Abstract
Immune checkpoint inhibitors have become standard of care in metastatic malignant melanoma management. Despite superior effectiveness to chemotherapy, significant immune-related adverse events (irAE) may occur, particularly if used in combination. Gastrointestinal irAEs were reported with different patterns of involvement. Here, we report the case of a patient who had ileal perforation as a complication of terminal ileitis, without colitis, induced by combination immune checkpoint blockade.
Keywords: skin cancer, unwanted effects / adverse reactions, small intestine, malignant disease and immunosuppression, radiology
Description
Immune checkpoint inhibitors have become standard of care in metastatic malignant melanoma management. Despite superior effectiveness to chemotherapy, significant immune-related adverse events (irAE) may occur, particularly if used in combination.1 Gastrointestinal irAEs induced by anti-CTLA-4 and anti-PD-1 agents and their patterns were reported by Marthey et al and Collins et al, respectively.2 3 Although a few patients had evidence of ileitis, none of these cases reported resulted in ileal perforation. There were five cases of colonic perforation in the study by Marthey et al.2 Here, we report the case of a patient who had ileal perforation as a complication of terminal ileitis, without colitis, induced by combination immune checkpoint blockade.
A 52-year-old woman with a diagnosis of metastatic vulval malignant melanoma presented prior to cycle three of combination ipilimumab–nivolumab treatment, with severe abdominal pain, mouth ulcers, nausea and appetite loss. Examination revealed generalised abdominal tenderness with guarding but no rigidity and bowel sounds present. Vital signs were within normal range. Laboratory tests were satisfactory except an elevated C reactive protein (89 mg/L). A prompt CT scan of the chest to pelvis revealed evidence of terminal ileitis but no colitis, perforation or bowel obstruction (figure 1). An excellent tumour response was also reported. She commenced intravenous methylprednisolone (1.5 mg/kg/day) and fluids. She initially responded to steroid treatment both clinically and biochemically. However, on night 6 after admission, while an inpatient she had a sudden and significant increase in her abdominal pain. A repeat CT scan of the abdomen confirmed our clinical suspicion of bowel perforation. She underwent an urgent laparotomy where her terminal ileum was resected and an end ileostomy fashioned. At surgery, throughout the ileum, there were multiple circular areas measuring 10–20 mm in diameter with significant inflammation. Only the most distal lesions were resected, as removal of all lesions would have risked short bowel syndrome. Histologically, each lesion was associated with complete loss of the mucosa—only attenuated muscularis propria and serosa remained—with florid neovascularisation at the periphery of each lesion (figure 2). A single small ileal perforation was present in the centre of one lesion. There was no vasculitis. Two days postoperatively, a single dose of intravenous infliximab (5 mg/kg) was administered, aiming to prevent further perforation of known residual inflamed sites. She made a rapid, unremarkable recovery, being discharged home 10 days postoperatively.
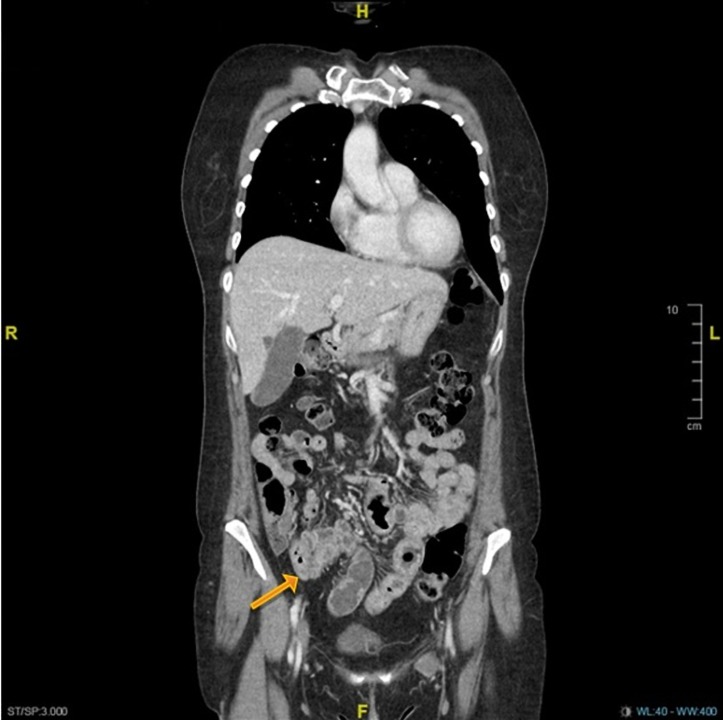
Figure 1.
Contrast-enhanced coronal-view CT scan of the lower chest, abdomen and pelvis showing bowel wall thickening of the distal ileum (arrow) with numerous small volume ileocolic lymph nodes suggestive of enteritis.
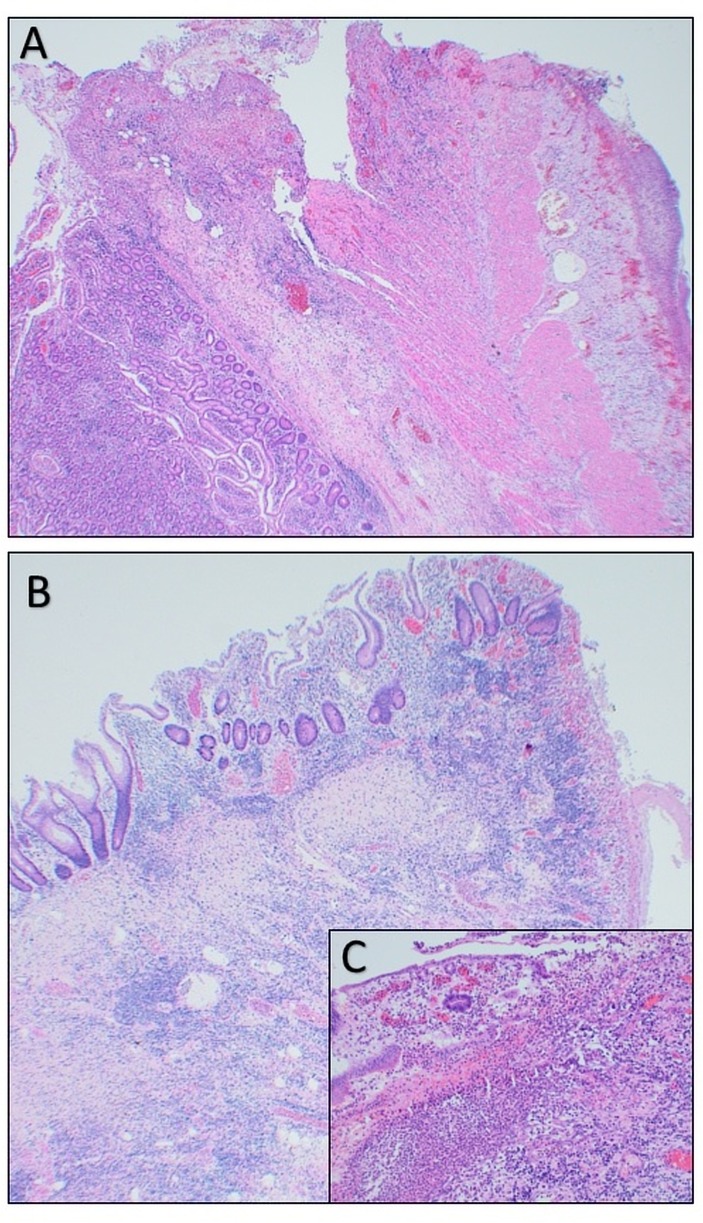
Figure 2.
A shows acute transmural ischaemic necrosis of the small bowel with perforation and peritonitis (H&E magnification ×40). B shows acute ischaemic changes to the small bowel with ulceration (H&E magnification ×40). C is a higher power image of acute ischaemic changes to the small bowel mucosa with ulceration (H&E magnification ×250).
To our knowledge, only one case of ipilimumab-induced ileitis without colitis that resulted in ileal perforation has been reported in the literature.4 The authors hypothesise that there could be two different pathways activated against colic and ileal epitopes resulting in two distinguished pattern of gastrointestinal involvement.
In conclusion, our case study highlights the importance of early recognition of gastrointestinal irAEs of checkpoint inhibitors particularly in patients receiving combination immunotherapy. Spain et al have outlined the general approach to the management of irAEs, and this needs to be expanded.5 We call for expert consensus on clear guidelines for the management of the gastrointestinal irAEs to try preventing complications and the need for surgical intervention.
Patient’s perspective.
I was offered immunotherapy after my melanoma returned, despite surgery in 2016. It was explained that the combined ipilimumab–nivolumab treatment would have side effects that could be very severe but that it was more effective than single treatment.
After the first treatment there were no major side effects other than the lymph node in my left groin swelled up and was very painful. I had a few itchy spots develop on my legs similar to mosquito bites. There were some occasional stomach cramps and my appetite started to decline just before my second treatment.
After the second treatment I developed mouth ulcers and the stomach cramps increased in severity, I was also sick on occasions—this reduced my appetite to zero. I was admitted to LRI and the symptoms seemed to indicate influenza —I started on steroids and once my temperature came down I was discharged.
On the week I was due for the third treatment I was in so much abdominal pain and had not eaten properly for weeks I was admitted again. The pain seemed to increase at night and the few nights before my surgery it was unbearable and the pain relief was ineffective—I kept the other patients awake with my screaming which is not like me as I have quite a high pain threshold.
I woke after the emergency surgery not sure of what had happened but grateful that the pain had decreased. The rest of the stay in hospital was spent getting moving and learning how to look after my stoma.
Having a stoma is proving troublesome as I’ve developed a fistula and this is causing pain where the stomach contents are burning the skin. After discussion with surgery team it may be as early as May for surgery reversing the stoma.
I am aware that I am very lucky to receive the treatment and to be clear of tumours, and that although the stoma is troublesome it could have been so much worse. I am back at work now after a phased return in January. I have also been on holiday abroad and plan another holiday before getting the stoma reversed.
It has been very distressing but I am truly grateful and I can’t thank all the people involved in my treatment enough, especially my oncology team, who were all excellent.
Learning points.
Immune checkpoint inhibitors are effective new generation of anticancer therapies but can be associated with significant immune-related adverse events (irAEs).
Autoimmune ileitis, without colitis, is a relatively rare gastrointestinal irAE in association with immune checkpoint inhibitor.
Careful reporting of irAEs helps to inform judgement of benefits and risks of cancer immunotherapies and aids early recognition and intervention.
Footnotes
Contributors: AAM conceived the case report, reviewed the literature and prepared the first manuscript draft. CJR contributed with the histopathological notes and images and reviewed the manuscript. KB contributed with the surgical notes and reviewed the manuscript. GF contributed to the conception of the case report and reviewed the manuscript. All authors approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marthey L, Mateus C, Mussini C, et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis 2016;10:395–401. 10.1093/ecco-jcc/jjv227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins M, Michot JM, Danlos FX, et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol 2017;28:2860–5. 10.1093/annonc/mdx403 [DOI] [PubMed] [Google Scholar]
- 4. Venditti O, De Lisi D, Caricato M, et al. Ipilimumab and immune-mediated adverse events: a case report of anti-CTLA4 induced ileitis. BMC Cancer 2015;15:87 10.1186/s12885-015-1074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44(Supplement C):51–60. 10.1016/j.ctrv.2016.02.001 [DOI] [PubMed] [Google Scholar]