Abstract
Perianal fistulae are commonly seen clinical entity. Development of malignancy within a perianal fistula is rare. Even rarer is the development of mucinous adenocarcinoma in a chronic fistula-in-ano. Only a handful of such cases have been reported in the past. A case of mucinous adenocarcinoma arising in chronic perianal fistula in a 34-year-old woman is being described. She presented with complaints of perineal fullness, pain and recurrent pus discharge from perianal fistula for 4 years. On radiological workup, a large solid-cystic pelvic mass was seen in relation to the fistula. On MRI, the lesion was mimicking a large horseshoe abscess. Transrectal ultrasound-guided biopsy and subsequent histopathological examination confirmed the presence of mucinous adenocarcinoma with tumour cells immunopositive for CK7 and CK20.
Keywords: radiology (diagnostics), colon cancer, radiology, gastrointestinal surgery, surgical oncology
Background
Mucinous adenocarcinoma arising from chronic perianal fistula is a rare occurrence with only few cases reported in the literature.1–12 Since it is a slow-growing neoplasm and does not invade bowel mucosa, it usually does not cause obstruction or bleeding per rectum. Hence, presentation is delayed and the tumours attain large size by the time they are diagnosed.1 3 8 A high degree of clinical suspicion is required for early diagnosis and subsequent treatment.
Case presentation
A 34-year-old woman presented with low back pain and perianal purulent discharge for a total duration of 4 years. Initially, she was managed conservatively with analgesics and antibiotics, but her symptoms persisted. Subsequently, she was operated twice with fistulotomy and drainage for this condition within an interval of 10 month but her symptoms were not relieved. She continued to experience local fullness and purulent discharge from the fistula.
On clinical examination, two discharging sinuses were seen in the perianal location at 9 and 3 o’clock positions, respectively. Scars from the previous surgery were present. Ultrasound (USG) evaluation revealed a large (11 cm×9 cm×6 cm) solid-cystic mass in pelvis with significant internal vascularity on colour Doppler evaluation. Contrast-enhanced CT scan showed a large lobulated predominantly cystic lesion with few enhancing foci within the pelvis (figure 1A–B). On MRI, the mass was hyperintense on T2-weighted images (T2WI), impinging and compressing over mid and lower rectum, displacing it to the left in the process. Both the ischiorectal fossae were involved and the mass was mimicking a large horseshoe-shaped abscess. Internal opening of the fistula was at 8 o’clock position, below the level of levator ani muscle (figure 1C–D). Proctoscopy revealed circumferential ulceration of the lower anal canal mucosa just proximal to the anal verge. Biopsy taken from this area showed features of acute or chronic proctitis without any evidence of malignancy. Colonoscopic evaluation was normal. Serum carcinoembryonic antigen (CEA) level was within normal limits. Transrectal ultrasound-guided biopsy was performed and subsequent histopathological examination revealed mucinous adenocarcinoma with tumour cells immunopositive for CK7 and CK20 (figure 2). Further work up with contrast-enhanced CT scan of thorax and abdomen did not show any other bowel lesion, lymphadenopathy or evidence of metastasis. Based on clinical, histopathological and radiological assessment, preoperative staging of the tumour was T3N0M0 according to American Joint Committee on Cancer seventh edition manual.
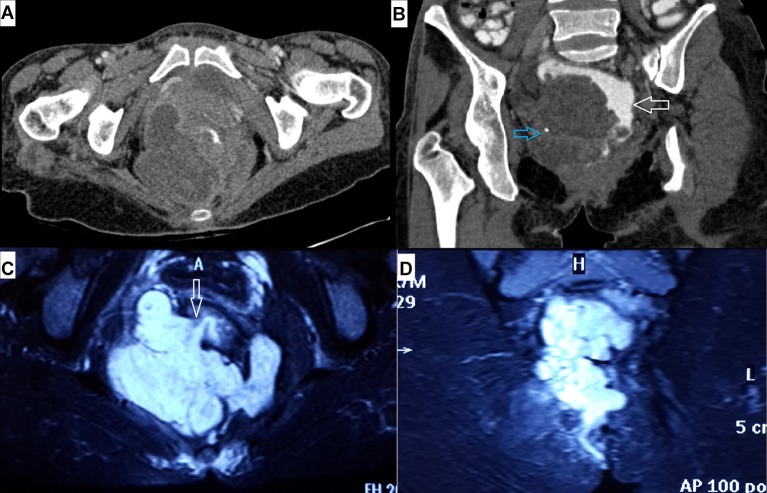
Figure 1.
(A) Axial and (B) oblique coronal contrast-enhanced CT images showing large hypodense pelvic mass impinging on rectum (white arrow in B), displacing it towards left. Also noted a tiny flake of calcification within the mass (blue arrow in B). Fat-suppressed MRI T2-weighted images in (C) axial and (D) coronal plane showing large lobulated T2 hyperintense mass involving both ischiorectal fossae mimicking horseshoe abscess. The mass is in relation to the tract of the fistula, and tumour-free internal opening is communicating with anal canal at 8 o’clock position (arrow in C).
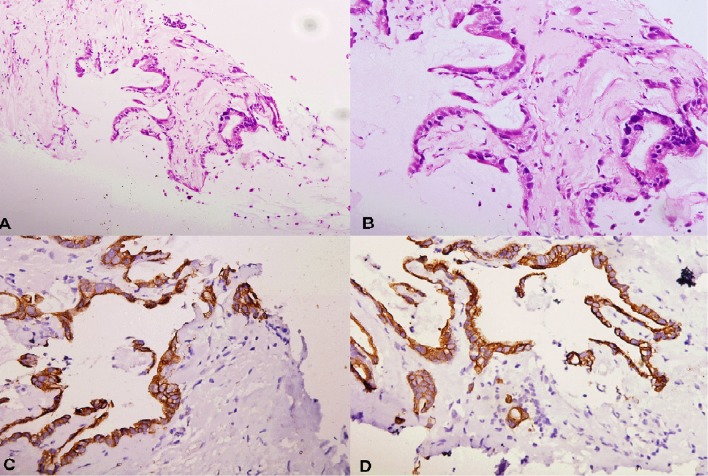
Figure 2.
Biopsy from pelvic mass shows mucinous adenocarcinoma invading surrounding fibrotic stroma (2A, 10×, H&E). The tumour cells show hyperchromatic nuclei and high nucleocytoplasmic ratio (1B, 20×, H&E). The tumour cells are immunopositive for CK20 (1C, 20×, IHC) and CK7 (1D, 20×, IHC). IHC, immunohistochemistry.
Treatment
Keeping in view the large size of the mass and extensive involvement of pelvis, patient was subjected to neoadjuvant chemoradiation. Chemotherapy started with oxaliplatin, capecitabine and dexamethasone regimen followed by radiotherapy and subsequent adequate abdominoperineal resection with wide excision of the tumour. Post surgery, resection margins were negative for malignancy (R0).
Outcome and follow-up
Postsurgery, the patient was discharged and kept under regular follow-up. She is live and free from disease for the last 23 months, both clinically and radiologically. No evidence of recurrence or metastases noted on follow-up assessment.
Discussion
Perianal fistula is a common entity seen in clinical practice; however, mucinous adenocarcinoma developing secondarily in a chronic perianal fistula is rare with less than 200 cases reported in the literature.5 7 Three criteria are required for the diagnosis of this condition: (1) presence of fistula should usually antedate that of carcinoma by 10 years, (2) any tumorous involvement of the rectum or anal canal should only be a secondary extension of the primary mass involving the fistula and (3) internal opening of the fistula within the rectum or anal canal should not be involved.3 9 Our case fulfils the last two criteria completely; however, duration of the presence of chronic pus discharging perianal fistula in the current case is 4 years. On thorough review of available literature, it becomes obvious that the first criterion stating time duration is arbitrary only and was proposed to exclude the possibility of malignancy preceding the fistula. Cases have been reported with the fistula antedating malignancy even by up to 3 years only.3 5 6 Though exact aetiology of this condition is not known, it is hypothesised that chronic inflammation-induced changes may be responsible at least in part for development of malignancy.3 This condition can also occur in long-standing perianal fistula of Crohn’s disease.13 On histopathology, malignant cells line large pools of extracellular mucin and on immunohistochemistry, these malignant cells are immunopositive for CK20 and sometimes for CK7 (figure 2). Since both pus collection and mucin are hyperintense on T2WI, these tumours may mimic abscesses on MRI as in our case. The patient may present with persistent symptoms for long duration which are not resolving even after repeated surgeries. A high level of clinical suspicion coupled with USG evaluation and biopsy from the mass is essential for early diagnosis and treatment in these cases. Also the fistula communicating with both the mass and the anal canal on MRI is considered a characteristic feature of mucinous adenocarcinoma arising from chronic perianal fistula (figure 1C).3
These tumours have indolent course, present late and hence attain a large size by the time they are diagnosed as in current case. This is because of infiltrative growth pattern of the tumour without having endoluminal extension into the bowel loop. Thus, luminal obstruction and bleeding per rectum are not usual presentations in these cases.1 3 8 Even on digital rectal examination, the mass might not be perceived and only a thickened indurated area is appreciated towards the side of fistula.7 One important clue to diagnosis can be obtained from mucinous instead of purulent discharge from the fistula.12 Gaertner et al in a series of 15 cases of fistula-associated adenocarcinoma informs that one should strongly suspect underlying mucinous adenocarcinoma in cases of recurrent or complex perianal fistula, if the discharge contains mucin.13 In another instance, Okada et al reported a case where repeated biopsies from the fistula were negative but cytology of mucinous discharge from the tract was positive for malignancy.14 Metastases to inguinal lymph nodes may be present in advanced cases; however, distant metastasis is uncommon.3 13 MRI best demonstrates extent of the disease and also helps in preoperative staging and further management.2–5
Since this condition is extremely rare, there is no consensus on the proper strategy for treating these cases.1 3 5 11 However, in general, adenocarcinomas of anorectal area are treated with abdominoperineal resection with wide local excision of the perirectal soft tissue, adjuvant/neoadjuvant chemotherapy and radiation.1 4–8 On review of literature, it becomes obvious that different strategies have been tried for the treatment of mucinous adenocarcinomas associated with perianal fistula with variable success. Better results have been obtained where aggressive approach with adjuvant chemoradiotherapy along with abdominoperineal resection were used as in our case.11 13 15 Hongo et al reported treatment and follow-up of 11 cases of perianal adenocarcinoma associated with perianal fistula and concluded that there is better outcome and more disease-free survival in cases where neoadjuvant chemoradiotherapy was given prior to surgery.11 Similarly Gaertner et al showed that using aggressive treatment strategy, these tumours can be cured in a considerable proportion of cases.13 Prognosis is usually poor if the tumour is larger than 5 cm, CEA is elevated or if lymph nodal or haematogeneous metastasis are there at the time of presentation.3 4 6 13
Learning points.
Mucinous adenocarcinoma arising from chronic perianal fistula is a rare occurrence. The presentation is often delayed or occult and a high degree of clinical suspicion is required for early diagnosis, which increases the scope for curative surgery.
Imaging is extremely useful in identifying development of malignancy and staging the disease, thereby guiding early and effective treatment. On MRI, it may mimic abscess and one must go for biopsy and histopathological examination, if solid components and/or internal vascularity are present on ultrasound Doppler study or there is mucinous discharge from the fistula.
Histopathological evaluation of tissue from cases of even benign looking perianal fistula undergoing surgery is advisable wherever possible to omit the possibility of missing an underlying malignancy.
Treatment includes surgery (abdominoperineal resection) with or without adjuvant/neoadjuvant chemotherapy and radiation. Better outcome has been seen in cases treated with more aggressive therapeutic approach.
Prognosis seems to be worse when the tumour is larger than 5 cm size, carcinoembryonic antigen is elevated or lymph nodal or haematogeneous metastasis is present at the time of diagnosis.
Footnotes
Contributors: SNP and HL conceived the manuscript. SNP prepared the manuscript. AR and FS collected the images and also helped in manuscript preparation. SNP and HL edited the manuscript. The final manuscript was read and approved by all the authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ohta R, Sekikawa K, Goto M, et al. . A case of perianal mucinous adenocarcinoma arising from an anorectal fistula successfully resected after preoperative radiotherapy. Case Rep Gastroenterol 2013;7:219–23. 10.1159/000351830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman HJ, Perry T, Webber DL, et al. . Mucinous carcinoma in Crohn’s disease originating in a fistulous tract. World J Gastrointest Oncol 2010;2:307–10. 10.4251/wjgo.v2.i7.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang BL, Shao WJ, Sun GD, et al. . Perianal mucinous adenocarcinoma arising from chronic anorectal fistulae: a review from single institution. Int J Colorectal Dis 2009;24:1001–6. 10.1007/s00384-009-0657-7 [DOI] [PubMed] [Google Scholar]
- 4.Venclauskas L, Saladzinskas Z, Tamelis A, et al. . Mucinous adenocarcinoma arising in an anorectal fistula. Med Kaunas Lith 2009;45:286–90. [PubMed] [Google Scholar]
- 5.Leal RF, Ayrizono ML, Coy CS, Csr C, et al. . Mucinous adenocarcinoma derived from chronic perianal fistulas: report of a case and review of the literature. Tech Coloproctol 2007;11:155–7. 10.1007/s10151-007-0348-8 [DOI] [PubMed] [Google Scholar]
- 6.Ong J, Jit-Fong L, Ming-Hian K, et al. . Perianal mucinous adenocarcinoma arising from chronic anorectal fistulae: a review from a single institution. Tech Coloproctol 2007;11:34–8. 10.1007/s10151-007-0322-5 [DOI] [PubMed] [Google Scholar]
- 7.Sierra EM, Villanueva Saenz E, Martínez PH, et al. . Mucinous adenocarcinoma associated with fistula in ano: report of a case. Tech Coloproctol 2006;10:51–3. 10.1007/s10151-006-0251-8 [DOI] [PubMed] [Google Scholar]
- 8.Papapolychroniadis C, Kaimakis D, Giannoulis K, et al. . A case of mucinous adenocarcinoma arising in long-standing multiple perianal and presacral fistulas. Tech Coloproctol 2004;8:s138–40. 10.1007/s10151-004-0136-7 [DOI] [PubMed] [Google Scholar]
- 9.Erhan Y, Sakarya A, Aydede H, et al. . A case of large mucinous adenocarcinoma arising in a long-standing fistula-in-ano. Dig Surg 2003;20:69–71. 10.1159/000068857 [DOI] [PubMed] [Google Scholar]
- 10.Ho CM, Tan CH, Ho BC. Clinics in diagnostic imaging (143). Perianal mucinous adenocarcinoma arising from chronic fistula-in-ano. Singapore Med J 2012;53:843–8. quiz p. 849. [PubMed] [Google Scholar]
- 11.Hongo K, Kazama S, Sunami E, et al. . Perianal adenocarcinoma associated with anal fistula: a report of 11 cases in a single institution focusing on treatment and literature review. Hepatogastroenterology 2013;60:720–6. [PubMed] [Google Scholar]
- 12.Santos MD, Nogueira C, Lopes C. Mucinous adenocarcinoma arising in chronic perianal fistula: good results with neoadjuvant chemoradiotherapy followed by surgery. Case Rep Surg 2014;2014:1–5. 10.1155/2014/386150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaertner WB, Hagerman GF, Finne CO, et al. . Fistula-associated anal adenocarcinoma: good results with aggressive therapy. Dis Colon Rectum 2008;51:1061–7. 10.1007/s10350-008-9294-4 [DOI] [PubMed] [Google Scholar]
- 14.Okada K, Shatari T, Sasaki T, et al. . Is histopathological evidence really essential for making a surgical decision about mucinous carcinoma arising in a perianal fistula? Report of a case. Surg Today 2008;38:555–8. 10.1007/s00595-007-3651-0 [DOI] [PubMed] [Google Scholar]
- 15.Inoue Y, Kawamoto A, Okigami M, et al. . Multimodality therapy in fistula-associated perianal mucinous adenocarcinoma. Am Surg 2013;79:E286. [PubMed] [Google Scholar]