Abstract
Results of an environmental assessment conducted in a newly emergent focus of murine typhus in southern California are described. Opossums, Didelphis virginiana Kerr, infested with cat fleas, Ctenocephalides felis Buché, in the suburban area were abundant. Animal and flea specimens were tested for the DNA of two flea-borne rickettsiae, Rickettsia typhi and Rickettsia felis. R. felis was commonly detected in fleas collected throughout this area while R. typhi was found at a much lower prevalence in the vicinity of just 7 of 14 case-patient homes identified. DNA of R. felis, but not R. typhi, was detected in renal, hepatic, and pulmonary tissues of opossums. In contrast, there were no hematologic polymerase chain reaction findings of R. felis or R. typhi in opossums, rats, and cats within the endemic area studied. Our data suggest a significant probability of human exposure to R. felis in the area studied; however, disease caused by this agent is not recognized by the medical community and may be misdiagnosed as murine typhus using nondiscriminatory serologic methods.
Keywords: flea-borne rickettsiosis, Rickettsia typhi, Rickettsia felis, flea, opossum
Murine typhus is a febrile disease caused by the gram-negative bacterium Rickettsia typhi. It is often referred to as a mild illness with clinical symptoms of fever, headache, rash, meningitis, and retinitis; however, murine typhus has also been described as a master of disguise, causing a variety of atypical symptoms with a fatality rate of up to 4% (Dumler et al. 1991, Azad et al. 1997, Gikas et al. 2002, Khairallah et al. 2009). R. typhi is classically maintained in a rat-flea-rat transmission cycle that involves urban rats, Rattus norvegicus Berkenhout and Rattus rattus L., and their flea, Xenopsylla cheopis Rothschild (Azad 1990). However, other rodents and ectoparasites have been reported to be infected with R. typhi (Traub et al. 1978, Azad 1990). As earlier reports regarding the ecology and atypical cycles of R. typhi were often based on biological or serological characteristics of R. typhi, some of these associations may need to be reevaluated with additional testing using modern molecular tools to ensure that other rickettsiae were not actually being detected.
Humans become infected with R. typhi through accidental contact with infected fleas and their feces by inhalation or scarification into damaged skin (Azad 1990). While the number of cases reported in the United States has decreased from a peak of several thousand cases per year during the early 1940s to <100 cases per year currently (Civen and Ngo 2008), murine typhus is still an endemic disease and a public health concern in Hawaii, the gulf coast of Texas, and southern California (Fergie et al. 2000; Centers for Disease Control and Prevention 2003, 2009; Purcell et al. 2007; Civen and Ngo 2008; Green et al. 2011).
After the identification of the first case of murine typhus in California in 1919, the number of annual cases increased until the middle of 1940s when 62 cases were reported in both 1945 and 1946 (California Department of Public Health 1950). In total, 473 cases and 21 deaths were attributed to murine typhus in California during 1919–1948, and all cases occurred in five southern counties: Los Angeles, Orange, San Bernardino, San Diego, and Santa Barbara (California Department of Public Health 1950). Since the early 1950s a shift has been seen in the geographic distribution of murine typhus cases in California from urban settings to more suburban areas (Adams et al. 1970). Interestingly, X. cheopis was rarely found in association with these suburban murine typhus cases (Adams et al. 1970). Subsequent epidemiological investigations led to the belief that this suburban transmission cycle of murine typhus may differ significantly from the classic urban cycle and involves cats (Felis catus L.), opossums (Didelphis virginiana Kerr), and the cat flea, Ctenocephalides felis Bouché (Beck and Van Allen 1947, Adams et al. 1970, Azad et al. 1997, Sorvillo et al. 1993, Schriefer et al. 1994a). Over the last two decades, it has been established that cat fleas are commonly infected with a different species of Rickettsia, Rickettsia felis (reviewed in Reif and Macaluso 2009), which belongs to the spotted fever group (Bouyer et al. 2001, La Scola et al. 2002). However, to the best of our knowledge, no confirmed human infections because of R. felis have been reported in California. Only murine typhus is currently diagnosed using serological methods with R. typhi antigen and reported in California (Green et al. 2011), despite the significant presence and wide distribution of R. felis in both urban and suburban environments (Civen and Ngo 2008, Abramowicz et al. 2011). Sera from humans infected with R. felis contain antibody reacting with R. typhi antigen detectable by both microimmunofluorescence assay and western blotting assay; similarly, sera from patients with confirmed diagnosis of murine typhus cross-react with R. felis antigen (Znasen et al. 2006, Wiggers et al. 2005, Pérez–Arellano et al. 2005, Raoult et al. 2001).
In response to an increased number of reported cases of murine typhus in suburban Los Angeles and Orange counties during 2007, an investigation was conducted to assess the contemporary ecological situation regarding the presence and circulation of flea-borne rickettsiae. We report the prevalence and distribution of R. typhi and R. felis at sites previously not known for murine typhus endemicity and discuss the ecological and epidemiological implications of these findings.
Materials and Methods
Animal and Flea Collections
Rats (R. rattus), opossums (D. virginiana), and cats (F. catus) were trapped in the vicinity of households with serologically confirmed human murine typhus cases in Orange and Los Angeles counties, CA, by the staff of the Vector-borne Disease Section, California Department of Public Health, the Long Beach Public Health Department, and the Orange County Vector Control District. After notification of each case, Sherman (H.B. Sherman Traps, Tallahassee, FL) and Tomahawk (Tomahawk Live Trap, Tomahawk, WI) live-traps were set up as close to the suspected human exposure site as possible, not to exceed a 1 mile (1,609 m) radius of the case household, and checked daily. Trapped animals were handled according to approved protocols. Fleas were combed from the animals and sent to the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. Fleas from the city of Long Beach were shipped on ice packs while those from Orange County were shipped on dry ice. Blood was drawn from the captured rats and opossums, and shipped to the CDC on cold packs in 2 ml EDTA blood tubes. Sixteen of 79 opossums from Orange County were euthanized and the brain, spleen, lung, liver, adrenal gland, and kidneys removed, frozen, and shipped to the CDC on dry ice. If cats were present in the case household, and owner approval was obtained, flea and blood samples were also collected and shipped to the CDC.
Serological Testing
Blood was centrifuged; plasma was transferred into a clean vial and the buffy coat was collected according to standard laboratory procedures. The plasma was used for serological testing and the buffy coat was used for DNA extraction as described below.
Serology was conducted using indirect immunofluorescent antibody assay (IFA) against R. typhi grown in embryonated chicken yolk sacs that were air-dried and acetone fixed onto template slide wells. In each assay, antibodies bound to the antigens were detected using species specific fluorescein-isothiocyanate (FITC) labeled conjugates. FITC-labeled goat anti-rat IgG (H + L) were purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD) and used at 1:100 dilution. Rabbit anti-opossum IgG (H + L) was obtained from Bethyl Laboratories (Montgomery, TX) and used at a final dilution of 1:100; the final detection was completed using FITC labeled goat anti-rabbit IgG (H + L) antibody at a final dilution of 1:100. The assay format, buffers, and other reagents were used according to the method described previously (Nicholson et al. 1997). Sera were tested at consecutive two-fold serum dilutions starting at 1:64 in dilution buffer (PBS, pH 7.4 supplemented with 1% BSA and 1% normal goat serum); the last well demonstrating specific fluorescence of the R. typhi organisms was recorded as the endpoint titer.
DNA Extraction from Buffy Coat and Tissues
DNA was extracted from the buffy coat preparations and tissue samples using the QiaAmp Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. The entire buffy coat was used for the DNA extractions. The opossum organs were thawed and ≈20 mg of tissue used for each extraction; DNA was eluted using AE buffer and stored at 4°C.
Flea Processing
Upon receipt at the CDC, fleas were identified to species using standard taxonomic keys. Fleas from animals were usually placed into 1.8 ml Sarstedt vials in pools of 2–3 to reduce testing costs; however, different species of fleas, or from different animals, were never combined together. The DNA was extracted as previously reported (Eremeeva et al. 2008, Karpathy et al. 2009). Briefly, the fleas were disinfected with 10% bleach and 70% ethanol, pulverized after freezing in liquid nitrogen, and the DNA extracted using the Wizard SV96 Genomic DNA kit (Promega, Madison, WI) on a Biomek 2000 Laboratory Automation Workstation (Beckman Coulter, Fullerton, CA). DNA was eluted with 100 μl of sterile distilled water and stored at 4°C.
Nested Polymerase Chain Reaction
Nested polymerase chain reaction (PCR) was used to test flea samples for the presence of rickettsial DNA as previously published (Massung et al. 2001, Tzianabos et al. 1989). Briefly, 2 μl of sample DNA was used in a 25 μl primary reaction along with primers Rr17.122 and Rr17.500. One microliter of the primary reaction was used as the template for the secondary reaction along with primers RP1D and RP2 for the detection of R. typhi, or primers TZ15 and TZ16 for the detection of R. felis. All products were resolved on a 1% agarose gel in 1× TAE and sequenced.
Taqman Assay
Primers were designed to amplify a 266 bp region of the citrate synthase gene (gltA) of Rickettsia (Eremeeva et al. 2008, Karpathy et al. 2009). These primers (gltA-F.287-GAT TTT TTA GAA GTG GCA TAT TTG; gltA-R.552-GGK ATY TTA GCW ATC ATT CTA ATA GC) were used in conjunction with species specific probes to simultaneously detect and differentiate R. typhi and R. felis DNA. To increase the melting temperature and binding specificity, locked nucleic acid (LNA) nucleotides (in parenthesis) (Koshkin et al. 1998) were incorporated into the probes (R. typhi gltA: 5′ CalRed 610-TT(T) A(C)T A(C)A (A)AG (A)T(T) G(C)T (C)A-BHQ2; R. felis gltA: 5′ Cy-5-CTA (C)GG A(G)A ATT (G)CC A-BHQ3) (Eremeeva et al. 2008, Karpathy et al. 2009). Reagents from the Brilliant qPCR kit (Stratagene, LA Jolla, CA) were used as previously described (Eremeeva et al. 2008, Karpathy et al. 2009). All samples, including both positive and negative control samples placed on every plate, were tested in duplicate.
DNA Sequencing and Analysis
All nested PCR products and selected qPCR products were purified using the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI) according to the manufacturer’s instructions. Purified amplicons were sequenced using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions on an ABI 3130xl genetic analyzer (Applied Biosystems). The sequencing reads were assembled and analyzed using Sequencher 4.8 (Gene Codes Corporation, Ann Arbor, MI) and compared with the nr database of the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/).
Data Analysis
Minimum infection rates (MIR) were calculated for both R. typhi and R. felis by dividing the total number of flea pools positive for that agent (pools positive for that agent alone plus those pools positive for both agents) by the total number of fleas tested. To assess the distribution of flea infection rates in proximity to the human case households, trap locations were classified as being within areas of one acre or closer to a case household (≈36 m radius), a half mile radius from a household (>36–800 m), or between 0.5 and 1 mile radius (>800–1,600 m) from a case household. Opossum home-range was estimated between 10 and 50 acres (113–252 m radius or 4–20 ha area) (Jackson 2011). Flea infection rates obtained from animals trapped further than a mile from any case household were grouped together and were used to determine the background level of R. typhi and R. felis in the area. Statistical assessment was done using Student’s t-test and χ2 test.
Results
Characteristics of Trapped Animals and Collections of Fleas
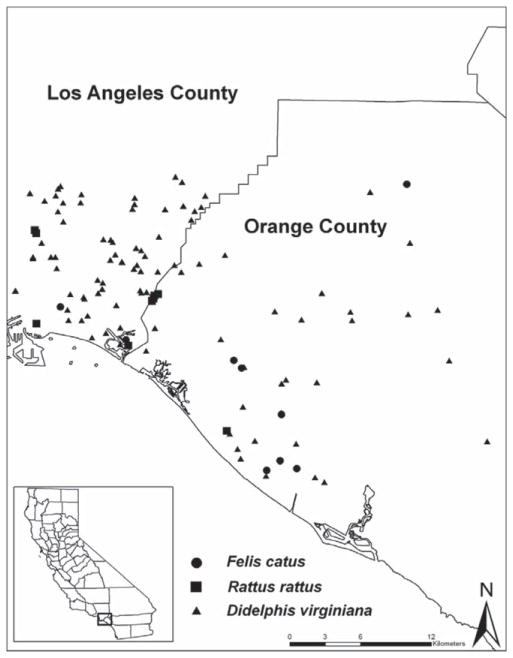
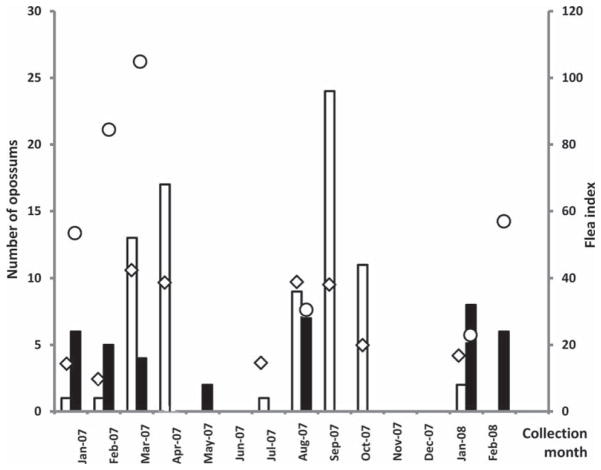
After reporting of diseases, an environmental investigation was conducted for each of 14 serologically diagnosed human cases of murine typhus in the City of Long Beach area of Los Angeles County and the neighboring part of Orange County. In total, 177 opossums (D. virginiana), 20 rats (R. rattus), and 16 cats (F. catus) were captured during 14 mo between January of 2007 and February of 2008 (Table 1; Figs. 1 and 2). Most of the opossums were caught in the City of Long Beach during March–April and August–October months of 2007 (30 and 44 animals, respectively, 38 and 56% of the total number trapped), while in Orange County, most animals were trapped in January–February of 2007 (11 opossums, 29%) and 2008 (14 opossums, 37%) (Fig. 2).
Table 1.
Summary of trapping success and laboratory findings in animal blood
| Location and animal | Characteristic (no.) | Flea species collected | Flea index (range)a | Laboratory findings in animals | |
|---|---|---|---|---|---|
|
| |||||
| IFA | PCRb | ||||
| Los Angeles County | |||||
| Didelphis virginiana (79) | Adult, female (40) | Ctenocephalides felis (1,189) | 40 (2–144) | 0/24 | 0/38 |
| Xenopsylla cheopis (5) | |||||
| Pulex irritans (1) | |||||
| Echidnophaga gallinaceae (2) | |||||
| Adult, male (25) | Ctenocephalides felis (1,151) | 66 (4–136) | 0/18 | 0/24 | |
| Pulex irritans (1) | |||||
| Echidnophaga gallinaceae (2) | |||||
| Young (10) | Ctenocephalides felis (174) | 17 (1–50) | 0/10 | 0/10 | |
| Pulex irritans (1) | |||||
| Echidnophaga gallinaceae (13) | |||||
| Unclassified (4) | Ctenocephalides felis (99) | 25 (14–42) | 0/1 | 0/4 | |
| Rattus rattus (6) | Adult, female (3) | None | 0/3 | 0/3 | |
| Adult, male (3) | None | 0/3 | 0/3 | ||
| Felis catus (3) | Adult (3) | Ctenocephalides felis (27) | 9 (3–11) | NDc | 0/3 |
| Orange County | |||||
| Didelphis virginiana (38) | Adult, female (11) | Ctenocephalides felis (504) | 46 (0–197) | 0/6 | 0/10 |
| Diamanus montanus (2) | |||||
| Adult, male (7) | Ctenocephalides felis (606) | 87 (0–216) | 0/6 | 0/7 | |
| Echidnophaga gallinaceae (2) | |||||
| Diamanus montanus (2) | |||||
| Pulex irritans (104) | |||||
| Young (5) | Ctenocephalides felis (103) | 21 (0–51) | 0/3 | 0/2 | |
| Unclassified (15) | Ctenocephalides felis (550) | 37 (2–221) | 0/3 | 0/4 | |
| Pulex irritans (6) | |||||
| Rattus rattus (14) | Adult, female (6) | None | 0/6 | 0/6 | |
| Adult, male (4) | Ctenocephalides felis (11) | 0/2 | 0/4 | ||
| Pulex irritans (1) | |||||
| Young (3) | None | 0/3 | 0/3 | ||
| Unclassified (1) | Leptosylla segnis (1) | 0/1 | 0/1 | ||
| Felis catus (13) | Adult (13) | Ctenocephalides felis (29) | 2 (0–29) | ND | 0/13 |
Flea index and range were calculated for C. felis only because of the low collection sizes of other flea species collections.
PCR only blood results are shown. R. felis PCR positive issue samples were found in 4 of 16 opossums trapped in Orange County (details are provided in the text).
ND, testing not performed.
Fig. 1.
Map of animal trap sites in Los Angeles and Orange Counties.
Fig. 2.
Seasonal distribution of opossum trapping in Los Angeles and Orange Counties, CA. White bars, number of opossums captured in Los Angeles County; black bars, numbers of opossums captured in Orange County; diamond (◇) indicates flea index in Los Angeles County; circle (○) indicates flea index in Orange County.
In total, 4,586 fleas were collected including C. felis (n=4443, 97%), 114 Pulex irritans L., 19 Echidnophaga gallinacea Westwood, 5 Xenopsylla cheopis, 4 Diamanus montanus Baker, and 1 Leptosylla segnis Schoenherr (Table 1). Many animals had severe flea infestations particularly opossums. Male adult opossums typically had a higher flea index than females (P=0.04 and P = 0.006 [χ2 test], respectively, in Los Angeles and Orange counties). In total, 56 C. felis fleas were collected from 6 of 16 cats examined. Only two rats from Orange County of the 16 rats captured in both locations had fleas, of which one was infested by C. felis (n = 11) and P. irritans (n = 1), the other rat had only one L. segnis, and neither harbored X. cheopis.
Laboratory Testing of Animal Specimens
DNA of buffy coats from 138 animals was tested for the presence of DNA from R. typhi and R. felis using the multiplex TaqMan assay. No rickettsial DNA was detected in any of the blood samples tested (Table 1). Serological testing did not detect the presence of antirickettsial antibodies reacting with R. typhi antigen in the plasma of these animals (Table 1). In contrast, DNA of R. felis was detected in tissue samples from several opossums captured in Orange County. Of the 81 tissue samples from 16 animals examined, five DNA samples from four animals tested positive for R. felis DNA by TaqMan assay: lung (n = 1), kidney (n = 1), liver (n = 1), and adrenal gland (n = 2). DNA of R. typhi was not detected in any of the 81 animal tissue samples tested.
Testing of Fleas
In total, 4,586 fleas were removed from opossums (n=112 of 117), cats (n=6 of 16), and rats (n = 2 of 20) captured at peridomestic sites in the vicinity of households of patients with suspected or serologically confirmed cases of murine typhus in Los Angeles and Orange Counties, CA; 3,690 fleas were tested in 1,950 pools for the presence of DNA from R. typhi and R. felis (Table 2). The fleas were tested in samples of 1, 2, or 3 fleas (average =1.9 fleas per pool) with each pool consisting of a single species of flea, all from the same animal. Of the 1,950 pooled samples tested, 29 (1.5%) were positive for the presence of only R. typhi DNA and 900 (46.2%) were positive only for R. felis DNA. Furthermore, 33 (1.7%) samples tested positive for both R. typhi and R. felis DNA.
Table 2.
Detection of R. typhi and R. felis DNA in flea DNA samples
| Location, flea species | No. of flea pools tested (total fleas) | No. of positive pools (%) | Minimum infection rate (%)a | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| R. typhi | R. felis | Dual positive | R. typhi | R. felis | ||
| Orange County | ||||||
| Ctenocephalides felis | 727 (1405) | 7 (0.9) | 428 (58.9) | 22 (3.0) | 2.1 | 32 |
| Pulex irritans | 55 (109) | 4 (7.3) | 9 (16.4) | 0 | 3.7 | 8.3 |
| Echidnophaga gallinacea | 2 (2) | 0 | 0 | 0 | 0 | 0 |
| Diamanus montanus | 3 (4) | 0 | 2 (66.7) | 0 | 0 | 50 |
| Leptosylla segnis | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Los Angeles County | ||||||
| Ctenocephalides felis | 1,146 (2144) | 18 (1.6) | 454 (39.6) | 10 (0.9) | 1.3 | 21.6 |
| Pulex irritans | 3 (3) | 0 | 1 (33.3) | 0 | 0 | 33.3 |
| Xenopsylla cheopis | 4 (5) | 0 | 2 (50.0) | 0 | 0 | 40 |
| Echidnophaga gallinacea | 9 (17) | 0 | 4 (44.4) | 1 (11.1) | 5.9 | 29.4 |
Minimum infection rate: no. of PCR positive flea pools/total no. of fleas tested. Assumes that each PCR positive pool contains DNA of at least one flea infected with either R. typhi or R. felis, respectively.
When analyzed based on the host animal species, significant differences in flea infestation and prevalence of infected fleas were detected (Tables 1 and 2). Only 2 of the 20 rats captured were infested with fleas including 1 L. segnis, 1 P. irritans, and 11 C. felis. No rickettsial DNA was detected from the P. irritans or L. segnis collected from rodents, while 1 of the 6 C. felis pools was positive for R. felis DNA. In total, 56 C. felis were collected from 6 cats and screened in 29 pools. Of these, 22 pools (75.9%) tested positive for the presence of R. felis DNA alone, while one pool was positive for the DNA of both R. felis and R. typhi. The remaining 3,621 fleas, including 3,472 C. felis, 111 P. irritans, 19 E. gallinaceae, 5 X. cheopis, and 2 D. montanus, were collected from 112 opossums. These fleas were tested in 1,914 pools and 877 (45.8%) tested positive only for R. felis DNA, 29 (1.5%) tested positive only for R. typhi DNA, and 32 pools (1.7%) tested positive for the presence of both DNAs.
For C. felis, 47.1% (n = 882) of the pools tested positive for R. felis DNA only, 1.3% (n = 25) were positive for R. typhi DNA only, and 1.7% (n=32) were positive for DNA from both species. Of the 58 P. irritans pools, 17.2% (n = 10) were positive for R. felis DNA only and 6.9% (n = 4) were positive for R. typhi DNA only. The 19 E. gallinacea fleas were tested in 11 pools; 4 (36.4%) were positive for R. felis DNA only, and 1 (9.1%) tested positive for both R. typhi and R. felis DNA. Two (50%) of the four X. cheopis flea pools tested positive for R. felis DNA only, as did two (66.7%) of the three D. montanus pools. No R. typhi DNA was detected in any of the X. cheopis or D. montanus fleas tested. No rickettsial DNA was detected in the lone L. segnis flea tested.
Spatial Distribution of R. felis and R. typhi in Fleas Relative to Case Households
Of the 749 fleas removed from 26 animals captured within a one acre area around the case household, 63.7% of flea pools were positive for R. felis DNA and 4.1% were positive for R. typhi DNA. Nine animals were captured within 0.002 mile to 0.5 mile radius from a case household from which 354 fleas were collected. Of these, 35.9% of the pools tested positive for R. felis DNA and 4.9% tested positive for R. typhi DNA. Thirteen animals were captured within a 0.5–1 mile radius zone from a case household, from which 294 fleas were removed. Rickettsia felis DNA was detected in 37.7% of such flea pools, while R. typhi DNA was not detected in any fleas. In total, 2,293 fleas were removed from 69 animals trapped further than 1 mile from any reported human cases; 46.9% of flea pools were positive for the presence of R. felis DNA while 3.9% were positive for R. typhi DNA. Respectively, there was no significant difference in the MIRs for R. typhi of fleas collected within a 1 mile radius zone from cases compared with the MIR in fleas collected more than a mile from any case (P = 0.33; χ2 test). Similarly, the MIR of R. felis in the same fleas was 26.7% and did not exhibit significant differences compared with 26.2% MIR detected in the fleas collected from remote locations (P = 0.73; χ2) (Table 3).
Table 3.
Flea minimum infection rates vs distance to case household
| Distance to nearest case | Fleas | Animals | MIR R. felis |
MIR R. typhi |
|---|---|---|---|---|
| 36 m (0.02 mi) | 749 | 26 | 32.9 | 2.1 |
| 37–800 m (<0.5 mi) | 354 | 9 | 18.6 | 2.5 |
| 801–1,600 m (0.5–1 mi) | 294 | 13 | 20.7 | 0 |
| >1,600 m (>1 mi) | 2,293 | 69 | 26.2 | 2.2 |
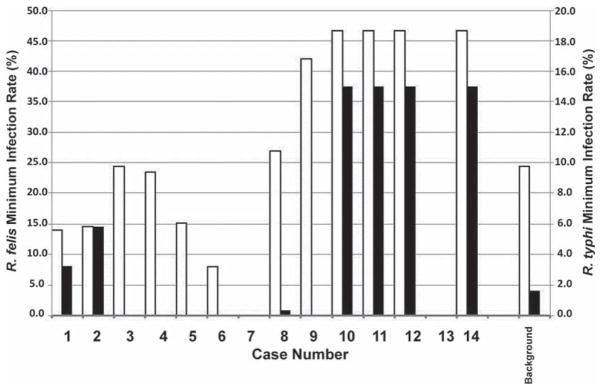
To further evaluate these observations, we analyzed the same parameters on a case by case basis (Fig. 3). R. typhi was detected in fleas near 7 of 14 case households but was not detected in any flea collected within a mile of cases 3, 4, 5, 6, and 9, and only infrequently (MIR = 0.8%) in fleas collected within an acre area of case 8. However, R. felis was detected in these same fleas in varying rates (MIR = 34.3–48.1%), often exceeding or even nearly doubling the background rate (MIR =26.2%). Fleas removed from animals captured 0.5 miles (37–800 m) from cases one and two had MIRs for R. typhi (3.2 and 6.9%, respectively) much higher than the background rate (1.6%). Cases 10, 11, 12, and 14 represent a cluster of neighboring cases and the same fleas were associated with all four cases. Both R. typhi (MIR = 15.0%) and R. felis (MIR = 46.6%) were detected at rates much higher than the background rate and either agent could have caused disease. No animals were trapped within a mile from either case 7 or 13.
Fig. 3.
Minimum infection rates for R. typhi and R. felis in fleas collected within one mile of individual case households. Minimum infection rates were calculated as the ratio of the numbers of PCR positive flea DNA samples divided by total number of flea DNA samples tested. The background prevalence was calculated from all fleas collected more than 1 mile from any known case household. No animals were collected within 1 mile of cases 7 and 13.
Discussion
The state of California is one of two mainland U.S. states that continues surveillance and reporting of murine typhus because of its recognized potential public health impact. By the end of the 20th century the number of reported cases fell off dramatically because of efforts aimed at controlling the populations of rats and their associated ectoparasites. Current endemic foci are mostly confined to a few southern counties, with Los Angeles County accounting for 42 to 91% of all cases in California (Civen and Ngo 2008). Observations made during the 1970–1990s led to the conclusion that the distribution of human cases has shifted from urban to suburban foci and, respectively, associated with two different ecological cycles, the classic urban cycle involving R. typhi circulating in rat and rat fleas and the suburban cycle involving opossums, cats, and cat fleas that were later found to be primarily infected with R. felis (Adams et al. 1970, Reif and Macaluso 2009). The classic endemic focus continues to be associated with its historic area in North Central Los Angeles County and central Los Angeles. Starting in 2006, murine typhus cases were observed first in the western part of Los Angeles County and then south and eastward in areas where murine typhus had not typically been reported (Adams et al. 1970, Civen and Ngo 2008). Here we provide the details of our ecological assessment of areas in the City of Long Beach area of Los Angeles County and an adjacent region of Orange County where emerging cases of murine typhus have been reported.
To ensure sensitive high throughput testing we developed a multiplex TaqMan assay that allows simultaneous detection of two flea-borne rickettsiae, R. typhi and R. felis (Eremeeva et al. 2008, Karpathy et al. 2009). First, 216 C. felis DNA samples were used to compare the performance of our TaqMan assay which targets a GltA gene fragment (Eremeeva et al. 2008, Karpathy et al. 2009) with a nested PCR assay targeting the 17 kDa protein gene of Rickettsia (Tzianabos et al. 1989, Massung et al. 2001). We established that the TaqMan assay is more sensitive than the nested PCR for detection of R. felis in DNA samples of wild-caught cat fleas as it detected ≥3 times as many positive samples as did the nested PCR assay. The sensitivity for detection of R. typhi DNA was demonstrated using a homologous recombinant plasmid, because R. typhi DNA was not detected in this set of samples and is typically much less prevalent in cat fleas as shown here and reported by others (Boostrom et al. 2002, Henry et al. 2007). The sensitivity of this R. typhi TaqMan assay was similar as that established for R. felis DNA with limits of detection 1–2.5 DNA copies per reaction (Karpathy et al. 2009).
In total, 3,690 fleas that had been removed from 120 animals captured in the suburban environment associated with suspected or serologically confirmed cases of human murine typhus were tested for R. typhi and R. felis DNA. Overall the MIR in these samples was 1.7% for R. typhi and 25.3% for R. felis (Table 2). When the samples from two counties were compared, the MIR of fleas with R. typhi was estimated to be 1.3% in Los Angeles County and 2.2% in Orange County and did not exhibit significant differences (P = 0.72; χ2), while the MIR of fleas with R. felis was much lower in Los Angeles County (21.8%) than that found in Orange County (30.3%). When the presence and prevalence of R. felis and R. typhi in fleas captured within a 1 mile distance from each case household was estimated, we found R. typhi only in the vicinity of seven patient households (Fig. 3). No R. typhi (five cases) or even animals (two cases) were found for the other seven cases also diagnosed as murine typhus using serologic methods (Fig. 3). The reported home range of Norway rats is within a 25–50 m radius, but may vary depending on habitat conditions and density of rodent population (Russell et al. 2010). In Southern California, the roof rat home range varies from 0.5 to 1.25 acres (a 2.5–12 m radius) and shape depending on the microstructure of the habitat (Recht 1988). While we expected opossums to range much more widely (Jackson 2011), our trapping results suggested that their distribution around case households is patchy so some exposures may have actually occurred at sites other than their households.
Our data indicate a very significant prevalence of R. felis in multiple species of fleas collected from two suburban areas of southern California identified as newly emergent foci of murine typhus. Furthermore, the significant presence of opossums with a high rate infestation by C. felis was also established, while attempts to detect the classic rat–flea cycle of R. typhi were not successful in this study. Indeed, only a few roof rats were captured and they had very limited flea infestation, a result in good agreement with previous findings in 1985 reporting lack of ectoparasites on these animals (Schwan et al. 1985). However, C. felis that were recovered from case homes with pet cats exhibited a high MIR infection rate by R. felis only (38 and 41% from three infested cats each in Orange and Los Angeles Counties, respectively), suggesting the possible importance of domestic cats as transport hosts of fleas to humans. Cat fleas are very promiscuous feeders and can establish transient parasitism on many different animals; furthermore, once infected they maintain persistent rickettsial infection through very effective transovarial transmission (Azad 1990). To the contrary, the efficiency of horizontal transmission is low and it appears that only a small portion of animals infested with infected fleas may develop a transient rickettsiaemia (Wedincamp and Foil 2000, Boostrom et al. 2002, Adjemian et al. 2010, Horta et al. 2010). Seroconversion in animals to R. felis has also been reported; however, it appears to require a prolonged exposure before reaching detectable levels in cats and opossums, and infection does not always correlate with the degree of flea infestation (Reif et al. 2008, Horta et al. 2010) and occurs at a low prevalence in cats (Case et al. 2006). Therefore, it is plausible to conclude that the absence of detectable antirickettsial antibodies in the blood of animals tested in this study may be because of limited transmission from cat fleas that were mostly infected with R. felis. The source and mechanism of acquisition and maintenance of R. typhi by cat fleas in this area is not known and it may be because of spillover from wildlife that was not sampled. Norway rats and mice are particularly susceptible to R. typhi infection and may establish a long-term persistent intracerebral infection after a short-term transient rickettsiemia (Traub et al. 1978). Although roof rats are often described as a natural reservoir of R. typhi (Traub et al. 1978), their significance in the maintenance of R. typhi in California appears to be less important and may require further evaluation as noted previously (Schwann et al. 1985) and found in this study. Another source of R. typhi might be P. irritans, a very promiscuous feeder. In this study we obtained two large collections of 62 and 42 P. irritans both from two opossums from Orange County that were also infested with C. felis. For one of these animals, P. irritans tested positive either for R. felis or R. typhi but not both while C. felis were positive for R. felis alone or tested positive for both organisms.
R. felis has been detected on several occasions in the same animal and flea populations as R. typhi, and its presence often correlates with identification of human cases of murine typhus (Boostrom et al. 2002, Abramowicz et al. 2011). Experimentally infected fleas were shown to be able to maintain co-infection with both rickettsiae (Noden et al. 1998), and wild caught X. cheopis can be also coinfected with R. felis and R. typhi (Eremeeva et al. 2008). Those observations raise questions about the role of both bacteria in the pathogenesis and epidemiology of human infections diagnosed as murine typhus. Although, R. felis is a recognized human pathogen with well-confirmed cases reported from many parts of the world (Pérez–Osorio et al. 2008, Richards et al. 2010), in the United States only one single confirmed case due to R. felis was identified in 1994 in Texas (Schriefer et al. 1994b). The relatively low incidence of R. felis cases reported in other countries either suggests that only a small portion of the human population may be susceptible to this pathogen or other factors may affect susceptibility to infection including genetic variations in the pathogen itself (Bauer et al. 2006, Rolain et al. 2009). It is unknown whether there is a human genetic factor associated with susceptibility to this infection. Molecular diagnostic practices and procedures should be implemented to ensure accurate clinical treatment and etiological identification of cases suggestive of flea-borne rickettsioses because these infections may be because of either R. typhi or R. felis based on the presence of both agents in fleas in many locations. PCR based diagnostic assays are recommended to detect the presence of rickettsial DNA in EDTA whole-blood samples collected during the acute stage of illness before administration of antibiotics. If only serological testing can be performed, simultaneous testing of acute and convalescent serum specimens against both R. typhi and R. felis antigens is needed, ideally using cross-absorption of the sera to ensure the accurate differential detection of the correct etiological agent (Pérez–Arellano et al. 2005).
Acknowledgments
We thank Maria L. Zambrano, Kyle F. Abramowicz, and Michele M. Sturgeon of the CDC for laboratory assistance; Wesley Moore, Lamar Rush, and John Holguin of City of Long Beach for assistance with field work, the CDC Biotechnology Core Facility Branch for synthesis of primers and probes, and cell culture. This research was supported in part by an appointment of S.E.K. to the Emerging Infectious Diseases Fellowship Program administered by the Association of Public Health Laboratories and funded by the Centers for Disease Control and Prevention. E.K.H. was supported through the James A. Ferguson Emerging Infectious Diseases Minority Program Fellows, CDC. A.M.W. was supported through the Bevier Training Program of Agnes Scott College, Atlanta, GA. Y.Z. was supported through the Partners of the Americas, Department of State, Washington, DC. Preliminary reports of this investigation were presented in 2007 at the 21st Meeting of the American Society for Rickettsiology (Colorado Springs, CO) and in 2008 at the fifth International Meeting on Rickettsiae and Rickettsial Diseases (Marseille, France).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control or the Department of Health and Human Services of the United States.
References Cited
- Abramowicz KF, Rood MP, Krueger L, Eremeeva ME. Urban focus of Rickettsia typhi and Rickettsia felis in Los Angeles, California. Vector-Borne Zoon Dis. 2011;11:979–984. doi: 10.1089/vbz.2010.0117. [DOI] [PubMed] [Google Scholar]
- Adams WH, Emmons RW, Brooks JE. The changing ecology of murine (endemic) typhus in Southern California. Am J Trop Med Hyg. 1970;19:311–318. doi: 10.4269/ajtmh.1970.19.311. [DOI] [PubMed] [Google Scholar]
- Adjemian J, Parks S, McElroy K, Campbell J, Eremeeva M, Nicholson W, McQuiston J, Taylor J. Murine typhus in Austin, Texas, USA, 2008. Emerg Infect Dis. 2010;16:412–417. doi: 10.3201/eid1603.091028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad AF. Epidemiology of murine typhus. Annu Rev Entomol. 1990;35:553–569. doi: 10.1146/annurev.en.35.010190.003005. [DOI] [PubMed] [Google Scholar]
- Azad AF, Webb L, Carl M, Dasch GA. Detection of rickettsiae in arthropod vectors by DNA amplification using the polymerase chain reaction. Ann N Y Acad Sci. 1990;590:557–563. doi: 10.1111/j.1749-6632.1990.tb42266.x. [DOI] [PubMed] [Google Scholar]
- Azad AF, Radulovic S, Higgins JA, Noden BH, Troyer JM. Flea-borne Rickettsioses: ecologic considerations. Emerg Infect Dis. 1997;3:319–327. doi: 10.3201/eid0303.970308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer O, Baneth GAD, Eshkol T, Shaw SE, Harrus S. Polygenic detection of Rickettsia felis in cat fleas (Ctenocephalides felis) from Israel. Am J Trop Med Hyg. 2006;74:444–448. [PubMed] [Google Scholar]
- Beck MD, Van Allen A. Typhus fever in California, 1916–1945, inclusive: an epidemiologic and field laboratory study. Am J Epidemiol. 1947;45:335–354. doi: 10.1093/oxfordjournals.aje.a119140. [DOI] [PubMed] [Google Scholar]
- Boostrom A, Beier MS, Macaluso JA, Macaluso KR, Sprenger D, Hayes J, Radulovic S, Azad AF. Geographic association of Rickettsia felis-infected opossums with human murine typhus, Texas. Emerg Infect Dis. 2002;8:549–554. doi: 10.3201/eid0806.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer D, Stenos J, Crocquet–Valdes P, Moron C, Popov V, Zavala–Velazquez J, Foil L, Stothard D, Azad A, Walker D. Rickettsia felis: molecular characterization of a new member of the spotted fever group. Int J Syst Evol Microbiol. 2001;51(3):39–347. doi: 10.1099/00207713-51-2-339. [DOI] [PubMed] [Google Scholar]
- California Department of Public Health. Typhus fever in California: 1916–1948, inclusive. State of California Department of Public Health; San Francisco, CA: 1950. [Google Scholar]
- Case JB, Chomel B, Nicholson W, Foley JE. Serological survey of vector-borne zoonotic pathogens in pet cats and cats from animal shelter and feral colonies. J Fel Med Surg. 2006;8:111–117. doi: 10.1016/j.jfms.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Murine typhus–Hawaii, 2002. MMWR. 2003;52:1224–1226. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Outbreak of Rickettsia typhi infection–Austin, Texas, 2008. MMWR. 2009;58:1267–1270. [PubMed] [Google Scholar]
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913–918. doi: 10.1086/527443. [DOI] [PubMed] [Google Scholar]
- Dumler JS, Taylor JP, Walker DH. Clinical and laboratory features of murine typhus in south Texas, 1980 through 1987. JAMA. 1991;266:1365–1370. [PubMed] [Google Scholar]
- Eremeeva ME, Warachina WR, Sturgeon MM, Buchholz AE, Olmsted GK, Park SY, Effler PV, Karpathy SE. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2008;14:1613–1615. doi: 10.3201/eid1410.080571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergie JE, Purcell K, Wanat D. Murine typhus in South Texas children. Pediatr Infect Dis J. 2000;19:535–538. doi: 10.1097/00006454-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Gikas A, Doukakis S, Pediaditis J, Kastanakis S, Psaroulaki A, Tselentis Y. Murine typhus in Greece: epidemiological, clinical, and therapeutic data from 83 cases. Trans R Soc Trop Med Hyg. 2002;96:250–253. doi: 10.1016/s0035-9203(02)90090-8. [DOI] [PubMed] [Google Scholar]
- Green J, Singh J, Cheung M, Adler F, Ashouri N. A cluster of pediatric endemic typhus cases in Orange County, California. Pediatr Infect Dis J. 2011;30:163–165. doi: 10.1097/inf.0b013e3181f4cc25. [DOI] [PubMed] [Google Scholar]
- Henry KM, Jiang J, Rozmajzl PJ, Azad AF, Macaluso KR, Richards AL. Development of quantitative real-time PCR assays to detect Rickettsia typhi and Rickettsia felis, the causative agents of murine typhus and flea-borne spotted fever. Mol Cell Probes. 2007;21:17–23. doi: 10.1016/j.mcp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Horta MC, Sabatini GS, Moraes–Filho J, Ogrzewalska M, Canal RB, Pacheco RC, Martins TF, Matushima ER, Labruna MB. Experimental infection of the opossum Didelphis aurita by Rickettsia felis, Rickettsia bellii, and Rickettsia parkeri and evaluation of the transmission of the infection to ticks Amblyomma cajennense and Amblyomma dubitatum. Vector-Borne Zoonot. 2010;10:959–967. doi: 10.1089/vbz.2009.0149. [DOI] [PubMed] [Google Scholar]
- Jackson JJ. Internet Center for Wildlife Damage Management. 2011 ( http://icwdm.org/handbook/mammals/opossums.asp)
- Karpathy SE, Hayes EK, Williams AM, Hu R, Krueger L, Bennett S, Tilzer A, Velten RK, Kerr N, Moore W, Eremeeva ME. Detection of Rickettsia felis and Rickettsia typhi in an area of California endemic for murine typhus. Clin Microbiol Infect. 2009;15(Suppl 2):218–219. doi: 10.1111/j.1469-0691.2008.02140.x. [DOI] [PubMed] [Google Scholar]
- Khairallah M, Ben Yahia S, Toumi A, Jelliti B, Loussaief C, Ben Romdhane F, Messaoud R, Chakroun M. Ocular manifestations associated with murine typhus. Br J Ophthalmol: BJO. 2009 doi: 10.1136/bjo.2008.156059. 2008.156059. [DOI] [PubMed] [Google Scholar]
- Koshkin AA, Singh SK, Nielsen P, Rajwanshi VK, Kumar R, Meldgaard M, Olsen CE, Wengel J. LNA (Locked Nucleic Acids): synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine, and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. Tetrahedron. 1998;54:3607–3630. [Google Scholar]
- LaScola B, Meconi S, Fenollar F, Rolain JM, Roux V, Raoult D. Emended description of Rickettsia felis (Bouyer et al. 2001), a temperature-dependent cultured bacterium. Int J Syst Evol Microbiol. 2002;52:2035–2041. doi: 10.1099/00207713-52-6-2035. [DOI] [PubMed] [Google Scholar]
- Massung RF, Davis LE, Slater K, McKechnie DB, Puerzer M. Epidemic typhus meningitis in the Southwestern United States. Clin Infect Dis. 2001;32:979–982. doi: 10.1086/319351. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Comer JA, Sumner JW, Gingrich Baker C, Coughlin RT, Magnarelli LA, Olson JG, Childs JE. An indirect immunofluorescence assay using a cell culture-derived antigen for detection of antibodies to the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1510–1516. doi: 10.1128/jcm.35.6.1510-1516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden BH, Radulovic S, Higgins JA, Azad AF. Molecular identification of Rickettsia typhi and R. felis in co-infected Ctenocephalides felis (Siphonaptera: Pulicidae) J Med Entomol. 1998;35:410–414. doi: 10.1093/jmedent/35.4.410. [DOI] [PubMed] [Google Scholar]
- Pérez–Arellano J-L, Fenollar F, Angel–Moreno A, Bolaños M, Herández M, Santana E, Hemmersbach–Miller M, Martín A-M, Raoult D. Human Rickettsia felis infection, Canary Islands, Spain. Emerg Infect Dis. 2005;11:1961–1964. doi: 10.3201/eid1112.050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez–Osorio CE, Zavala–Velázquez JE, León JJA, Zavala–Castro JE. Rickettsia felis as emergent global threat for humans. Emerg Infect Dis. 2008;14:1019–1023. doi: 10.3201/eid1407.071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell K, Fergie J, Richman K, Rocha L. Murine typhus in children, south Texas. Emerg Infect Dis. 2007;13:926–927. doi: 10.3201/eid1306.061566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D, La Scola B, Enea M, Fournier PE, Fenollar F, Galvao MA, de Lamballerie X. A flea-associated Rickettsia pathogenic for humans. Emerg Infect Dis. 2001;7:73–81. doi: 10.3201/eid0701.010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht MA. The biology of domestic rats: telemetry yields insights for pest control. Proc Vertebr Pest Conf. 1988;13:98–100. [Google Scholar]
- Reif KE, Macaluso KR. Ecology of Rickettsia felis: a review. J Med Entomol. 2009;46:723–736. doi: 10.1603/033.046.0402. [DOI] [PubMed] [Google Scholar]
- Reif KE, Stout RW, Henry GC, Foil LD, Macaluso KR. Prevalence and infection load dynamics of Rickettsia felis in actively feeding cat fleas. PLoS One. 2008;3:e2805. doi: 10.1371/journal.pone.0002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A, Jiang J, Omulo S, Dare R, Abdirahman K, Ali A, Sharif S, Feikin D, Breiman R, Njenga MK. Human infection with Rickettsia felis, Kenya. Emerg Infect Dis. 2010;16:1081–1086. doi: 10.3201/eid1607.091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolain JM, Bitam I, Buffet S, Mari JL, Bourry O, Portelli Clerc C, Beaucournu JC, Parola P, Fournier PE, Davoust B, Raoult D. Presence or absence of plasmid in Rickettsia felis depending on the source of fleas. Clin Microbiol Infect. 2009;15(Suppl 2):296–297. doi: 10.1111/j.1469-0691.2008.02245.x. [DOI] [PubMed] [Google Scholar]
- Russell JC, McMorland AJC, MacKay JWB. Exploratory behaviour of colonizing rats in novel environments. Anim Behav. 2010;79:159–164. [Google Scholar]
- Schriefer ME, Sacci JB, Taylor JP, Higgins JA, Azad AF. Murine typhus: updated roles of multiple urban components and a second typhus like rickettsia. J Med Entomol. 1994a;31:681–685. doi: 10.1093/jmedent/31.5.681. [DOI] [PubMed] [Google Scholar]
- Schriefer ME, Sacci JB, Jr, Dumler JS, Bullen MG, Azad AF. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol. 1994b;32:949–954. doi: 10.1128/jcm.32.4.949-954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Thompson D, Nelson BC. Fleas on roof rats in six areas of Los Angeles County, California: their potential role in the transmission of plague and murine typhus to humans. Am J Trop Med Hyg. 1985;34:372–379. doi: 10.4269/ajtmh.1985.34.372. [DOI] [PubMed] [Google Scholar]
- Sorvillo FFJ, Gondo BB, Emmons RR, Ryan PP, Waterman SSH, Tilzer AA, Andersen EEM, Murray RRA, Barr RR. A suburban focus of endemic typhus in Los Angeles County: association with seropositive domestic cats and opossums. Am J Trop Med Hyg. 1993;48:269–273. doi: 10.4269/ajtmh.1993.48.269. [DOI] [PubMed] [Google Scholar]
- Traub R, Wisseman CL, Farhang–Azad A. The ecology of murine typhus-a critical review. Trop Dis Bull. 1978;75:237–317. [PubMed] [Google Scholar]
- Tzianabos T, Anderson BE, McDade JE. Detection of Rickettsia rickettsii DNA in clinical specimens by using polymerase chain reaction technology. J Clin Microbiol. 1989;27:2866–2868. doi: 10.1128/jcm.27.12.2866-2868.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedincamp JJ, Foil LLD. Infection and seroconversion of cats exposed to cat fleas (Ctenocephalides felis Bouché) infected with Rickettsia felis. J Vector Ecol. 2000;25:123–126. [PubMed] [Google Scholar]
- Wiggers RJ, Martin MC, Bouyer D. Rickettsia felis infection in an East Texas population. Tex Med. 2005;101:56–58. [PubMed] [Google Scholar]
- Znazen A, Rolain JM, Hammami N, Hammami A, Ben Jemaa M, Raoult D. Rickettsia felis infection, Tunisia. Emerg Infect Dis. 2006;12(1):38–140. doi: 10.3201/eid1201.050876. [DOI] [PMC free article] [PubMed] [Google Scholar]