is beyond the scope of the study to assess actual screening usage at these facilities and the impact on lung cancer mortality reduction.
Conclusion
Despite heterogeneity in lung cancer screening service distribution and the tendency toward facility clustering for the vast majority of states, encouragingly, the number of screening facilities in each state correlated with the rate of screening-eligible individuals per 100,000, a measure of the at-risk population. However, there remains a need to evaluate the extent to which geographic clustering or dispersal impacts service access. The lack of correlation with other variables such as state-specific lung cancer mortality or smoking prevalence remain opportunities for aligning service provision with clinically relevant epidemiologic factors.
Take-home points.
At the state level, the number of lung cancer screening facilities correlate with the rate of screening-eligible individuals per 100,000, a measure of the at-risk population.
Despite correlation of the number of screening facilities with the size of the at-risk population, the geographic distribution of the screening sites may still result in disparities to screening access.
The potential mismatch in screening facility distribution with other clinically relevant epidemiologic factors, such as lung cancer mortality and smoking prevalence, remain a public health opportunity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure:
|
| |||||||
| By-line position | Position Type | Author’s Full Name | Institution | City, State | Grant Number(s) | Role | |
|
| |||||||
| 1 | Lead | Paniz Charkhchi | University of Michigan | Ann Arbor, Michigan | CA180801 | Primary author | panizcharkhchi@yahoo.com |
|
| |||||||
| 2 | Lead | Giselle E. Kolenic | University of Michigan | Ann Arbor, Michigan | CA180801 | Co-author | gkolenic@med.umich.edu |
|
| |||||||
| 3 | Senior | Ruth C. Carlos | University of Michigan | Ann Arbor, Michigan | CA180801 | Corresponding author | rcarlos@med.umich.edu |
|
| |||||||
| Required Acknowledgement: | This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA189828, CA180801. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. | ||||||
|
| |||||||
Potential conflict of interest unrelated to the submitted work: RCC receives salary support as deputy editor of the JACR.
References
- 1.Suresh R, Ali S, Ahmad A, Philip PA, Sarkar FH. Lung Cancer and Personalized Medicine. 2016;893:57–74. doi: 10.1007/978-3-319-24932-2_4. Available from: http://link.springer.com/10.1007/978-3-319-24223-1. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin [Internet] 2016;66(1):7–30. doi: 10.3322/caac.21332. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26742998. [DOI] [PubMed] [Google Scholar]
- 3.National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med [Internet] 2011 Aug 4;365(5):395–409. doi: 10.1056/NEJMoa1102873. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21714641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research Team. Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, et al. The National Lung Screening Trial: overview and study design. Radiology [Internet] 2011 Jan;258(1):243–53. doi: 10.1148/radiol.10091808. Available from: http://radiology.rsna.org/content/early/2010/10/28/radiol.10091808.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lung-cancer-screening. Accessed 2/13/2017.
- 6.Affordable Care Act. https://www.medicare.gov/about-us/affordable-care-act/affordable-care-act.html. Accessed 2/13/2017.
- 7.CMS.gov. https://www.cms.gov/Medicare/Medicare-General-Information/MedicareApprovedFacilitie/Lung-Cancer-Screening-Registries.html. Accessed 2/13/2017.
- 8.The American College of Radiology. https://www.acr.org/Quality-Safety/National-Radiology-Data-Registry/Lung-Cancer-Screening-Registry. Accessed 2/13/2017.
- 9.List of LCSR Registrants. https://www.acr.org/Quality-Safety/National-Radiology-Data-Registry/Lung-Cancer-Screening-Registry. Accessed 2/13/2017.
- 10.CDC. Methodologic Changes in the Behavioral Risk Factor Surveillance System in 2011 and Potential Effects on Prevalence Estimates. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a3.htm. [PubMed]
- 11.CDC. About the Behavioral Risk Factor Surveillance System (BRFSS) https://www.cdc.gov/brfss/about/about_brfss.htm.
- 12.Pierannunzi C, Hu SS, Balluz L. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004 – 2011. BMC Med Res Methodol [Internet] 2013;13(1):1. doi: 10.1186/1471-2288-13-49. Available from: BMC Medical Research Methodology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2013 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; p. 201. [Google Scholar]
- 14.https://www.cdc.gov/statesystem/cigaretteuseadult.html. Accessed 2/13/2017.
- 15.2015 State Physician Workforce Data Book. Center for Workforce Studies. American Association of Medical Colleges; 2015. [Google Scholar]
- 16.Centers for Medicare and Medicaid Services. Geographic Variation Public Use Files. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Geographic-Variation/GV_PUF.html. Accessed 4.11.2017.
- 17.Liu L, Edland S, Myers MG, Hofstetter CR, Al-Delaimy WK. Smoking prevalence in urban and rural populations: findings from California between 2001 and 2012. Am J Drug Alcohol Abuse [Internet] 2016 May;2990:1–10. doi: 10.3109/00952990.2015.1125494. Available from: http://www.tandfonline.com/doi/full/10.3109/00952990.2015.1125494. [DOI] [PubMed] [Google Scholar]
- 18.Aloise-Young PA, Wayman JC, Edwards RW. Prevalence of Cigarette Smoking Among Rural Adolescents in the United States. Subst Use Misuse [Internet] 2002;37(5–7):613–30. doi: 10.1081/ja-120004276. Available from: http://www.tandfonline.com/doi/full/10.1081/JA-120004276. [DOI] [PubMed] [Google Scholar]
- 19.Dwyer-Lindgren L, Mokdad AH, Srebotnjak T, Flaxman AD, Hansen GM, Murray CJ. Cigarette smoking prevalence in US counties: 1996–2012. Popul Health Metr [Internet] 2014;12(1):5. doi: 10.1186/1478-7954-12-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24661401%5Cnhttp://pophealthmetrics.biomedcentral.com/articles/10.1186/1478-7954-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan-Gates JA, Ersek JL, Eberth JM, Adams SA, Pruitt SL. Geographic Access to Mammography and Its Relationship to Breast Cancer Screening and Stage at Diagnosis: A Systematic Review. Women’s Heal Issues. 2015;25(5):482–93. doi: 10.1016/j.whi.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkin EB, Ishill NM, Snow JG, Panageas KS, Bach PB, Liberman L, et al. Geographic access and the use of screening mammography. Med Care [Internet] 2010 Apr;48(4):349–56. doi: 10.1097/MLR.0b013e3181ca3ecb. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20195174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smieliauskas F, MacMahon H, Salgia R, Shih Y-CT. Geographic variation in radiologist capacity and widespread implementation of lung cancer CT screening. J Med Screen [Internet] 2014;21(4):207–15. doi: 10.1177/0969141314548055. Available from: http://msc.sagepub.com/content/21/4/207.full. [DOI] [PMC free article] [PubMed] [Google Scholar]


