Abstract
In 1973, investigators isolated a rickettsial organism, designated strain WB-8-2T, from an adult Amblyomma americanum tick collected at Land Between the Lakes National Recreation Area, TN, USA. This organism is now recognized as highly prevalent in A. americanum, as well as several other Amblyomma species found throughout the Western hemisphere. It has been suggested that cross-reactivity to WB-8-2T and similar strains contributes to the increasing number of spotted fever cases reported in the USA. In 1995, investigators provided preliminary evidence that this strain, as well as another strain from Missouri, represented a distinct taxonomic unit within the genus Rickettsia by evaluating sequences of the 16S rRNA and 17 kDa protein genes. However, the bacterium was never formally named, despite the use of the designation ‘Rickettsia amblyommii’ and later ‘Candidatus Rickettsia amblyommii’, for more than 20 years in the scientific literature. Herein, we provide additional molecular evidence to identify strain WB-8-2T as a representative strain of a unique rickettsial species and present a formal description for the species, with the proposed name modified to Rickettsia amblyommatis sp. nov. to conform to the International Code of Nomenclature of Prokaryotes. We also establish a pure culture of strain WB-8-2T and designate it as the type strain for the species. The type strain is WB-8-2T (=CRIRC RAM004T=CSURP2882T).
In 1973, investigators isolated a novel spotted fever group (SFG) Rickettsia strain from an adult Amblyomma americanum tick collected from vegetation in Land Between the Lakes National Recreation Area, TN, USA (Burgdorfer et al., 1974, 1981). This strain, designated WB-8-2T, was subsequently identified in 16 % of A. americanum from SC, USA, in 11 % of the A. americanum ticks from TN, USA and in 41 % of ticks from AR, USA. It was determined that WB-8-2T was a member of SFG of the genus Rickettsia and distinguishable from other Rickettsia species by using mouse serotyping and SDS-PAGE protein analyses (Burgdorfer et al., 1981). Two decades later, Stothard (1995) further characterized this and another strain, also isolated from A. americanum (MO 85-1084), by using newly developed molecular tools. Although the rrs sequence was similar to other spotted fever group rickettsiae (SFGR), analysis of the 17 kDa antigen gene indicated that WB-8-2T and MO 85-1084 were distinct from other named species of the genus and that these strains represented a novel Rickettsia species (Stothard, 1995). In her dissertation, Stothard described the bacterium as ‘Rickettsia amblyommii’, Unfortunately, this name was never validly published. Nonetheless, Stothard and Fuerst used this name in their pioneering phylogenetic studies of this organism, and the designation ‘Rickettsia amblyommii’ (Stothard & Fuerst, 1995) has since been used regularly in scientific literature. Herein, we confirm previous findings that this organism is a distinct taxonomic entity; however, we propose the novel species name amblyommatis to conform to the rules of the International Code of Nomenclature of Prokaryotes. As this bacterial species is named for the tick genus Amblyomma (Greek third declension), the species epithet must be in the Latin third declension; thus, we propose the name Rickettsia amblyommatis sp. nov. (Oren et al., 2016).
‘Rickettsia amblyommatis’ occurs in several tick species of the genus Amblyomma throughout the Western hemisphere, but it is most commonly detected in A. americanum, with rates of infection that often exceed 40 % of questing adult ticks (Table 1). Both transstadial and transovarial transmissions occur in A. americanum ticks, and filial infection rates range between 30 and 100 % depending on the level of rickettsiae in ovarial tissues (Burgdorfer et al., 1981). Other tick species in which ‘Rickettsia amblyommatis’ has been detected include Amblyomma longirostre in Brazil (Labruna et al., 2004), Amblyomma neumannii and Amblyomma hadanii in Argentina (Labruna et al., 2007; Mastropaolo et al., 2016), Amblyomma cajennense in Mexico, Costa Rica and Colombia (Faccini-Martínez et al., 2016; Hun et al., 2011; Medina et al., 2007), Amblyomma mixtum and Haemaphysalis juxtakochi in Panama (Castro et al., 2015), Amblyomma coelebs in French Guyana (Parola et al., 2007) and Dermacentor variabilis in the USA (Moncayo et al., 2010). In this context, ‘Rickettsia amblyommatis’ likely represents the most prevalent and widely distributed SFG rickettsial species in the Americas.
Table 1.
Frequency of ‘Rickettsia amblyommatis’ infection in A. americanum ticks in the USA
| State | No. of ticks tested (% positive for ‘Rickettsia amblyommatis’) | Reference |
|---|---|---|
| AR | 463 (41.9 %) | Burgdorfer et al. (1981) |
| AR | 653 (37 %) | Trout Fryxell et al. (2015) |
| FL | 151 (37.1 %) | Mixson et al. (2006) |
| FL | 1479 (57.1 %) | Sayler et al. (2014) |
| GA | 704 (44.7 %) | Mixson et al. (2006) |
| IA | 19 (57.9 %) | Mixson et al. (2006) |
| MD | 502 (64.1%) | Zhang et al. (2012) |
| MO | 74 (2.7 %) | Hermance et al. (2014) |
| NC | 391 (55.2 %) | Mixson et al. (2006) |
| NC | 3695 (56.4 %) | Smith et al. (2010) |
| NJ | 121 (6.6 %) | Mixson et al. (2006) |
| NY | 475 (41.7 %) | Mixson et al. (2006) |
| OH | 21 (38 %) | Kelly et al. (2005) |
| OH | 308 (30.2 %) | Fitak et al. (2014) |
| OK | 60 (10 %) | Mixson et al. (2006) |
| RI | 38 (47.4 %) | Mixson et al. (2006) |
| SC | 545 (11.7 %) | Burgdorfer et al. (1981) |
| SC | 79 (45.6 %) | Mixson et al. (2006) |
| TN | 96 (16.6 %) | Burgdorfer et al. (1981) |
| TN | 655 (39.5 %) | Moncayo et al. (2010) |
Strains of this organism can be cultivated in chicken fibro-blasts, primary embryonated chicken eggs, Vero cells, ISE6 tick cells and AAE2 tick cells (Burgdorfer et al., 1981; Carmichael, 2008; Stothard, 1995; Stothard & Fuerst, 1995). In their initial studies, Burgdorfer et al. found that WB-8-2T was non-pathogenic to guinea pigs (Cavia porcellus), but the strain produced mild and transient infections in the tunica vaginalis of male meadow voles (Microtus pennsylvanicus) when inoculated with densely infected cell culture suspensions. They concluded that the organism was not likely pathogenic to humans due to its inability to cause disease in guinea pigs and the lack of epidemiological evidence of fever, rash or headache in humans from areas with high prevalence of WB-8-2T and high population densities of A. americanum (Burgdorfer et al., 1981). Nonetheless, some serological evidence suggests that humans develop a robust immune response to this organism (Apperson et al., 2008; Medina et al., 2007) and it may be associated with disease manifestations in some patients (Delisle et al., 2016). Repeated exposure to infected ticks can induce very high serological titres in dogs, and there is recent molecular evidence that this organism can infect dogs (Barrett et al., 2014). Although Burgdorfer et al. (1981) did not observe complement-fixing antibody development in inoculated guinea pigs and voles, Blanton et al. (2014) demonstrated that guinea pigs developed very high titres by indirect immunofluorescence assay, despite no obvious clinical signs of infection. Recently, investigators demonstrated that an isolate from Costa Rica causes fever and pathological signs of disease in guinea pigs and that ‘Rickettsia amblyommatis’ DNA can be detected in guinea pig testicles up to 2 days after I.P inoculation (Rivas et al., 2015).
Strains of ‘Rickettsia amblyommatis’ may also play a role in the ecology and epidemiology of other pathogenic SFGR. A. americanum is a potential vector of at least two confirmed rickettsial pathogens, Rickettsia rickettsii and Rickettsia parkeri (Berrada et al., 2011; Cohen et al., 2009; Parker et al., 1933, 1943), yet in nature the prevalence of these bacteria in A. americanum is extremely low (Berrada et al., 2011; Cohen et al., 2009; Gaines et al., 2014; Wright et al., 2015) In this context, it is possible that the observed high rates of ‘Rickettsia amblyommatis’ infection could inhibit the transovarial transmission of other SFGR, including R. rickettsii and R. parkeri (Macaluso et al., 2002). Infection with ‘Rickettsia amblyommatis’ in A. americanum has been shown to inhibit, but not completely block, the acquisition of R. parkeri during experimental co-feeding with infected ticks (Wright et al., 2015). Infection with ‘Rickettsia amblyommatis’ may also provide some level of protection against subsequent infection with R. rickettsii in guinea pigs (Blanton et al., 2014; Rivas et al., 2015).
A americanum is a common human-biting tick and the high prevalence of ‘Rickettsia amblyommatis’ in this tick may complicate the diagnosis and surveillance of other SFG rickettsial infections in humans. Most cases of Rocky Mountain spotted fever (RMSF) are diagnosed using serological assays, and it has been suggested that the cross-reactivity of antibodies to strains like WB-8-2T against R. rickettsii antigens may help explain the large numbers of so-called ‘mild RMSF’ cases in areas where A. americanum is prevalent (Apperson et al., 2008; Delisle et al., 2016; Openshaw et al., 2010; Parola et al., 2013; Stromdahl et al., 2008). Multiple serological assessments of military personnel involved in training exercises in A. americanum-infested habitats demonstrated high rates of seroconversion to antigens of SFG rickettsial species among individuals who had developed asymptomatic or relatively mild illnesses not characteristic of RMSF (McCall et al., 2001; Sanchez et al., 1992; Yevich et al., 1995). Recently, a correlation between the presence of A. americanum in a geographical area and decreased hospitalization rates of RMSF (i.e., less severe or mild RMSF cases) in that same area was identified (Dahlgren et al., 2016). These data suggest the influence of a less pathogenic SFG rickettsial species on human disease reports.
The high prevalence of WB-8-2T and other strains in ticks poses a problem for investigators using molecular assays to assess rickettsial levels in tick populations. Cohen et al. (2009) found low levels of dually infected ticks; however, these individual ticks contained much higher bacterial loads of ‘Rickettsia amblyommatis’ than R. rickettsii, and common PCR and sequencing techniques only revealed the presence of the more abundant ‘Rickettsia amblyommatis’ (Cohen et al., 2009). It was only after the PCR amplicons were cloned and sequenced that R. rickettsii-specific sequences were identified. Berrada et al. (2011) also relied on cloning to reveal the presence of R. rickettsii-specific sequences in extracts of ‘Rickettsia amblyommatis’-infected A. americanum ticks from KS, USA (Berrada et al., 2011).
Molecular testing using the Universal Mycoplasma Detection Kit (ATCC) revealed the presence of a contaminating Mycoplasma species in the available stocks of strain WB-8-2T. To obtain a Mycoplasma-free stock of strain WB-8-2T for deposition and distribution, 50 μg ml−1 lincomycin was added to the medium and cell cultures were exposed to the antibiotic-supplemented medium for 6 weeks (Ogawa et al., 2013). Cultures were shown to be negative for infection with Mycoplasma species at 4, 5 and 6 weeks by using the PCR assay (data not shown), after which time the antibiotic was removed from the medium. Cultures were evaluated again after additional 4 weeks of antibiotic-free growth and showed no evidence of re-infection by Mycoplasma species.
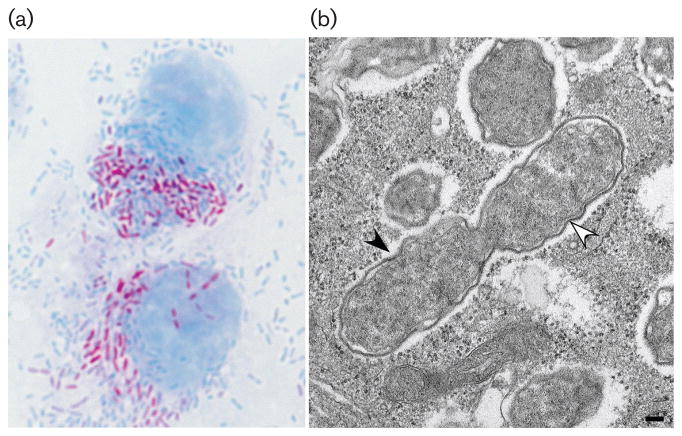
After isolation from tick haemolymph into primary chicken fibroblasts, strain WB-8-2T forms plaques of 1.7–2.0 mm in diameter. Passage into embryonated chicken eggs results in embryo death in 5–7 days (Burgdorfer et al., 1981). The organisms stain a pink colour with Giménez stain and appear round to oval in shape, often arranged in pairs (Fig. 1a). Immunofluorescence of the organisms in similar shape and arrangement may be seen by using mouse polyclonal conjugate made to R. rickettsii. All tick tissues may be infected, with ovary and Malpighian tubules being more heavily infected (Burgdorfer et al., 1981).
Fig. 1.
Light and electron microscopical appearance of ‘Rickettsia amblyommatis’ in Vero E6 cells. (a) Giménez stain of ‘Rickettsia amblyommatis’ in Vero E6 cells. (b) Transmission electron microscopy image of ‘Rickettsia amblyommatis’ infecting Vero E6 cells. White arrow, inner and outer membranes separated by a periplasmic space; black arrow, outer slime layer.
In this study, five geographically unique isolates (Table 2) were propagated in Vero E6 cells grown in Minimal Essential Media (Gibco) supplemented with 0.1 mM Minimal Essential Media non-essential amino acids (Gibco), 10 mM HEPES buffer (Gibco), 2 mM L-glutamine (Gibco), 10 mM sodium pyruvate (Gibco) and 5 % (w/v) heat-inactivated foetal bovine serum (Atlas Biologicals) at 32 °C with 5 % (v/v) CO2. When observed under bright-field microscopy, WB-8-2T appeared as small bacilli found free-living in the host cell cytoplasm (Fig. 1a). To prepare Rickettsia-infected cells for electron microscopy, an infected monolayer was washed in 0.1 M phosphate buffer, pH 7.3, and fixed in buffered 2.5 % (w/v) glutaraldehyde for 5 min at 4 °C. The monolayer was gently removed from the flask using a cell scrapper, and the cells were centrifuged at 1000 g for 5 min at 4 °C. The remaining glutaraldehyde was removed, and the cell pellet was covered with 0.1 M phosphate buffer and stored at 4 °C. The cells were post-fixed in 1 % (w/v) buffered osmium tetroxide, stained in 4 % (w/v) uranyl acetate, dehydrated through a graded series of alcohols and acetone and embedded in a mixture of Epon substitute and Araldite. Thin sections were stained with 4 % (w/v) uranyl acetate and Reynold’s lead citrate.
Table 2.
Isolates characterized as Rickettsia amblyommatis sp. nov.
| Strain | Source | Geographic origin | CRIRC isolate number | Reference |
|---|---|---|---|---|
| WB-8-2T | A. americanum | Land Between the Lakes National Recreation Area, TN, USA | RAM004T | Burgdorfer et al. (1974) |
| GAT-3OV | A. americanum | Stockbridge, GA, USA | RAM007 | Unpublished |
| Darkwater | A. americanum | Sopchoppy, FL, USA | RAM003 | Baldridge et al. (2010) |
| Line Creek | A. americanum | Peachtree City, GA, USA | RAM005 | This study |
| Ac/Pa | A. cajennense | Panama | RAM002 | Baldridge et al. (2010) |
Electron microscopy of WB-8-2T revealed a Gram-negative, bacillary morphology consistent with other members of the genus Rickettsia. A comparison of 45 individual bacterial cells revealed an average length of 0.832 μm (median, 0.798 μm) and an average width of 0.427 μm (median, 0.416 μm). These dimensions are compatible with the observations of Burgdorfer et al. (1981) when examining infected tick tissues (Burgdorfer et al., 1981). Strain WB-8-2T has a cell wall with inner and outer membranes separated by a peri-plasmic space (white arrow, Fig. 1b). The cell wall is surrounded by a translucent area consistent with an outer slime layer (black arrow, Fig. 1b) (Silverman, 1991) which is in agreement with the original description of WB-8-2T (Burgdorfer et al., 1981).
Traditionally, DNA–DNA hybridization techniques have been used to define bacterial species, with a 70 % relatedness cutoff generally used to differentiate species (Wayne et al., 1987). However, DNA sequences are highly conserved between different rickettsial species, and if the traditional 70 % relatedness criteria were applied to the genus, many defined species would be consolidated into a single species (Myers & Wisseman, 1981). In 2003, investigators proposed a molecular scheme for the classification of rickettsial species to maintain the established species structure of the genus (Fournier et al., 2003). This scheme utilizes a multi-locus sequence typing (MLST) approach based on the sequences of five rickettsial genes; rrs, gltA, sca0 (ompA), sca5 (ompB) and sca4 (Gene D). PCR amplification and DNA sequencing were performed on WB-8-2T and three other strains (Table 2). All reactions were performed as described previously (Fournier et al., 2003) with the exception of the gltA PCR, which used an annealing temperature of 55 °C. PCR amplicons were sequenced using a BigDye Terminator V3.1 kit and an ABI 3130xl genetic analyser (Applied Biosystems, Carlsbad, CA, USA). Sequences were assembled using Sequencher 5.1 (Gene Codes, Ann Arbor, MI, USA), and MEGA 6.05 (Tamura et al., 2013) was used to create alignments so that the individual sequences from the four sequenced isolates could be compared along with that of the available sequenced full genome of strain GAT-30V (GenBank accession number NC_017028.1).
According to the proposed MLST classification scheme, for an isolate to be confirmed as a new rickettsial species, it should have no more than one locus with an identity equal to or greater than 99.8, 99.9, 98.8, 99.2 and 99.3 % identities to an established rickettsial species for rrs, gltA, sca0, sca5 and sca4, respectively (Fournier et al., 2003). When the sequences for WB-8-2T are compared to the sequences of other rickettsial type strains, Rickettsia raoultii strain KhabarovskT often is the most similar, with 99.3, 99.1, 97.4 and 97.3 % identities for rrs, gltA, sca0 and sca5, respectively. The WB-8-2T gene sequence for sca4 is most similar to Rickettsia japonica YHT, with 97.6 % identity. All five of the WB-8-2T loci sequence comparisons fall below the cutoffs suggested by the MLST classification scheme, confirming that WB-8-2T represents a novel SFG rickettsial species.
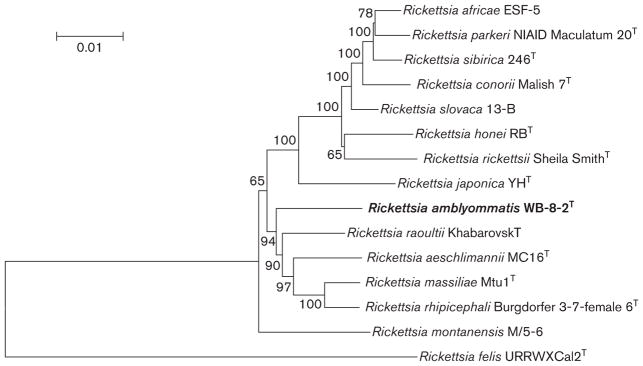
To better assess the genetic relationship of strain WB-8-2T compared to recognized SFG Rickettsia species, a phylogenetic analysis was performed using the concatenated sequences of the five loci. Homologous sequences were obtained from National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/) for validated species of SFGR and for Rickettsia felis, a member of the transitional group rickettsiae (Table 3) (Gillespie et al., 2008), which was included to be used as an outgroup. The nucleotide sequences were concatenated and aligned using MEGA 6.05 (Tamura et al., 2013) in the following order: rrs, gltA, sca0, sca5 and sca4; gaps were removed from the alignment using the simple indel coding method (Ogden & Rosenberg, 2007). The MEGA 6.05 software package was also used to infer the evolutionary history. The neighbour-joining phylogenetic analysis agrees with the MLST analysis and clearly shows that strain WB-8-2T is a distinct member of the genus Rickettsia (Fig. 2). Analyses utilizing the maximum-parsimony and maximum-likelihood methods are in agreement with the neighbour-joining analysis (Figs S1 and S2, available in the online Supplementary Material).
Table 3.
GenBank accession numbers of rickettsial gene sequences used in the phylogenetic analysis
| Strain species and strain | rrs | gltA | sca0 | sca5 | sca4 |
|---|---|---|---|---|---|
| R. aeschlimannii MC16T | U74757 | U59722 | U43800 | AF123705 | AF163006 |
| R. africae ESF-5 | l36098 | U59733 | U43790 | AF123706 | AF151724 |
| R. conorii Malish 7T | AF541999 | U59730 | U43806 | AF123721 | AF163008 |
| R. felis URRWXCal2T | L28944 | AF210692 | AF210694 | AF210695 | AF196973 |
| R. honei RBT | U17645 | AF018074 | AF018075 | AF123711 | AF163004 |
| R. japonica YHT | NR_074459 | AP011533 | AP011533 | AP011533 | AP011533 |
| R. massiliae Mtu1T | L36214 | U59719 | U43799 | AF123714 | AF163003 |
| R. montanensis M/5–6 | L36215 | U74756 | U43801 | AF123716 | AF163002 |
| R. parkeri NIAID Maculatum 20T | L36673 | U59732 | U43802 | AF123717 | AF155059 |
| R. rhipicephali Burgdorfer 3–7-female 6T | L36216 | U59721 | U43803 | AF123719 | AF155053 |
| R. rickettsii Sheila SmithT | NR_102941 | CP000848 | CP000848 | CP000848 | CP000848 |
| R. sibirica 246T | L36218 | U59734 | U43807 | AF123722 | AF155057 |
| R. slovaca 13-B | L36224 | U59725 | U43808 | AF123723 | AF155054 |
| R. raoultii KhabarovskT | DQ365810 | DQ365804 | DQ365801 | DQ365798 | DQ365808 |
Fig. 2.
Phylogenetic relationship of ‘Rickettsia amblyommatis’ to other SFGR using five concatenated sequences. The evolutionary history was inferred using the neighbour-joining method while the evolutionary distances were computed using the maximum-composite-likelihood method. A total of 10 640 positions were included in the analysis. The scale bar is in units of the number of base substitutions per site. Bootstrap values for 1000 replicates are displayed next to the branches.
Sequencing of the five loci was completed for strains and compared to the genome sequence for strain GAT-3OV in GenBank (accession number NC_017028.1) in order to identify genetic variation among these isolates. The sequences for all five isolates were 100 % identical to each other in the regions of rrs and sca0 that were compared. Four of the five sequences were 100 % identical to gltA, with the exception of strain Ac/Pa, which contains one single-nucleotide polymorphism in the 1048 bp examined. When the sca5 sequences were compared, again strain Ac/Pa was the only strain to exhibit variation, with four single-nucleotide polymorphisms identified across the 4845 bp examined. Strain Ac/Pa was also the only strain to have sequence variation in the 2941 bp examined in sca4, with Ac/Pa containing six single-nucleotide polymorphisms compared to the other four strains. Thus, very little genetic variation was observed in the loci examined, with the five isolates maintaining 99.9 % or higher sequence identity in each locus.
The results of phylogenetic, phenotypic, and biologic features of strain WB-8-2T indicate that this is a novel species. Additional strains belong to this species, and this taxon can be distinguished from previously described species of the genus Rickettsia. The original species name, ‘Rickettsia amblyommii’, was proposed in a dissertation by Diane Stothard (Stothard, 1995) and has been accepted by many authors since then. However, this name does not conform to the International Code of Nomenclature of Prokaryotes; thus, we propose strain WB-8-2T as the type strain for the species Rickettsia amblyommatis sp. nov.
Description of Rickettsia amblyommatis sp. nov
Rickettsia amblyommatis (am.bly.om’ma.tis. N.L. gen. neut. n. amblyommatis of Amblyomma, the genus of hard ticks from which the type strain was isolated).
In Vero E6 cells, these Gram-negative bacilli have an average length of 0.832 μm and an average width of 0.427 μm. In ticks, the round- or oval-shaped organisms are often in pairs. This intracellular organism infects and replicates in eukaryotic cells where it is found free in the cytoplasm. In nature, a variety of tick species from North, Central and South America are infected with ‘Rickettsia amblyommatis’ including A. americanum Linneaus (1758), A. longirostre Koch (1844), A. neumannii Ribga (1902), A. cajennense Fabricius (1787) and A. coelebs Neumann (1899). Other tick genera are infected occasionally including the species Dermacentor and Haemaphysalis. The infection occurs in all tick tissues, but ovaries and Malpighian tubules are more intensely infected. This organism is cultivated in several established cell lines including Vero cells. Antigens of ‘Rickettsia amblyommatis’ react with polyclonal antisera raised to other SFG Rickettsia species; however, microimmunofluorescence assay with specific mouse antisera shows no cross-reaction with other SFG rickettsial serotypes. Although the pathogenic potential of this organism remains unclear for humans, experimentally infected guinea pigs and dogs mount a robust serological response by IFA assay when infected with ‘Rickettsia amblyommatis’. The WB-8-2T DNA sequences analysed in regions of the rrs, sca0, sca5, gltA and sca4 genes are 100 % identical to those publicly available for strain GAT-3OV. The complete genome sequence of ‘Rickettsia amblyommatis’ strain GAT-3OV is available in GenBank (accession number NC_017028) and consists of one circular chromosome (1 407 796 bp; 32.4 mol% G+C) and three circular plasmids containing 31 974, 18 263 and 22 851 bp, respectively.
The type strain, WB-8-2T(=CRIRC RAM004T=CSUR-P2882T), was isolated from an adult A. americanum tick collected from vegetation at Land Between the Lakes National Recreation Area, Stewart County, TN, USA, in 1973.
Supplementary Material
Acknowledgments
We wish to thank Dr Allen Richards from the Navy Medical Research Center for the donation of strain WB-8-2T and Drs Ulrike Munderloh and Marcelo Labruna from the University of Minnesota and the Universidade de São Paulo, respectively, for the donation of strain Ac/Pa. The authors also acknowledge the seminal studies of Diane Stothard, Jennifer Carmichael and Paul Fuerst in the characterization of ‘Rickettsia amblyommatis’. Finally, the authors would like to thank Dr Aharon Oren of The Hebrew University of Jerusalem and Dr Bernard Schink of the University of Konstanz for helpful discussions and advice regarding bacterial nomenclature. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations
- MLST
multi-locus sequence typing
- RMSF
Rocky Mountain spotted fever
- SFG
spotted fever group
- SFGR
spotted fever group rickettsiae
Footnotes
The GenBank accession numbers for Rickettsia amblyommatis Ac/Pa are KX151486 (gltA), KX151487 (sca5) and KX151488 (sca4).
Two supplementary figures are available with the online Supplementary Material.
References
- Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. Tick-borne diseases in North Carolina: is ‘Rickettsia amblyommii’ a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- Baldridge GD, Burkhardt NY, Labruna MB, Pacheco RC, Paddock CD, Williamson PC, Billingsley PM, Felsheim RF, Kurtti TJ, Munderloh UG. Wide dispersal and possible multiple origins of low-copy-number plasmids in Rickettsia species associated with blood-feeding arthropods. Appl Environ Microbiol. 2010;76:1718–1731. doi: 10.1128/AEM.02988-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A, Little SE, Shaw E. ‘Rickettsia amblyommii’ and R. montanensis infection in dogs following natural exposure to ticks. Vector Borne Zoonotic Dis. 2014;14:20–25. doi: 10.1089/vbz.2013.1325. [DOI] [PubMed] [Google Scholar]
- Berrada ZL, Goethert HK, Cunningham J, Telford SR. Rickettsia rickettsii (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from Kansas. J Med Entomol. 2011;48:461–467. doi: 10.1603/me10130. [DOI] [PubMed] [Google Scholar]
- Blanton LS, Mendell NL, Walker DH, Bouyer DH. ‘Rickettsia amblyommii’ induces cross protection against lethal Rocky Mountain spotted fever in a guinea pig model. Vector Borne Zoonotic Dis. 2014;14:557–562. doi: 10.1089/vbz.2014.1575. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Cooney JC, Thomas LA. Zoonotic potential (Rocky Mountain spotted fever and tularemia) in the Tennessee Valley region. Am J Trop Med Hyg. 1974;23:109–117. doi: 10.4269/ajtmh.1974.23.109. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Hayes SF, Thomas LA. A new spotted fever group rickettsia from the lone star tick, Amblyomma americanum. In: Burgdorfer W, Anacker RL, editors. Rickettsiae and Rickettsial Diseases. New York: Academic Press, Inc; 1981. pp. 595–602. [Google Scholar]
- Carmichael JR. Ph.D. dissertation. The Ohio State University; 2008. The host–pathogen relationship in Rickettsia: epidemiological analysis of RMSF in Ohio and a comparative molecular analysis of four vir genes. [Google Scholar]
- Castro AM, García GG, Dzul-Rosado K, Aguilar A, Castillo J, Gabster A, Trejos D, Zavala-Castro J, Bermúdez SE. Questing Amblyomma mixtum and Haemaphysalis juxtakochi (Acari: Ixodidae) infected with Candidatus ‘Rickettsia amblyommii’ from the natural environment in Panama Canal Basin, Panama. Trop Med Health. 2015;43:217–222. doi: 10.2149/tmh.2015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SB, Yabsley MJ, Garrison LE, Freye JD, Dunlap BG, Dunn JR, Mead DG, Jones TF, Moncayo AC. Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, USA. Emerg Infect Dis. 2009;15:1471–1473. doi: 10.3201/eid1509.090330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren FS, Paddock CD, Springer YP, Eisen RJ, Behravesh CB. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am J Trop Med Hyg. 2016;94:35–42. doi: 10.4269/ajtmh.15-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle J, Mendell NL, Stull-Lane A, Bloch KC, Bouyer DH, Moncayo AC. Human infections by multiple spotted fever group Rickettsiae in Tennessee. Am J Trop Med Hyg. 2016;94:1212–1217. doi: 10.4269/ajtmh.15-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccini-Martinez Á, Ramírez-Hernández A, Forero-Beccerra E, Cortés-Vecino JA, Escandón P, Rodas JD, Palomar AM, Portillo A, Oteo JA, Hidalgo M. Molecular evidence of different Rickettsia species in Villeta, Colombia. Vector Borne Zoonotic Dis. 2016;16:85–87. doi: 10.1089/vbz.2015.1841. [DOI] [PubMed] [Google Scholar]
- Fitak RR, Kelly DJ, Daniels MK, Jiang J, Richards AL, Fuerst PA. The prevalence of rickettsial and ehrlichial organisms in Amblyomma americanum ticks collected from Ohio and surrounding areas between 2000 and 2010. Ticks Tick Borne Dis. 2014;5:797–800. doi: 10.1016/j.ttbdis.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines DN, Operario DJ, Stroup S, Stromdahl E, Wright C, Gaff H, Broyhill J, Smith J, Norris DE, et al. Ehrlichia and spotted fever group rickettsiae surveillance in Amblyomma americanum in Virginia through use of a novel six-plex real-time PCR assay. Vector Borne Zoonotic Dis. 2014;14:307–316. doi: 10.1089/vbz.2013.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JJ, Williams K, Shukla M, Snyder EE, Nordberg EK, Ceraul SM, Dharmanolla C, Rainey D, Soneja J, et al. Rickettsia phylogenomics: unwinding the intricacies of obligate intracellular life. PLoS One. 2008;3:e2018. doi: 10.1371/journal.pone.0002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermance M, Dos Santos RI, Heinze D, Hausser N, Bouyer DH, Thangamani S. Detection of Rickettsia amblyommii in ticks collected from Missouri, USA. Emerg Microbes Infect. 2014;3:e34. doi: 10.1038/emi.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hun L, Troyo A, Taylor L, Barbieri AM, Labruna MB. First report of the isolation and molecular characterization of Rickettsia amblyommii and Rickettsia felis in Central America. Vector Borne Zoonotic Dis. 2011;11:1395–1397. doi: 10.1089/vbz.2011.0641. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Carmichael JR, Booton GC, Poetter KF, Fuerst PA. Novel spotted fever group Rickettsiae (SFGR) infecting Amblyomma americanum ticks in Ohio, USA. Ann N Y Acad Sci. 2005;1063:352–355. doi: 10.1196/annals.1355.058. [DOI] [PubMed] [Google Scholar]
- Labruna MB, Whitworth T, Bouyer DH, McBride JW, Camargo LMA, Camargo EP, Popov V, Walker DH. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the state of Rondônia, Western Amazon, Brazil. J Med Entomol. 2004;41:1073–1081. doi: 10.1603/0022-2585-41.6.1073. [DOI] [PubMed] [Google Scholar]
- Labruna MB, Pacheco RC, Nava S, Brandão PE, Richtzenhain LJ, Guglielmone AA. Infection by Rickettsia bellii and Candidatus ‘Rickettsia amblyommii’ in Amblyomma neumannii ticks from Argentina. Microb Ecol. 2007;54:126–133. doi: 10.1007/s00248-006-9180-3. [DOI] [PubMed] [Google Scholar]
- Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J Med Entomol. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- Mastropaolo M, Tarragona EL, Silaghi C, Pfister K, Thiel C, Nava S. High prevalence of ‘Candidatus Rickettsia amblyommii’ in Amblyomma ticks from a spotted fever endemic region in north Argentina. Comp Immunol Microbiol Infect Dis. 2016;46:73–76. doi: 10.1016/j.cimid.2016.05.001. [DOI] [PubMed] [Google Scholar]
- McCall CL, Curns AT, Rotz LD, Singleton JA, Treadwell TA, Comer JA, Nicholson WL, Olson JG, Childs JE. Fort Chaffee revisited: the epidemiology of tick-borne rickettsial and ehrlichial diseases at a natural focus. Vector Borne Zoonotic Dis. 2001;1:119–127. doi: 10.1089/153036601316977723. [DOI] [PubMed] [Google Scholar]
- Medina A, Guevara E, Alcantara V, Garza C, Hunt F, Davis J, Gonzalez R, Bouyer D, Walker D. Isolation of Rickettsia amblyommii and seroprevalence of rickettsia in the state of Veracruz, Mexico. 21st Meeting of the American Society for Rickettsiology; Colorado Springs, CO. 2007. Abstract #132. [Google Scholar]
- Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, Dasch GA. Prevalence of Ehrlichia, Borrelia, and rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. Vector Borne Zoonotic Dis. 2006;43:1261–1268. doi: 10.1603/0022-2585(2006)43[1261:poebar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Moncayo AC, Cohen SB, Fritzen CM, Huang E, Yabsley MJ, Freye JD, Dunlap BG, Huang J, Mead DG, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653–657. doi: 10.4269/ajtmh.2010.09-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers WF, Wisseman CL. The taxonomic relationship of Rickettsia canada to the typhus and spotted fever groups of the genus Rickettsia. In: Burgdorfer W, Anacker RL, editors. Rickettsiae and Rickettsial Diseases. New York, NY: Academic Press, Inc; 1981. pp. 313–325. [Google Scholar]
- Ogawa M, Uchiyama T, Satoh M, Ando S. Decontamination of mycoplasma-contaminated Orientia tsutsugamushi strains by repeating passages through cell cultures with antibiotics. BMC Microbiol. 2013;13:32. doi: 10.1186/1471-2180-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden TH, Rosenberg MS. How should gaps be treated in parsimony? A comparison of approaches using simulation. Mol Phylogenet Evol. 2007;42:817–826. doi: 10.1016/j.ympev.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Openshaw JJ, Swerdlow DL, Krebs JW, Holman RC, Mandel E, Harvey A, Haberling D, Massung RF, McQuiston JH. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg. 2010;83:174–182. doi: 10.4269/ajtmh.2010.09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A, Vandamme P, Schink B. Notes on the use of Greek word roots in genus and species names of prokaryotes. Int J Syst Evol Microbiol. 2016;66:2129–2140. doi: 10.1099/ijsem.0.001063. [DOI] [PubMed] [Google Scholar]
- Parker RR, Philip CB, Jellison WL. Potentialities of tick transmission in relation to geographical occurrence in the United States. Am J Trop Med Hyg. 1933;13:341–379. [Google Scholar]
- Parker RR, Kohls GM, Steinhaus EA. Rocky Mountain spotted fever: spontaneous infection in the tick Amblyomma americanum. Public Health Reports. 1943;58:721–729. [Google Scholar]
- Parola P, Matsumoto K, Socolovschi C, Parzy D, Raoult D. A tick-borne rickettsia of the spotted-fever group, similar to Rickettsia amblyommii, in French Guyana. Ann Trop Med Parasitol. 2007;101:185–188. doi: 10.1179/136485907154557. [DOI] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, Abdad MY, Stenos J, Bitam I, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas JJ, Moreira-Soto A, Alvarado G, Taylor L, Calderón-Arguedas O, Hun L, Corrales-Aguilar E, Morales JA, Troyo A. Pathogenic potential of a Costa Rican strain of ‘Candidatus Rickettsia amblyommii’ in guinea pigs (Cavia porcellus) and protective immunity against Rickettsia rickettsii. Ticks Tick Borne Dis. 2015;6:805–811. doi: 10.1016/j.ttbdis.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Sanchez JL, Candler WH, Fishbein DB, Greene CR, Coté TR, Kelly DJ, Driggers DP, Johnson BJ. A cluster of tick-borne infections: association with military training and asymptomatic infections due to Rickettsia rickettsii. Trans R Soc Trop Med Hyg. 1992;86:321–325. doi: 10.1016/0035-9203(92)90330-f. [DOI] [PubMed] [Google Scholar]
- Sayler KA, Wamsley HL, Pate M, Barbet AF, Alleman AR. Cultivation of Rickettsia amblyommii in tick cells, prevalence in Florida lone star ticks (Amblyomma americanum) Parasit Vectors. 2014;7:270. doi: 10.1186/1756-3305-7-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DJ. Some contributions of electron microscopy to the study of the rickettsiae. Eur J Epidemiol. 1991;7:200–206. doi: 10.1007/BF00145667. [DOI] [PubMed] [Google Scholar]
- Smith MP, Ponnusamy L, Jiang J, Ayyash LA, Richards AL, Apperson CS. Bacterial pathogens in ixodid ticks from a Piedmont County in North Carolina: prevalence of rickettsial organisms. Vector Borne Zoonotic Dis. 2010;10:939–952. doi: 10.1089/vbz.2009.0178. [DOI] [PubMed] [Google Scholar]
- Stothard DR. PhD Dissertation. The Ohio State University; 1995. The evolutionary history of the genus Rickettsia as inferred from 16S and 23S ribosomal RNA genes and the 17 kilodalton cell surface antigen gene. [Google Scholar]
- Stothard DR, Fuerst PA. Evolutionary analysis of the spotted fever and thyphus [sic] groups of Rickettsia using 16S rRNA gene sequences. Syst Appl Microbiol. 1995;18:52–61. [Google Scholar]
- Stromdahl EY, Vince MA, Billingsley PM, Dobbs NA, Williamson PC. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15–24. doi: 10.1089/vbz.2007.0138. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout Fryxell RT, Steelman CD, Szalanski AL, Billingsley PM, Williamson PC. Molecular detection of Rickettsia species within ticks (Acari: Ixodidae) collected from Arkansas, United States. J Med Entomol. 2015;52:500–508. doi: 10.1093/jme/tjv027. [DOI] [PubMed] [Google Scholar]
- Wayne LG, Brenner DJ, Colwell RR, Grimont PAD, Kandler O, Krichevsky MI, Moore LH, Moore WEC, Murray RGE, et al. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Evol Microbiol. 1987;37:463–464. [Google Scholar]
- Wright CL, Sonenshine DE, Gaff HD, Hynes WL. Rickettsia parkeri transmission to Amblyomma americanum by cofeeding with Amblyomma maculatum (Acari: Ixodidae) and potential for spillover. J Med Entomol. 2015;52:1090–1095. doi: 10.1093/jme/tjv086. [DOI] [PubMed] [Google Scholar]
- Yevich SJ, Sánchez JL, DeFraites RF, Rives CC, Dawson JE, Uhaa IJ, Johnson BJ, Fishbein DB. Seroepidemiology of infections due to spotted fever group Rickettsiae and Ehrlichia species in military personnel exposed in areas of the United States where such infections are endemic. J Infect Dis. 1995;171:1266–1273. doi: 10.1093/infdis/171.5.1266. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ren X, Norris DE, Rasgon JL. Distribution and infection frequency of ‘Candidatus Rickettsia amblyommii’ in Maryland populations of the lone star tick (Amblyomma americanum) and culture in an Anopheles gambiae mosquito cell line. Ticks Tick Borne Dis. 2012;3:38–42. doi: 10.1016/j.ttbdis.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.