Abstract
B-cell development is characterized by a number of tightly regulated selection processes. Signals through the B-cell receptor (BCR) guide and are required for B-cell maturation, survival, and fate decision. Here, we review the role of the BCR during B-cell development, leading to the emergence of B1, marginal zone, and peripheral follicular B cells. Furthermore, we discuss BCR-derived signals on activated B cells that lead to germinal center and plasma cell differentiation.
Keywords: B cell receptor, B cell differentiation, B cell development
Introduction
B cells, the antibody-producing cells, have a protagonist role in the immune response. Although the presence and relevance of antibodies were established more than 100 years ago 1 and antibody-producing cells were identified in the mid-20th century 2, it took until 1965 for the distinctive B-cell lineage to be recognized 3, 4. Today, there are still many questions about B-cell differentiation during development and after activation and about the signals that govern such differentiation.
B cells undergo a diversification process during their development in bone marrow and fetal liver, and, as part of this differentiation, B cells rearrange the immunoglobulin (Ig) heavy (H) and light (L) chain gene loci to create a complete Ig molecule 5, 6. The commitment of the common lymphoid progenitor to the B-cell lineage can be recognized by the expression of the B220 isoform of CD45 6– 8. B cells then develop through several well-characterized stages, ending with the expression of surface IgM and IgD class Ig molecules, which, in association with Igα and Igβ, form the B-cell receptor (BCR) for the antigen 5, 6. BCR signaling is required for B-cell maturation and survival, and BCR must provide tonic signals, either spontaneously or on interaction with ligands in the environment 9– 12. After this, B cells emerge to recirculate through secondary lymphoid organs such as the spleen and lymph nodes 13. Being well positioned in the secondary lymphoid organs, mature naïve B cells are ready to respond to antigens. Recognizing the antigen through the BCR, B cells are activated and differentiate into plasma cells through extra-follicular differentiation 14, or they become germinal center (GC) precursor cells to start GC reactions 15, 16. The precise signaling mechanisms of B-cell fate decision during this stage are not entirely understood.
In this review, we will focus on B-cell subsets in the spleen, key steps of differentiation after activation, and, in particular, recent findings about the role of the BCR driving these distinct differentiation stages ( Figure 1).
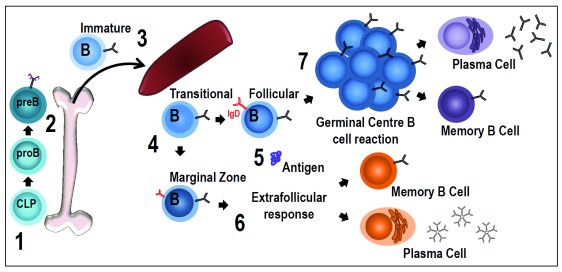
Figure 1. B-cell receptor signaling during B-cell development and for B-cell differentiation after the encounter with the antigen.
(1) Common lymphoid progenitor (CLP) cells commit to the B-cell lineage when they start expressing the B220 isoform of CD45. (2) Pro-B cells undergo DJ rearrangements and become pre-B cells when they express membrane forms of μ heavy chains with surrogate light chains (pink dotted lines) in the pre-B receptor. The transition between pro-B and pre-B cells has been described as being dependent on membrane association of Igα/Igβ complexes and their ability to generate basal signals. It seems that pre-B receptors do not have a ligand-recognition function 40, 41. (3) After VDJ recombination in the pre-B cell stage, immature B cells pair light chains with μ chains to form monomeric IgM, which is expressed at the cell surface in association with Igα/Igβ to form the B-cell receptor (BCR). Newly formed immature B cells exit the bone marrow to reach the spleen, where, as transitional B cells, they will complete their maturation before entering the follicles or the marginal zone (MZ). (4) BCR signaling strength appears to have a critical role during follicular (Fo) or MZ B-cell differentiation. There is evidence of at least two possibilities 29, 30, 36, 42– 44: increased BCR signaling could drive differentiation to Fo B cells or to MZ B cells. This could depend on other factors such as the microenvironment or the timing of BCR signaling. (5) Mature naïve B cells are ready to respond to antigens. By specifically binding antigen through the BCR, B cells are activated and differentiate into plasma cells through extra-follicular differentiation (6) or start the T-cell-dependent germinal center (GC) reaction (7). The mechanism of activated B cells entering the GC reaction or undergoing rapid plasma cell differentiation in extra-follicular proliferative foci is controlled by the nature of the interaction between the BCR and antigen. Responding clones that undergo a strong initial interaction with antigen can efficiently differentiate into extra-follicular plasma cells 45. However, it has also been shown that B cells expressing higher-affinity BCRs are more competitive to become pre-GC B cells during T–B interaction 46, 47. This seems contradictory, but timing may be a very important factor. B cells in the GC reaction are selected on the basis of their interaction with the antigen through BCRs. Based on how efficiently B cells present antigen to Fo T helper cells, they are allowed to further differentiate inside the GC. Whether this BCR–antigen interaction results in significant signaling and has a role for selection is not clear.
Brief overview of B-cell receptor signaling
BCR signaling has been intensely studied over the last 30 years, and details of this complex process are still not fully understood. In-depth reviews have been produced by others 17– 20. In brief, BCR is activated by binding of the antigen. This leads to phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) by the first kinase in the BCR signaling pathway, primarily LYN (part of the src-kinase family). After this, SYK is recruited through its SH2 domain to the phosphorylated Igα–Igβ heterodimer. The higher propensity of ligand-bound BCR molecules to aggregate can enhance their association with src-family PTKs 17, 20. Once SYK is activated, the BCR signal is propagated via a group of proteins associated with the adaptor protein B-cell linker (BLNK, SLP-65). Phospho-BLNK serves as a scaffold for the assembly of the other components, including Bruton’s tyrosine kinase (BTK), VAV 1, and phospholipase C-gamma 2 (PLCγ2) 19. The initiation of the BCR signal is indirectly regulated by at least two non-receptor-associated molecules: B220 and C-terminal src tyrosine kinase (CSK) 17.
Additionally, following BCR ligation, tyrosines of the cytoplasmic tail of CD19 are phosphorylated by LYN to create binding sites for the SH2 domains of the p85 adaptor subunit of PI-3K as well as other SH2 domain-containing effectors. Activated PLCγ2 cleaves membrane-associated phosphoinositide PI(4,5)P2 into the second messengers I(1,4,5)P3 and DAG. I(1,4,5)P3 generation causes the mobilization of Ca 2+ from intracellular and extracellular stores. Ca 2+ signaling is required for the activation of transcription factors such as nuclear factor kappa B (NF-κB) and nuclear factor of activated T cells (N-FAT) by protein kinase C (PKC) 21. DAG represents a classic activator of PKC isotypes which regulate the mitogen-activated protein kinase (MAPK) family (extracellular signal-regulated kinase [ERK], c-Jun NH2-terminal kinase [JNK/SAPK], and p38 MAPK); the overall result of these processes drives activation of the B cell, antigen presentation, cytokine production, and cell proliferation and differentiation 17– 19. In the following, we will discuss the effects of BCR signaling during B-cell development and after the encounter with the antigen.
B1 B cells
Three major populations of mature B cells have been described in mice: B1, marginal zone (MZ), and follicular (Fo) B cells. The phenotypic, microanatomic localization and functional differences that characterize them suggest specialized functions linked to the niches in which they reside 5. B1 cells produce polyreactive natural antibodies (NAbs) of relatively low affinity and primarily of the IgM isotype 22. NAbs play a critical role in the innate immune response against pathogens, and their absence can lead to increased susceptibility to microbial infections 23– 25. B1 cells are the major subpopulation in pleural and peritoneal cavities; however, they can also be found in the spleen and other lymphoid organs but at low frequency 26. B1 cells consist of two functional specialized subpopulations regarding the expression of CD5: CD5 + B1a and CD5 − B1b cells 27. However, expression of Blimp-1 also delineates two main coexisting B1 subpopulations in the bone marrow and spleen: B1 Blimp-1 hi cells that are dependent on Blimp-1 for maximal natural Ig production and those B1 cells that neither express Blimp-1 nor require it for steady-state antibody production 28. Recently, it has been shown that interleukin 17A (IL-17A) promotes B1-cell infiltration into lungs during viral infection, where B1a cells differentiate into IgM-producing plasma cells. This process was promoted through Blimp-1 expression and NF-κB activation 25. It is conceivable that the regulation of Blimp-1 expression would also drive the functional role of B1 subsets in sites such as the lung.
What is the role of BCR signaling in B1-cell differentiation? Studies with genetically modified mice indicate that the strength of BCR signaling may control the development or persistence of B cells (or both) 29– 36. Mutations that enhance BCR signaling strength through the specific deletion of SHP-1 in B cells expand the B1a population. SHP-1 is a protein-tyrosine phosphatase expressed in hematopoietic cells and plays a signal-attenuating role in pathways initiated by many ITAM-containing receptors 37– 39. The signal-attenuating effects of SHP-1 are mediated primarily via its binding to inhibitory receptors, such as CD5, expressed by B1a cells 34. Additionally, enhanced tonic BCR signaling results in an increased B1 B-cell subpopulation and a dysregulated homeostasis of other B-cell subsets 33. These findings indicate that BCR signaling is important in fate decisions during B1 cell development, and further studies are needed to better understand these mechanisms.
Marginal zone B cells
MZ B cells contribute about 5–10% of the B cells in the spleen. The “marginal zone” designation refers to the splenic structure that separates the red and the white pulp adjacent to the marginal sinus, where—in both mice and humans—these MZ B cells are in direct contact with blood and its contents 5, 48. The specialized localization and migration of B cells are strictly regulated under the guidance of different chemokine–chemokine receptor pairs, such as CXCL13–CXCR5, S1PR1, and S1P 49– 53.
Blood from the primary sinusoids in the spleen perfuses the MZ; the anatomic location of MZ B cells facilitates their role as a rapid first line of defense against blood-borne particulate antigens 52, 54. After MZ B cells capture the antigen, they transport it to the follicles and deliver it to follicular dendritic cells (FDCs) 52, 53, 55. Furthermore, MZ B cells respond to thymus-independent type 2 antigens producing high quantities of IgM and IgG3 14, 56, 57.
Newly formed B cells exiting the bone marrow reach the spleen at a relatively immature stage; these are termed transitional B cells and they need to complete their maturation in the spleen before entering the follicles or the MZ 58. It has been described that B cells in the transitional 2 (T2) stage face a decision to mature into either Fo or MZ B cells 5, 48. However, very recently, it was shown that T1 B cells can differentiate to MZ B cells 32. During this differentiation, signaling through the BCR is important for the Taok3-mediated acquisition of membrane expression of ADAM10, which cleaves Notch2 and CD23 31, 32. MZ B-cell instruction requires triggering of Notch2 on developing B cells by Delta-like 1 (Dll1) expressed by splenic red pulp sinus endothelial cells or MZ reticular cells 35, 59. How exactly B-cell-positive selection and BCR signaling are causing Taok3 activation and ADAM10 surface expression will require further study 32. Furthermore, BCR signaling strength appears to have a critical role during MZ B-cell development; mice lacking secreted IgM displaying increased BCR signaling had increased MZ and decreased Fo B-cell numbers 36, 42, 43. However, some studies reported that MZ B cells need low BCR signaling strength for their differentiation but that transitional B cells with higher BCR signaling strength favor differentiation into Fo B cells 29, 30, 44.
Follicular B cells
Fo B cells are the most prevalent of the three subsets of B cells and the better-studied subpopulation. Their anatomic enrichment in primary follicles gives them their name; however, they are not confined to the follicles and also predominate among the mature populations of B cells in the bone marrow, blood, and other lymphoid organs 5. Fo B cells are involved mainly in interactions with T cells, and their responses to T-cell-dependent antigens eventually originate GC reactions 16. Many of the mechanisms producing the selection of B-cell subsets, the roles of self- and environmental antigens, and survival signals that drive or maintain them in their proper anatomic and functional niches remain to be elucidated. BCR signaling has been proposed to be crucial in the selection of B1, MZ, and Fo B cells, supported by different genetically manipulated mice where the altered BCR signaling affected different B-cell subsets 32– 34, 36.
Follicular B-cell activation
After Fo B cells encounter the antigen through the BCR, CXCL13–CXCR5 slows down the motility of B cells by promoting membrane ruffling and LFA-1-supported adhesion to facilitate the antigen-recognizing process and enhance B-cell activation 60. Meanwhile, surface expression levels of CCR7 on responding B cells increase rapidly to make them more sensitive to CCL19/CCL21 51. CCL19/CCL21 is expressed by T-zone reticular cells and extends a gradient to the Fo region. Along the chemokine gradient, antigen-engaged B cells migrate from follicles to T–B border in order to get signals from primed T cells 51, 61, 62. EBI2 drives B cells to move back to the outer follicle and inter-follicular regions 63, 64. These results indicate interplay between activated BCR downstream signaling and surface chemokine receptors 60, 61. Although the possibility is less studied on steady state, tonic BCR signaling could also regulate chemokine receptor expression during their anatomic enrichment in specific areas within the secondary lymphoid organs.
Germinal center B cells
After T–B cell interaction, activated B cells either differentiate into plasma cells in the extra-follicular response 14 or become GC precursor cells, migrating back into follicles to start GC reactions 16. To get co-stimulation from T cells, B cells need to present cognate antigens to T cells in the major histocompatibility complex II (MHC-II) context. B-cell-captured antigen goes through both extracellular and intracellular degradation to become peptides 65. These peptides then are assembled with MHC-II molecules and expressed on the B-cell surface as peptide–MHC (pMHC). pMHC–T-cell receptor recognition has an essential role in the process of T–B “pairing” to ensure the specificity of later reactions. Schwickert et al. showed that a higher amount of pMHC help activated B cells, locking T-cell help on the T–B border, thus enabling them to become GC precursor B cells 46. CD40L is the most important cognate signal delivered from T cells. In mice and humans, CD40–CD40L ligation is indispensable for the initial formation of GCs 66, 67 and is needed to maintain ongoing GC reactions 68.
BCR affinity also plays an important role during the initial GC B-cell fate decision. The mechanism of activated B cells entering the GC reaction or undergoing rapid plasma cell differentiation in extra-follicular proliferative foci is controlled by the nature of the interaction between the BCR and antigen. Responding clones that undergo a strong initial interaction with antigen can efficiently differentiate into extra-follicular plasma cells and contribute to the rapid early thymus-dependent antibody response 45. Although the requirements for GC entry are not stringent 69, responding B cells expressing higher-affinity BCRs on their surface are more competitive to become pre-GC B cells during their T–B interaction 46, 47. High-affinity BCR captures more soluble antigens 70 and leads to a higher amount of pMHC expressed on the B-cell surface, which results in a competitive cognate interaction with T cells. The durations of T–B interactions have critical roles in B-cell fate decisions. Recent research shows that ICAM-1/2 adhesion molecules on B cells can secure long-lasting T–B interactions to enhance T-cell help. The expression of ICAM-1/2 could compensate the lack of MHC-II signaling to form GC B cells 71.
BCRs of B cells differentiating in GCs have to interact with antigen repetitively. Whether the BCR-antigen interaction in the GC results in significant signaling and what the role of this is are under debate 72– 74. The interaction is certainly important to test BCR specificity and binding competitiveness, probably mainly in competition with antibodies present in immune complexes on the FDC networks 75, 76. More important than BCR signaling may be that the affinity of the BCR–antigen interaction will let B cells take up more or less antigen, resulting in more or less efficient positive selection by Fo T helper cells 47, 74, 76.
Much of the knowledge on B-cell differentiation in response to antigen has been gained by using BCR knock-in animals specific for haptens or single epitopes on model proteins 46, 77. Recent attempts to use complex protein antigens such as influenza hemagglutinin should be better suited to understand the complex competition and crosstalk of many different clones interacting with different epitopes on a natural complex protein antigen 78, 79. However, for such experiments, it is important to develop methods that will allow the specific analysis of epitope-specific interactions of different B-cell clones. Without an understanding of whether clonal interactions happen because of BCR binding of overlapping epitopes or whether clones develop with less competition because they do bind different epitopes, it is impossible to conclude whether clones with different affinities compete for the antigen 80.
Ultimately, it has been estimated that nearly 50% of newly produced auto-reactive B cells can avoid deletion and are induced into an anergic state in peripheral lymphoid tissues 81, 82. Thus, perhaps B cells in the periphery are not in the same basal state as we thought they should be. The surface expression level of membrane IgM is downregulated on anergic B cells 83, 84, indicating that their BCR signaling is somehow different from that of normal naïve B cells. Indeed, anergic B cells maintain a distinct gene expression profile 81, 84 and have been observed preferentially residing in T-zone areas 85, indicating that their surface chemokine receptor expression pattern is also different from that of naïve B cells, possibly because of their different signaling. Several studies have found that anergic B cells can be selected to become GC B cells, although these cells previously were thought to be non-responsive 84, 86. By recruiting anergic B cells into the GC response involving many rounds of mutation, these B cells may mutate away from their original auto-reactivity and become specific for antigens that may have a close relationship with autoantigens 86– 89. The mechanism—for example, whether anergic and normal B cells will follow the same rules of B-cell selection or which difference an anergic state can bring to B-cell activation and fate decisions at the T–B border—is still not clear.
Concluding remarks
Co-stimulation from T cells plays indispensable roles in B-cell fate decisions after activation and has been studied in some detail. However, BCR signaling strength and patterns not only affect B-cell selection during development but also have been shown to affect the differentiation of B1, MZ, or Fo B-cell populations. Furthermore, BCR signaling strength may affect antigen-induced B-cell activation, migration, and surface co-stimulator molecule expression levels. It seems that there is still work to be done on how differential BCR signaling influences the differential development of B cells and how the various stages of antigen-induced T–B cell interactions affect further B-cell fate decisions.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Wanli Liu, Tsinghua University, Beijing, China
Pavel Tolar, Immune Receptor Activation Laboratory, The Francis Crick Institute, London, UK; Division of Immunology and Inflammation, Imperial College London, London, UK
Funding Statement
This work was supported by grants from the BBSRC UK BB/M025292/1 and a postdoctoral fellowship from the National Council of Science and Technology Mexico (CONACYT-Mexico) to JCY-P (CVU 49896).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Ueber das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. Dtsch med Wochenschr. 1890;16(49):1113–4. 10.1055/s-0029-1207589 [DOI] [PubMed] [Google Scholar]
- 2. Fagraeus A: The plasma cellular reaction and its relation to the formation of antibodies in vitro. J Immunol. 1948;58(1):1–13. [PubMed] [Google Scholar]
- 3. Cooper MD, Peterson RD, Good RA: Delineation of the Thymic and Bursal Lymphoid Systems in the Chicken. Nature. 1965;205:143–6. 10.1038/205143a0 [DOI] [PubMed] [Google Scholar]
- 4. Cooper MD: The early history of B cells. Nat Rev Immunol. 2015;15(3):191–7. 10.1038/nri3801 [DOI] [PubMed] [Google Scholar]
- 5. Vale AM, Kearney JF, Nobrega A, et al. : Development and Function of B Cell Subsets, Molecular Biology of B Cells.in Alt FW, et alEditors. Elsevier.2015;99–119. 10.1016/B978-0-12-397933-9.00007-2 [DOI] [Google Scholar]
- 6. Hardy RR, Hayakawa K: B cell development pathways. Annu Rev Immunol. 2001;19:595–621. 10.1146/annurev.immunol.19.1.595 [DOI] [PubMed] [Google Scholar]
- 7. Kondo M, Weissman IL, Akashi K: Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91(5):661–72. 10.1016/S0092-8674(00)80453-5 [DOI] [PubMed] [Google Scholar]
- 8. Li YS, Wasserman R, Hayakawa K, et al. : Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5(6):527–35. 10.1016/S1074-7613(00)80268-X [DOI] [PubMed] [Google Scholar]
- 9. Kraus M, Alimzhanov MB, Rajewsky N, et al. : Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117(6):787–800. 10.1016/j.cell.2004.05.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Lam KP, Kühn R, Rajewsky K: In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–83. 10.1016/S0092-8674(00)80373-6 [DOI] [PubMed] [Google Scholar]
- 11. Srinivasan L, Sasaki Y, Calado DP, et al. : PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–86. 10.1016/j.cell.2009.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Schweighoffer E, Tybulewicz VL: Signalling for B cell survival. Curr Opin Cell Biol. 2017;51:8–14. 10.1016/j.ceb.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 13. Ichii M, Oritani K, Kanakura Y: Early B lymphocyte development: Similarities and differences in human and mouse. World J Stem Cells. 2014;6(4):421–31. 10.4252/wjsc.v6.i4.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacLennan IC, Toellner KM, Cunningham AF, et al. : Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. 10.1034/j.1600-065X.2003.00058.x [DOI] [PubMed] [Google Scholar]
- 15. MacLennan IC: Germinal centers. Annu Rev Immunol. 1994;12:117–39. 10.1146/annurev.iy.12.040194.001001 [DOI] [PubMed] [Google Scholar]
- 16. Victora GD, Nussenzweig MC: Germinal centers. Annu Rev Immunol. 2012;30:429–57. 10.1146/annurev-immunol-020711-075032 [DOI] [PubMed] [Google Scholar]
- 17. Dal Porto JM, Gauld SB, Merrell KT, et al. : B cell antigen receptor signaling 101. Mol Immunol. 2004;41(6–7):599–613. 10.1016/j.molimm.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 18. Kurosaki T, Shinohara H, Baba Y: B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55. 10.1146/annurev.immunol.021908.132541 [DOI] [PubMed] [Google Scholar]
- 19. Packard TA, Cambier JC: B lymphocyte antigen receptor signaling: initiation, amplification, and regulation. F1000Prime Rep. 2013;5:40. 10.12703/P5-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pierce SK, Liu W: The tipping points in the initiation of B cell signalling: how small changes make big differences. Nat Rev Immunol. 2010;10(11):767–77. 10.1038/nri2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Su TT, Guo B, Kawakami Y, et al. : PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat Immunol. 2002;3(8):780–6. 10.1038/ni823 [DOI] [PubMed] [Google Scholar]
- 22. Avrameas S: Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunol Today. 1991;12(5):154–9. 10.1016/S0167-5699(05)80045-3 [DOI] [PubMed] [Google Scholar]
- 23. Ochsenbein AF, Fehr T, Lutz C, et al. : Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286(5447):2156–9. 10.1126/science.286.5447.2156 [DOI] [PubMed] [Google Scholar]
- 24. McKay JT, Haro MA, Daly CA, et al. : PD-L2 Regulates B-1 Cell Antibody Production against Phosphorylcholine through an IL-5-Dependent Mechanism. J Immunol. 2017;199(6):2020–9. 10.4049/jimmunol.1700555 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Wang X, Ma K, Chen M, et al. : IL-17A Promotes Pulmonary B-1a Cell Differentiation via Induction of Blimp-1 Expression during Influenza Virus Infection. PLoS Pathog. 2016;12(1):e1005367. 10.1371/journal.ppat.1005367 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Baumgarth N: Innate-like B cells and their rules of engagement. Adv Exp Med Biol. 2013;785:57–66. 10.1007/978-1-4614-6217-0_7 [DOI] [PubMed] [Google Scholar]
- 27. Ghosn EE, Sadate-Ngatchou P, Yang Y, et al. : Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci U S A. 2011;108(7):2879–84. 10.1073/pnas.1019764108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Savage HP, Yenson VM, Sawhney SS, et al. : Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J Exp Med. 2017;214(9):2777–94. 10.1084/jem.20161122 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Cariappa A, Takematsu H, Liu H, et al. : B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. J Exp Med. 2009;206(1):125–38. 10.1084/jem.20081399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cariappa A, Tang M, Parng C, et al. : The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14(5):603–15. 10.1016/S1074-7613(01)00135-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Gibb DR, El Shikh M, Kang DJ, et al. : ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage In vivo. J Exp Med. 2010;207(3):623–35. 10.1084/jem.20091990 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Hammad H, Vanderkerken M, Pouliot P, et al. : Transitional B cells commit to marginal zone B cell fate by Taok3-mediated surface expression of ADAM10. Nat Immunol. 2017;18(3):313–20. 10.1038/ni.3657 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Nguyen TT, Kläsener K, Zürn C, et al. : The IgM receptor FcμR limits tonic BCR signaling by regulating expression of the IgM BCR. Nat Immunol. 2017;18(3):321–33. 10.1038/ni.3677 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Pao LI, Lam KP, Henderson JM, et al. : B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27(1):35–48. 10.1016/j.immuni.2007.04.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Tanigaki K, Han H, Yamamoto N, et al. : Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3(5):443–50. 10.1038/ni793 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Tsiantoulas D, Kiss M, Bartolini-Gritti B, et al. : Secreted IgM deficiency leads to increased BCR signaling that results in abnormal splenic B cell development. Sci Rep. 2017;7(1): 3540. 10.1038/s41598-017-03688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Matthews RJ, Bowne DB, Flores E, et al. : Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992;12(5):2396–405. 10.1128/MCB.12.5.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Plutzky J, Neel BG, Rosenberg RD: Isolation of a src homology 2-containing tyrosine phosphatase. Proc Natl Acad Sci U S A. 1992;89(3):1123–7. 10.1073/pnas.89.3.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yi TL, Cleveland JL, Ihle JN: Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol Cell Biol. 1992;12(2):836–46. 10.1128/MCB.12.2.836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bannish G, Fuentes-Pananá EM, Cambier JC, et al. : Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J Exp Med. 2001;194(11):1583–96. 10.1084/jem.194.11.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mårtensson IL, Ceredig R: Review article: role of the surrogate light chain and the pre-B-cell receptor in mouse B-cell development. Immunology. 2000;101(4):435–41. 10.1046/j.1365-2567.2000.00151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waisman A, Kraus M, Seagal J, et al. : IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Ig alpha/beta. J Exp Med. 2007;204(4):747–58. 10.1084/jem.20062024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Horikawa K, Martin SW, Pogue SL, et al. : Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J Exp Med. 2007;204(4):759–69. 10.1084/jem.20061923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niiro H, Clark EA: Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2(12):945–56. 10.1038/nri955 [DOI] [PubMed] [Google Scholar]
- 45. Paus D, Phan TG, Chan TD, et al. : Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203(4):1081–91. 10.1084/jem.20060087 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Schwickert TA, Victora GD, Fooksman DR, et al. : A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med. 2011;208(6):1243–52. 10.1084/jem.20102477 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Victora GD, Schwickert TA, Fooksman DR, et al. : Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605. 10.1016/j.cell.2010.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Pillai S, Cariappa A, Moran ST: Marginal zone B cells. Annu Rev Immunol. 2005;23:161–96. 10.1146/annurev.immunol.23.021704.115728 [DOI] [PubMed] [Google Scholar]
- 49. Ansel KM, Ngo VN, Hyman PL, et al. : A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406(6793):309–14. 10.1038/35018581 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Förster R, Mattis AE, Kremmer E, et al. : A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87(6):1037–47. 10.1016/S0092-8674(00)81798-5 [DOI] [PubMed] [Google Scholar]
- 51. Reif K, Ekland EH, Ohl L, et al. : Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416(6876):94–9. 10.1038/416094a [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Arnon TI, Horton RM, Grigorova IL, et al. : Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature. 2013;493(7434):684–8. 10.1038/nature11738 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Cinamon G, Zachariah MA, Lam OM, et al. : Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9(1):54–62. 10.1038/ni1542 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Gray D, MacLennan IC, Bazin H, et al. : Migrant mu+ delta+ and static mu+ delta- B lymphocyte subsets. Eur J Immunol. 1982;12(7):564–9. 10.1002/eji.1830120707 [DOI] [PubMed] [Google Scholar]
- 55. Gray D, Kumararatne DS, Lortan J, et al. : Relation of intra-splenic migration of marginal zone B cells to antigen localization on follicular dendritic cells. Immunology. 1984;52(4):659–69. [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Oliver AM, Martin F, Gartland GL, et al. : Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997;27(9):2366–74. 10.1002/eji.1830270935 [DOI] [PubMed] [Google Scholar]
- 57. Vinuesa CG, Sze DM, Cook MC, et al. : Recirculating and germinal center B cells differentiate into cells responsive to polysaccharide antigens. Eur J Immunol. 2003;33(2):297–305. 10.1002/immu.200310003 [DOI] [PubMed] [Google Scholar]
- 58. Loder F, Mutschler B, Ray RJ, et al. : B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190(1):75–89. 10.1084/jem.190.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Tan JB, Xu K, Cretegny K, et al. : Lunatic and manic fringe cooperatively enhance marginal zone B cell precursor competition for delta-like 1 in splenic endothelial niches. Immunity. 2009;30(2):254–63. 10.1016/j.immuni.2008.12.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Sáez de Guinoa J, Barrio L, Mellado M, et al. : CXCL13/CXCR5 signaling enhances BCR-triggered B-cell activation by shaping cell dynamics. Blood. 2011;118(6):1560–9. 10.1182/blood-2011-01-332106 [DOI] [PubMed] [Google Scholar]
- 61. Calpe E, Codony C, Baptista MJ, et al. : ZAP-70 enhances migration of malignant B lymphocytes toward CCL21 by inducing CCR7 expression via IgM-ERK1/2 activation. Blood. 2011;118(16):4401–10. 10.1182/blood-2011-01-333682 [DOI] [PubMed] [Google Scholar]
- 62. Okada T, Cyster JG: B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18(3):278–85. 10.1016/j.coi.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 63. Gatto D, Paus D, Basten A, et al. : Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009;31(2):259–69. 10.1016/j.immuni.2009.06.016 [DOI] [PubMed] [Google Scholar]
- 64. Pereira JP, Kelly LM, Xu Y, et al. : EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460(7259):1122–6. 10.1038/nature08226 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Yuseff MI, Pierobon P, Reversat A, et al. : How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol. 2013;13(7):475–86. 10.1038/nri3469 [DOI] [PubMed] [Google Scholar]
- 66. Allen RC, Armitage RJ, Conley ME, et al. : CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259(5097):990–3. 10.1126/science.7679801 [DOI] [PubMed] [Google Scholar]
- 67. Ferrari S, Giliani S, Insalaco A, et al. : Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proc Natl Acad Sci U S A. 2001;98(22):12614–9. 10.1073/pnas.221456898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Han S, Hathcock K, Zheng B, et al. : Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155(2):556–67. [PubMed] [Google Scholar]
- 69. Dal Porto JM, Haberman AM, Kelsoe G, et al. : Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J Exp Med. 2002;195(9):1215–21. 10.1084/jem.20011550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Batista FD, Neuberger MS: B cells extract and present immobilized antigen: implications for affinity discrimination. EMBO J. 2000;19(4):513–20. 10.1093/emboj/19.4.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zaretsky I, Atrakchi O, Mazor RD, et al. : ICAMs support B cell interactions with T follicular helper cells and promote clonal selection. J Exp Med. 2017;214(11):3435–48. 10.1084/jem.20171129 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Khalil AM, Cambier JC, Shlomchik MJ: B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336(6085):1178–81. 10.1126/science.1213368 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Luo W, Weisel F, Shlomchik MJ: B Cell Receptor and CD40 Signaling Are Rewired for Synergistic Induction of the c-Myc Transcription Factor in Germinal Center B Cells. Immunity. 2018;48(2):313–326.e5. 10.1016/j.immuni.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Nowosad CR, Spillane KM, Tolar P: Germinal center B cells recognize antigen through a specialized immune synapse architecture. Nat Immunol. 2016;17(7):870–7. 10.1038/ni.3458 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Zhang Y, Garcia-Ibanez L, Toellner KM: Regulation of germinal center B-cell differentiation. Immunol Rev. 2016;270(1):8–19. 10.1111/imr.12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang Y, Meyer-Hermann M, George LA, et al. : Germinal center B cells govern their own fate via antibody feedback. J Exp Med. 2013;210(3):457–64. 10.1084/jem.20120150 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Phan TG, Amesbury M, Gardam S, et al. : B cell receptor-independent stimuli trigger immunoglobulin (Ig) class switch recombination and production of IgG autoantibodies by anergic self-reactive B cells. J Exp Med. 2003;197(7):845–60. 10.1084/jem.20022144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kuraoka M, Schmidt AG, Nojima T, et al. : Complex Antigens Drive Permissive Clonal Selection in Germinal Centers. Immunity. 2016;44(3):542–52. 10.1016/j.immuni.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Tas JM, Mesin L, Pasqual G, et al. : Visualizing antibody affinity maturation in germinal centers. Science. 2016;351(6277):1048–54. 10.1126/science.aad3439 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Toellner KM, Sze DM, Zhang Y: What Are the Primary Limitations in B-Cell Affinity Maturation, and How Much Affinity Maturation Can We Drive with Vaccination? A Role for Antibody Feedback. Cold Spring Harb Perspect Biol. 2017; pii: a028795. 10.1101/cshperspect.a028795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gauld SB, Benschop RJ, Merrell KT, et al. : Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6(11):1160–7. 10.1038/ni1256 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Grandien A, Fucs R, Nobrega A, et al. : Negative selection of multireactive B cell clones in normal adult mice. Eur J Immunol. 1994;24(6):1345–52. 10.1002/eji.1830240616 [DOI] [PubMed] [Google Scholar]
- 83. Quách TD, Manjarrez-Orduño N, Adlowitz DG, et al. : Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J Immunol. 2011;186(8):4640–8. 10.4049/jimmunol.1001946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sabouri Z, Perotti S, Spierings E, et al. : IgD attenuates the IgM-induced anergy response in transitional and mature B cells. Nat Commun. 2016;7:13381. 10.1038/ncomms13381 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Cyster JG, Hartley SB, Goodnow CC: Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371(6496):389–95. 10.1038/371389a0 [DOI] [PubMed] [Google Scholar]
- 86. Cappione A, 3rd, Anolik JH, Pugh-Bernard A, et al. : Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115(11):3205–16. 10.1172/JCI24179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Reed JH, Jackson J, Christ D, et al. : Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. J Exp Med. 2016;213(7):1255–65. 10.1084/jem.20151978 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Sabouri Z, Schofield P, Horikawa K, et al. : Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc Natl Acad Sci U S A. 2014;111(25):E2567–75. 10.1073/pnas.1406974111 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Williams JM, Bonami RH, Hulbert C, et al. : Reversing Tolerance in Isotype Switch-Competent Anti-Insulin B Lymphocytes. J Immunol. 2015;195(3):853–64. 10.4049/jimmunol.1403114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation