Summary
Chronic suppurative otitis media (CSOM) refers to the middle ear inflammation which is clinically characterized by the discharging ear, hearing deficit, fever and otalgia. Although a clinical diagnosis, imaging is imperative to rule out associated complications which apart from causing hearing deficit, may prove fatal at times.
Both high resolution computed tomography (HRCT) and MRI are helpful in evaluating middle ear pathologies, usage being indication specific. Due to its excellent spatial resolution, HRCT is invaluable in assessment of chronically discharging ears, especially to look for bone erosion and the integrity of the ossicles. Due to its better spatial resolution, HRCT is preferred in suspected intra temporal complications whereas MRI is more useful in evaluating intracranial extension.
Keywords: Cholesteatoma, Middle Ear; Otitis Media; Temporal Bone
Background
Aural infections are one of the major causes of morbidity in children that, if neglected, may lead to disabling complications or even death. Otitis media refers to the inflammation of the middle ear which may be further classified into acute or chronic suppurative otitis media (CSOM) on the basis of duration of symptoms. Chronic suppurative otitis media (CSOM) is the leading cause of chronic aural discharge. As per WHO estimates, it affects 65 to 330 million individuals globally. Its prevalence varies from nearly 1% in the developed to 30–45% in the underdeveloped countries.
HRCT and MRI are the two most important imaging modalities in the evaluation of chronically discharging ear. HRCT has a better spatial resolution needed for the evaluation of osseous structures, whereas MRI is ideal to evaluate intracranial complications. In this article, HRCT findings in CSOM and its complications are detailed along with a diagnostic approach to a chronically discharging ear, which can benefit both clinicians and radiologists [1–4].
Imaging Modalities for the Evaluation of CSOM [5–8]
Plain radiography
Schuller’s, Stenver’s, Towne’s and submentovertical projections are the most widely used radiographic projections in the imaging of the temporal bone in suspected CSOM. However, with the available imaging modalities (vide infra), plain radiography is rarely used in the evaluation of middle ear pathologies. Complex skull base anatomy along with poor spatial resolution make it easy to miss smaller lesion on the radiographs. Furthermore, the extent of involvement and bony erosion are not well visualized on radiographs.
HRCT (high resolution computed tomography)
With its excellent spatial resolution, HRCT is the imaging modality of choice for the evaluation of middle ear pathologies. HRCT is a highly sensitive screening tool, which if normal, virtually excludes any middle ear pathology [5,8]. It is the best modality to look for the integrity of the ossicles and the bony confines of the middle ear. As mentioned above, complications of CSOM mainly arise from bone erosion. HRCT is indicated in unsafe CSOM for its localization, extent delineation and to rule out associated complications. Although the status of the tympanic membrane (TM) may be evaluated on HRCT, otoscopy is more reliable for this purpose [5,8].
Limitations of HRCT
A variety of conditions such as chronic infection/inflammatory disease, glomus tympanicum, vascular anomalies, neoplasm or hemotympanum can cause middle ear opacification, of which CSOM is the leading cause. However, due to poor contrast resolution, HRCT lacks specificity in differentiating between various causes. Nonetheless, the presence of certain ancillary findings, e.g. retracted TM, rules out any mass lesion [5–7].
MRI
Due to its superior contrast resolution, MRI serves as a problem-solving tool in indeterminate, complicated CSOM cases to look for the involvement of facial nerve canal, inner ear structures and intracranial complications. It is of utmost utility in distinguishing residual/recurrent cholesteatoma from granulation or scar tissue in the post-operative ear [8–10].
This article illustrates HRCT findings in CSOM and its various complications. Due to the high incidence of complications, imaging is prudent in unsafe CSOM cases. A familiarity with the radiological appearances in certain clinical situations helps in early institution of treatment, thereby significantly reducing morbidity and mortality.
Acute otitis media
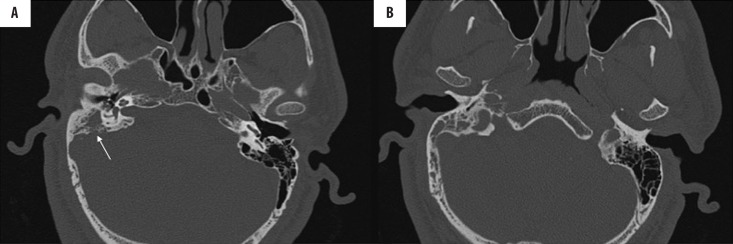
Acute otitis media (AOM) manifests with discharging ear, otalgia and fever of short duration. It results from the reactive accumulation of fluid in the middle ear cavity behind an intact tympanic membrane (TM). AOM is a clinical diagnosis and usually requires no imaging. HRCT, if performed, reveals opacification of the middle ear cavity with occasional presence of fluid levels. Bone and ossicular erosion are characteristically absent. At times, aditus ad antrum may be blocked, leading to concomitant mastoiditis, which is referred to as otomastoiditis (Figure 1). AOM and acute otomastoiditis usually resolve promptly with conservative management [2].
Figure 1.
(A, B) Acute otomastoiditis. Patient with leukaemia with bilateral aural discharge for 4 days; HRCT axial scans show soft tissue attenuation in both tympanic cavities and mastoids. Note the absence of ossicular erosion with preserved septations in mastoid air cells (arrow).
Chronic suppurative otitis media (CSOM)
CSOM is a sequela of unresolved acute otitis media. The chronological distinction between acute and chronic otitis media is variable; however, as per WHO definition, CSOM is defined by the persistence of otorrhea beyond 2 weeks [3]. It is the most common granulomatous condition of the middle ear and mastoid air cells. It is histopathologically characterized by advanced, irreversible mucosal changes [4]. Its permanent nature, resistance to conservative management and relentlessly dragging evolution differentiates it from simple AOM. If unhalted, it may lead to sclerotic-adhesive sequelae or life-threatening complications [4,5].
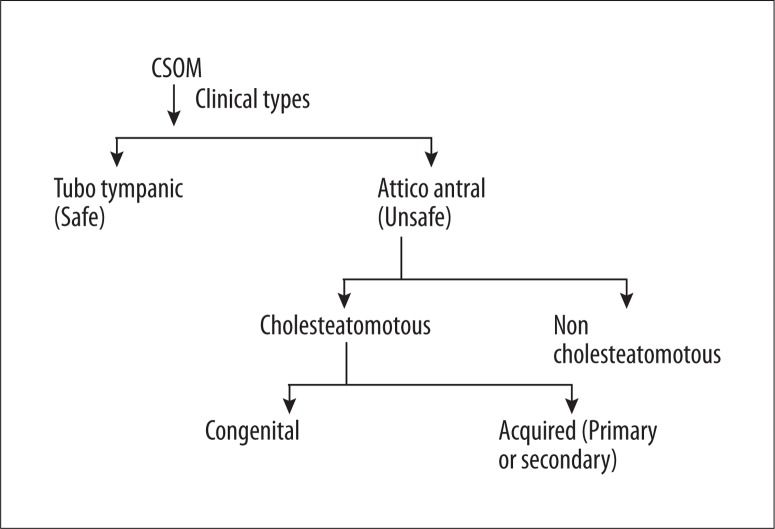
Clinically, CSOM is categorized into the following 2 types (Figure 2):
Figure 2.
Types of CSOM.
Tubotympanic (safe type) – there is central perforation of the tympanic membrane (TM), and the inflammatory process is restricted to the middle ear mucosa. HRCT reveals opacification of the middle ear cavity without erosion of the bone or ear ossicles (Figure 3).
Attico-antral (unsafe type) – marginal or attic type of TM perforation and involvement of the periosteum leads to bone erosions [6]. From the imaging perspective, attico-antral CSOM can be further classified into cholesteatomatous or non-cholesteatomatous types. In both these subtypes, granulation tissue is present; however, cholesteatoma characteristically leads to bone and ossicular erosion (in ~90% of the cases) [6,7].
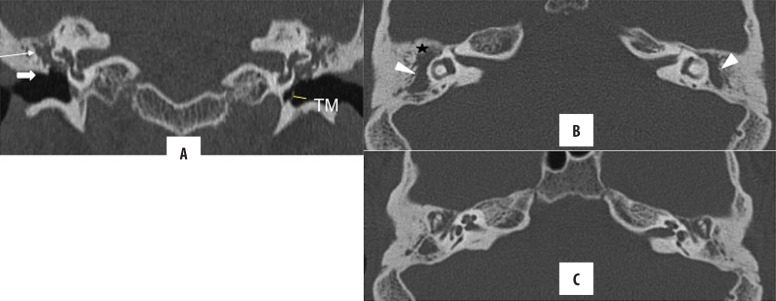
Figure 3.
Bilateral unsafe CSOM: atticoantral disease. Coronal and axial HRCT images. Nondependent soft tissue opacification in Prussak’s space (thin arrow), anterior epitympanic recess (star), aditus ad antrum (arrowhead) and mesotympanum, surrounding the ear ossicles. Scutum (block arrow in A) and ear ossicles are not eroded. TM is ruptured on the right, and thickened and retracted on the left. Note a concomitant mastoiditis with partial sclerosis.
The classical HRCT findings in attico-antral CSOM with cholesteatoma include 1) erosion and blunting of scutum (lateral attic wall), 2) widening of the aditus ad antrum, 3) displacement of ossicular chain and 4) destruction of ear ossicles [5–7].
Cholesteatoma
Cholesteatoma is the hallmark of unsafe CSOM and is classically described as “skin in wrong place”. Complications in CSOM develop mainly due to cholesteatoma owing to its propensity for bone erosion; hence, it is also called unsafe CSOM. Imaging evaluation of a complicated CSOM commences with HRCT, and MRI is performed to rule out any intracranial involvement.
Cholesteatoma can be congenital or acquired (Table 1), which may be distinguished based on the history, status of the TM and location of the lesion [8–10]. Congenital cholesteatoma (CC) develops from the embryonic epithelial rest which is most commonly located in the temporal bone but can be present elsewhere in the skull, including skin [8,11]. Unlike the acquired type, CC (Figure 4) is not a sequelae of CSOM; nonetheless, it needs to be mentioned due to similar imaging findings [12,13].
Table 1.
Classification of cholesteatomas.
| Cholesteatoma type Features | Congenital | Acquired | |
|---|---|---|---|
| Primary | Secondary | ||
| 1. Incidence (in%) | 2 | 80 | 18 |
| 2. Clinical feature | Asymptomatic | Chronic ear discharge with hearing deficit | Chronic ear discharge with hearing deficit |
| 3. Site predilection | Nonspecific | Middle ear (epitympanum) | Middle ear (mesotympanum) |
| 4. Tympanic membrane | Intact | Usually intact | Perforated |
| 5. Associated conditions | Microtia | – | – |
Figure 4.
(A, B) Bilateral microtia with congenital cholesteatoma. Axial and coronal HRCT images showing absent external auditory canals bilaterally (EAC) with soft tissue filling the middle ear and mastoid cavity, suggestive of a cholesteatoma (thin arrow); however, no bony erosion is present. Also, the incudo-malleal complex is dysplastic (block arrow).
On the other hand, acquired cholesteatoma can be primary or secondary, depending on the integrity of TM. In primary acquired cholesteatoma, TM is intact, whereas in the secondary type, there is epithelialization of the middle ear across the ruptured TM. Of its various subtypes, the incidence of primary acquired cholesteatoma is the highest (80%), followed by secondary acquired (18%) and congenital (2%) types [5].
The above-mentioned distinction between the types of cholesteatoma on the basis of aetiopathogenesis, is, however, of limited use to the otorhinolaryngologist. From the management perspective, location of the lesion with respect to the TM is more helpful for surgical planning. Accordingly, cholesteatoma may be divided into two types – pars flaccida (attic, 80%) or pars tensa (sinus, 18%) (Table 2).
Table 2.
Differentiating pars flaccida and pars tensa cholesteatomas.
| Pars flaccida cholesteatoma | Pars tensa cholesteatoma | |
|---|---|---|
| 1. Initial location | Epitympanum (Prussak’s space) | Mesotympanum (sinus tympani, facial recess, mastoid cavity) |
| 2. Distribution with respect to: a. TM b. Ossicles |
Upper 1/3rd
Lateral |
Lower 2/3rd Medial |
| 3. Pathogenesis | Usually primary acquired (attic cholesteatoma) Occasionally congential or secondary acquired |
Usually secondary acquired Can be congenital |
Pars flaccida cholesteatoma (Figure 5) is usually primary acquired (i.e. with intact TM). It initially fills the Prussak’s space, in the region of upper one-third of TM, displacing the ear ossicles medially. Thereafter, it grows posteriorly into the epitympanum, involving the posterolateral attic and may either widen the aditus and involve the mastoid air cells or may grow inferiorly involving the posterior tympanic recess. Aditus widening is a characteristic finding.
Figure 5.
(A, B) Right, pars flaccida cholesteatoma with ossicular erosion. Coronal and axial HRCT images of both temporal bones: thickened, right TM (arrowhead) with presence of nodular soft tissue with convex margins in Prussak’s space (arrow) causing scutum blunting and ossicular erosions. (C) Concomitant chronic mastoiditis. Contralateral normal ear for comparison.
Pars tensa cholesteatoma (Figure 6), on the other hand, is usually secondary acquired as a result of perforation of the lower two-thirds of TM. It is initially localized in the facial recess with later involvement of the sinus tympani and mastoid air cells. It may further extend superiorly to the attic and may displace the ear ossicles laterally. There is a tendency of pars tensa cholesteatoma to preferentially spread along the medial wall of the tympanic cavity; hence, there is early involvement of otic capsule and labyrinth. However, in advanced cases with extensive involvement, distinction of the cholesteatoma into pars flaccida and tensa may not be possible [6,8,14–18].
Figure 6.
Pars tensa cholesteatoma. Nodular soft tissue in the hypotympanum of the right ear in the region of sinus tympani (thin arrow) and facial nerve recess (thick arrow). Compare the normal sinus tympani and facial nerve recess on the left side.
At its inception, cholesteatoma is a well-circumscribed, nondependent, non-enhancing soft tissue lesion with convex or bulging margins on HRCT that may be associated with bone erosions [7,8]. Bone erosion (Figure 7) is nearly pathognomonic for cholesteatoma and is seen in approximately 90% of patients; however, it is rarely present in the congenital cholesteatoma. Pars tensa cholesteatoma (~90%) is more frequently associated with bone erosion than pars flaccida cholesteatoma (~75%). Moreover, bone and ossicular erosions are more common in children as compared to adults [8,18,19–22].
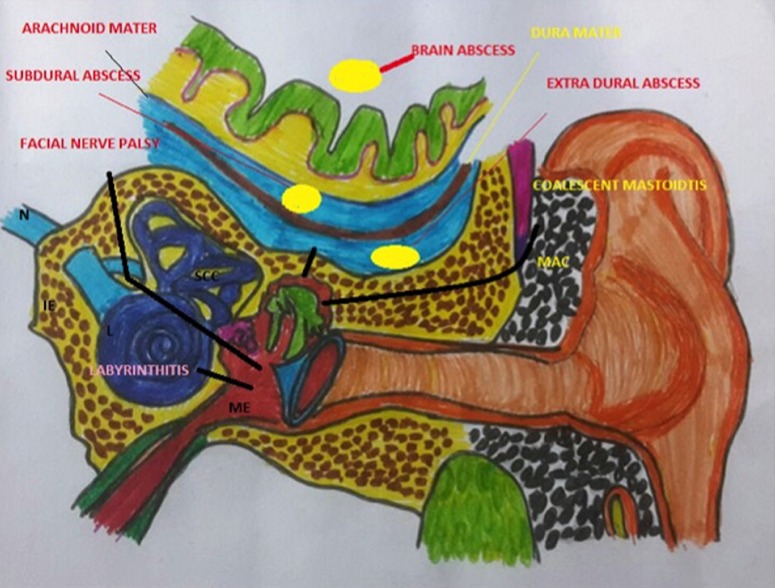
Figure 7.
Pathways of spread of CSOM leading to intra- and extratemporal complications. N – nerve (VII–VIIIth nerve complex); IE – inner ear; L – labyrinth; SCC – superior semicircular canal,; ME – middle ear; MAC – mastoid air cells.
Rosito et al. conducted a cross-sectional study comprising of 414 cases in order to classify cholesteatomas according to growth patterns and prevalence of various subtypes. It was observed that the posterior epitympanic cholesteatoma was more common in adults (41%), as compared to posterior mesotympanic type in children (43%). Furthermore, anterior epitympanic cholesteatoma was noted exclusively in children [9].
Ancillary findings of HRCT, such as attenuation values and pattern of contrast enhancement, are of limited use in diagnosing cholesteatoma. Cholesteatoma is usually nonenhancing, although there may be peripheral enhancement which goes unnoticed in smaller lesion. Lack of enhancement in the centre of a large lesion is one of the striking features of cholesteatoma, aiding in its distinction from neoplasms of the middle ear with otherwise similar morphological features [8].
Apart from the location of cholesteatoma with respect to the TM, it is also important to note its relation to the ear ossicles, i.e. medial or lateral, which is extremely helpful in determining the surgical approach [12]. Besides, due to a better preoperative extent delineation, HRCT improves the surgeon’s confidence in the preoperative counselling for various ancillary surgical procedures such as mastoidectomy or removal of ossicles [13].
Despite having the aforementioned utilities, HRCT may not always distinguish between safe and unsafe CSOM. For instance, it may be difficult to identify a small focus of cholesteatoma amidst an opacified tympanic cavity, especially in the absence of bone erosion. Attenuation values and contrast enhancement pattern of soft tissue on CT (unlike MRI) may not distinguish simple granulation tissue from the cholesteatoma. Also, HRCT is not precise in detecting residual cholesteatoma in a postoperative ear [5,7].
Imaging Findings in Complications of CSOM
Complications of CSOM can be broadly classified into 2 categories, as intra- or extratemporal (Table 3).
Table 3.
Complications of CSOM.
| Intratemporal | Extratemporal (extracranial) | |
|---|---|---|
| Extracranial | Intracranial | |
|
|
|
Ossicular erosion
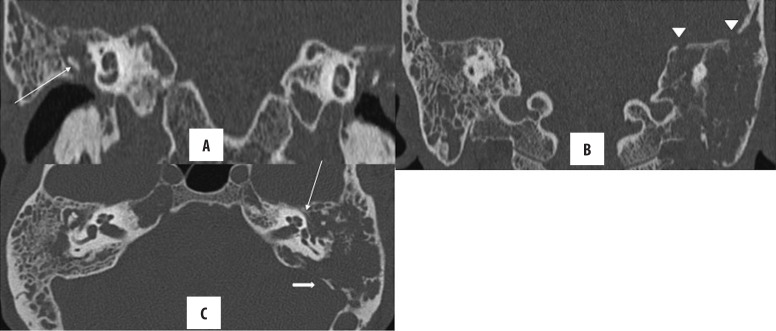
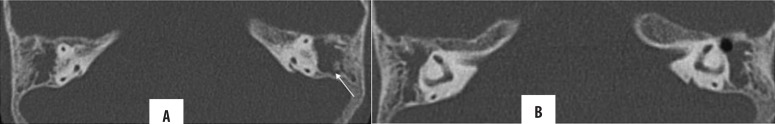
Cholesteatoma can erode the scutum, ear ossicles, tegmen tympani, bony labyrinth (lateral semicircular canal) and facial nerve canal (tympanic segment). Scutum erosion is classically seen in the attic cholesteatoma (Figures 8, 9). Of the ear ossicles, the long process of the incus is most commonly affected owing to its vicarious blood supply and limited ligamentous support, followed by the lenticular process of the incus and stapes footplate [5,19,21].
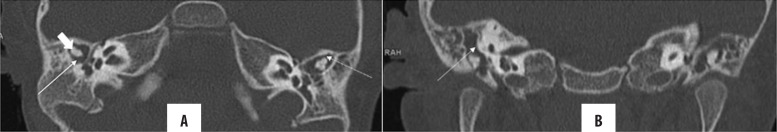
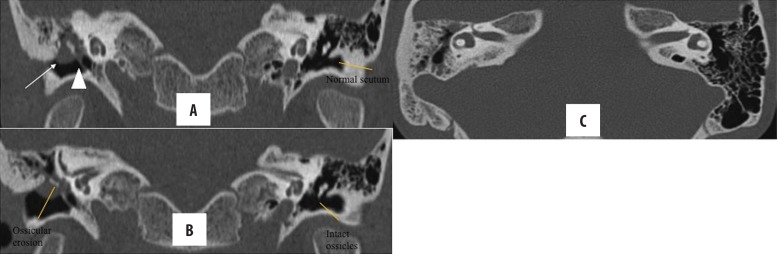
Figure 8.
Right-sided chronic otomastoiditis with bone erosion. Left cholesteatoma with automastoidectomy and erosion of ear ossicles, (A) lateral semicircular canal (arrow), sinus plate (star), facial nerve canal (arrowhead in B) and scutum (C). Note that the erosion of the lateral mastoid cortex (thick arrow) simulates modified radical mastoidectomy; however, the lateral attic spur is intact.
Figure 9.
Bilateral CSOM with cholesteatoma. Right-sided, holotympanic soft tissue opacification with erosion of the long process of the incus (arrow in A). Left-sided cholesteatoma with erosion of ear ossicles, tegmen tympani (arrowhead in B), tympanic segment of facial canal (thin arrow in C) and sinus plate (solid arrow in C). No jugular venous thrombosis was seen (not shown). Opacification of mastoid air cells on both sides is present with resorption of mastoid septa on the left and confluence into cavities, suggestive of coalescent mastoiditis.
As mentioned above, HRCT is the imaging modality of choice to detect bone erosions. The recommended window settings for the optimal assessment of ear ossicles include a large window width (e.g. 4000 HU) and a low window level (0–200 HU) [5]. Contrast-enhanced MRI is indicated if there is erosion of tegmen tympani or sinus plate due to potential intracranial complications because of the close proximity of the dura at these sites [24].
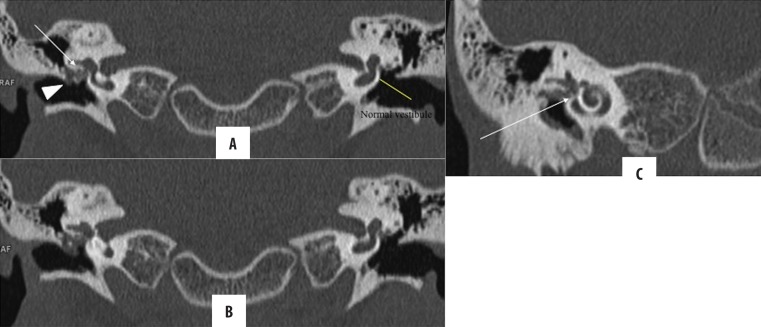
Labyrinthine fistula
It is one of the potentially serious complications of cholesteatoma with an incidence of 5–10%. Of the labyrinth apparatus, lateral semicircular canal (LSCC) is most commonly affected, followed by superior ampulla, posterior canal and cochlear promontory (Figures 10, 11). HRCT findings in labyrinthine fistula include direct apposition of the soft tissue to the lumen of labyrinth with associated thinning or erosion of the cortical bone. Subtle findings may be missed; hence, it is important to evaluate the labyrinthine structures in more than one plane [25,26].
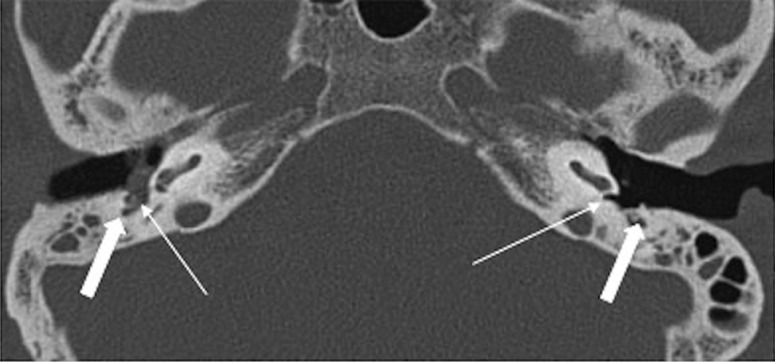
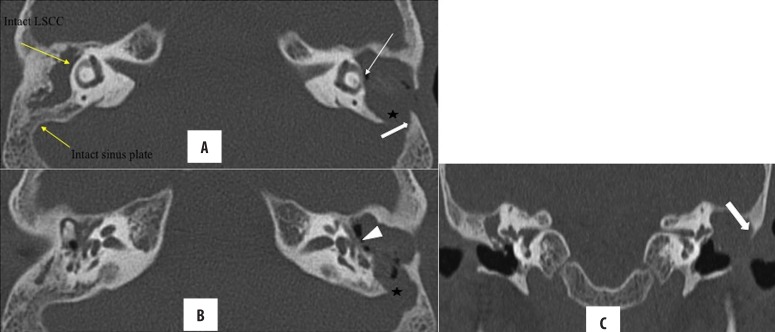
Figure 10.
(A–C) Right-sided cholesteatoma with vestibular fistula. Nodular soft tissue in the right epi- and mesotympanum causing ossicular erosions (arrowhead), suggestive of cholesteatoma. Also, it is in close apposition to vestibule (arrow) with loss of intervening bony labyrinth, suggestive of fistulous communication. Intact bony labyrinth on the left side for comparison.
Figire 11.
(A–C) Right-sided cholesteatoma with cochlear fistula: Post-MRM for bilateral cholesteatomatous CSOM. Soft tissue lining in the common cavity bilaterally, suggestive of residual disease. On the right side, the cholesteatoma (arrow) is abutting and eroding the basal and middle turn of the cochlea with loss of its internal architecture, suggestive of fistulous communication with the common cavity. Normal cochlea on the left is presented for comparison (arrowhead).
Sometimes, soft tissue may be simply adherent to the bony labyrinth without causing its erosion. Although not being a fistula, it still needs to be mentioned, as surgical removal of this soft tissue may cause iatrogenic fistulisation and unremitting vertigo. Ultimate resort in such cases may be labyrinthectomy which may cause complete deafness (4). On MRI, there is labyrinthine enhancement in the presence of a fistula [5,14,22–25].
Facial nerve palsy secondary to canal erosion is present in nearly 1% of cholesteatoma cases. The tympanic segment of the facial nerve is most commonly affected, as it is located in the vicinity of epitympanum where cholesteatomas frequently lodge. Diagnostic uncertainties may arise when there is congenital dehiscence or thinning of the bony canal, which may be indistinguishable from subtle erosions [24]. In a study comprising of 50 patients, conducted by Thukral et al. to evaluate the role of HRCT in temporal bone pathologies, HRCT was found to be highly sensitive in detecting cholesteatoma (89%) and erosions of the ear ossicles (83%). Furthermore, HRCT also aided in the detection of lateral semicircular canal erosions. However, it had a low sensitivity of detecting facial canal erosions (33%) [18].
Goma et al., in their study of 56 cases of cholesteatoma, observed that the scutum and lateral attic wall were eroded in nearly two-thirds of cases. The incus was the most commonly eroded ear ossicle (88%). The authors also noted that intra-temporal complications were more common than intracranial complications. Additionally, a comparison of HRCT and intra-operative findings revealed that HRCT had a high sensitivity and accuracy in detecting cholesteatomas (92%), ossicular erosions (98%), labyrinthine fistula as well as intracranial complications [20]. Rai et al. also observed similar results when demonstrating the accuracy of HRCT in diagnosing various complications of cholesteatoma which correlated well with operative findings. However, the sensitivity of HRCT was low (p<0.05) in detecting posterior fossa, dural plate and tegmen tympani erosions [26].
Post-inflammatory ossicular fixation (PIOF) is one of the causes of conductive hearing loss in CSOM. There are three subtypes of PIOF, representing the histopathological continuum from fibrosis to calcification:
Fibrous tissue fixation (chronic adhesive OM) – most common;
Collagen hyalinization (tympanosclerosis);
New bone formation (fibroosseous sclerosis).
HRCT is the modality of choice in suspected PIOF, irrespective of the pathological subtype. It not only helps in evaluating the lesion extent and its distribution but also predicts improvement after surgical correction..Fibrous tissue fixation may be either localized or diffuse. The localized form can occur anywhere; however, it most commonly involves the oval window niche leading to stapedial fixation (prestapedial stent) [6,27].
On HRCT, fibrosis appears as non-calcified, soft tissue debris encasing the ossicular chain. It is difficult to suggest fixation of ossicles on imaging, and findings remain indistinguishable from other causes of opacified middle ear, especially cholesteatoma. Nonetheless, the presence of bone erosion favours cholesteatoma, whereas absence of aural discharge favours PIOF.
Tympanosclerosis, which appears as punctate or diffuse calcified densities, may occur anywhere in the tympanic cavity, and depending upon the structure involved, may cause hearing loss or remain asymptomatic. It needs to be differentiated from calcified anatomical structures such as the cochleariform process. Tympanosclerosis of the TM is also referred to as myringosclerosis.
New bone formation (fibroosseous sclerosis) is the least common manifestation of PIOF and is characterized by lamellar bone deposition in the tympanic cavity, commonly in the epitympanum. Other imaging differential diagnoses for PIOF, apart from cholesteatoma, include otosclerosis, as both entities manifest with conductive hearing loss in a non-discharging ear. However, the presence of hypodense soft tissue in fissula ante fenestrum suggests otosclerosis [6,.27].
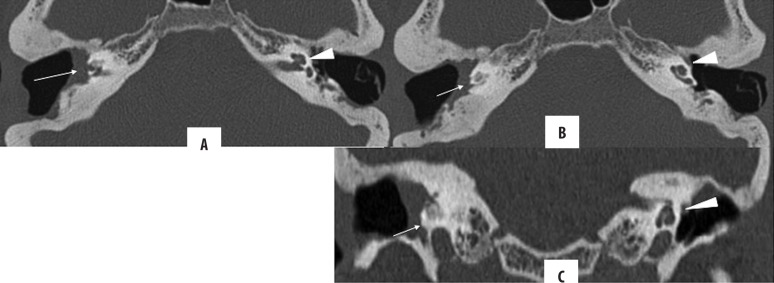
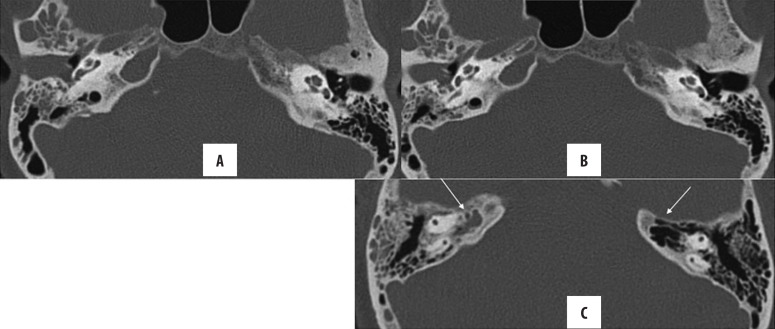
Mastoiditis is a frequent co-morbidity of both acute and chronic otitis media. In acute otomastoiditis, mastoid air cells become fluid-filled, and there is usually no bone erosion. On the other hand, mastoiditis (Figures 12, 13), occurring in conjunction with CSOM, may be coalescent or sclerosing. In sclerosing mastoiditis, there is a loss of pneumatisation of the mastoid air cells with their sclerosis. Coalescent mastoiditis is a more severe form with mucoperiosteal involvement, leading to the destruction of the mastoid septa and resulting in intramastoid empyema and cavity formation [7,12].
Figure 12.
Right CSOM with mastoiditis: Axial (A, C, D) and coronal (B) HRCT images of both temporal bones; (A) Ruptured TM on the right, intact on the left (arrow) (B) Coronal: soft tissue opacification of the right ear (epi-, meso- and hypotympanum). The scutum is not blunted (arrow), (C) Ear ossicles are intact, (D) mastoiditis with partial sclerosis of mastoid air cells. Contralateral normal ear is presented for comparison.
Figure 13.
(A, B) Bilateral CSOM with coalescent mastoiditis: soft tissue attenuation in both tympanic middle ears and mastoids. Also, there is resorption of mastoid septa (arrow) with formation of a large cavity. No bony erosion is seen.
HRCT is the imaging modality of choice for diagnosing coalescent mastoiditis, as bony septa, inner and outer mastoid cortices are best seen. Early in the course of the disease, erosion of the septa of mastoid air cells may be subtle; however, a comparison with the contralateral normal side may help to look for asymmetry.
Unresolved mastoiditis may further spread into the adjacent structures. Breach of the outer mastoid cortex leads to post-auricular abscess formation (Macewen’s triangle), whereas erosion of the mastoid tip may lead to the spread in the cervical region (Bezold’s abscess). Occasionally, a peri-auricular abscess may develop in the absence of cortical breach due to the involvement of mastoid emissary veins (Griesinger’s sign) [14,28,29].
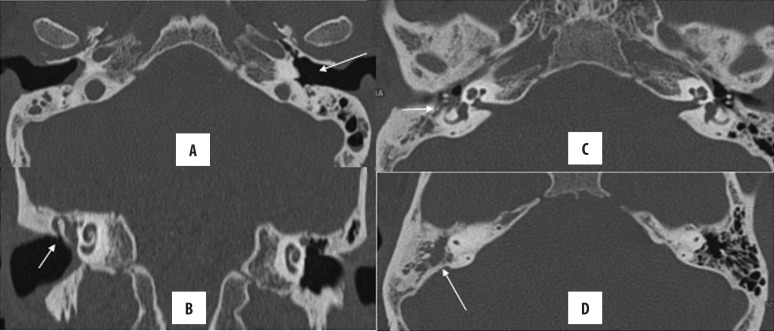
Petrous apicitis
The petrous apex is the anteromedial portion of the temporal bone and is pneumatized in ~30% of the general population. A pneumatized petrous apex freely communicates with the tympanic cavity and the mastoid air cells. Accordingly, CSOM may involve the pneumatized petrous apex leading to petrous apicitis (Figure 14). Clinically, patients may present with Gradenigo’s syndrome (triad of retro-orbital pain, aural discharge and sixth nerve palsy). Due to its location, an involvement of the petrous apex is of utmost clinical concern, as it may further spread to the middle and posterior cranial fossa. On HRCT, there is opacification of the pneumatized petrous apex [14,30].
Figure 14.
Right chronic otomastoiditis with petrous apicitis. (A, B) Soft tissue opacification in the external and middle ear (meso- and hypotympanum) as well as in the mastoid cavity; however, no bone erosion is seen. Note is made of pneumatisation of both petrous apices with presence of soft tissue density on the right, suggestive of petrous apicitis (arrow in C).
Intracranial complications
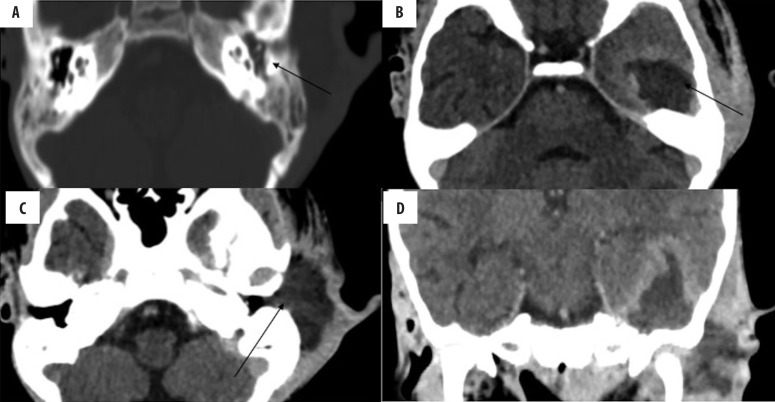
Meningitis is the most common intracranial complication of CSOM, followed by brain abscesses. Meningitis is relatively commoner in AOM than CSOM. Otogenic brain abscesses (Figure 15) are commonly located in the temporal lobe and cerebellum, which is explained by the contiguous spread of the disease process from the adjoining middle ear. CECT or MRI are used to diagnose brain abscesses [31].
Figure 15.
Left CSOM with otogenic brain and subperiosteal abscesses. (A) Soft tissue density in the left middle ear and mastoid cavity, (B) rim-enhancing abscess in the left temporal lobe and (C) subperiosteal abscess, (D) coronal image showing communicating brain and subperiosteal abscesses.
Dural venous sinus thrombosis is another frequent complication of CSOM and is seen in nearly 20% of cases. Of the venous sinuses, the lateral sinus is most frequently affected due to its proximity to the mastoid air cells. Lateral sinus thrombosis may result either from a direct spread of infection to the perisinus space after bone erosion or from retrograde transmission through the mastoid emissary vein. Acute sinus thrombosis is hyperdense on NCCT and appears as a filling defect on CECT/MRI. Lateral sinus thrombosis may extend further proximally to the sinus confluence (torcular herophili) and the sagittal sinus, leading to otitic hydrocephalus, or distally to the jugular vein, leading to formation of septic emboli. MR venography is of utmost utility in sinus thrombosis, especially when follow-up imaging is required due to the lack of radiation concerns and excellent contrast resolution. Moreover, associated venous infarcts are better evaluated on MRI [30].
Epidural abscess is another infectious complication which occurs due to the direct intracranial spread of the inflammatory process secondary to bone destruction. It manifests with vague clinical features such as headache or increasing otalgia, unlike the fulminant presentation in meningitis and brain abscess. Both CECT and CEMRI are nearly comparable in diagnosing this entity; however, bone erosions are better evaluated on HRCT, whereas MRI is more sensitive in detecting smaller abscesses [14].
Otitic hydrocephalus may follow otitis media (acute/chronic) and manifest with features of raised intracranial pressure. It is a sequelae of superior sagittal sinus thrombosis that alters the functioning of arachnoid villi; hence, preventing CSF absorption. This condition is a misnomer as it is neither exclusive to the otitis media nor there is true hydrocephalus as there is no ventriculomegaly. It is a diagnosis of exclusion and is suspected with clinical features of raised ICT but no corroborative imaging abnormality. Nonetheless, chronic venous sinus thrombosis may be variably present [31,32].
Meningo- or encephalocele is a rare complication resulting from the erosion of tegmen tympani or sinus plate, leading to herniation of the meninges or brain. As in other complications, HRCT is better suited for the detection of bone erosion, whereas MRI is indicated for the assessment of brain parenchyma [24].
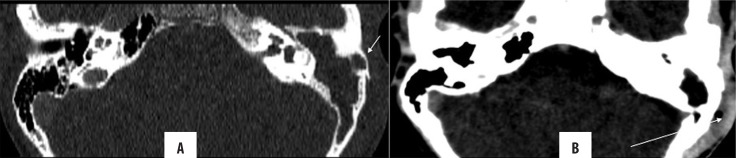
Subperiosteal abscesses result from the cortical breach leading to further spread of the inflammatory processes underneath an intact periosteum. On CECT/CEMRI, it presents as a well-defined, rim-enhancing collection (Figure 16). Osteomyelitis of the skull base is also an uncommon complication (Figure 17).
Figure 16.
Left unsafe CSOM with subperiosteal abscess. Axial and coronal HRCT images (A, B) showing soft tissue density in the left middle ear and mastoid cavity with destruction of ear ossicles, facial nerve canal and mastoid septa (automastoidectomy). Note the extremely thinned out lateral wall of the mastoid (arrow in A) with enhancing soft tissue lateral to it (arrow in B) in the overlying scalp, suggestive of a subperiosteal abscess.
Figure 17.
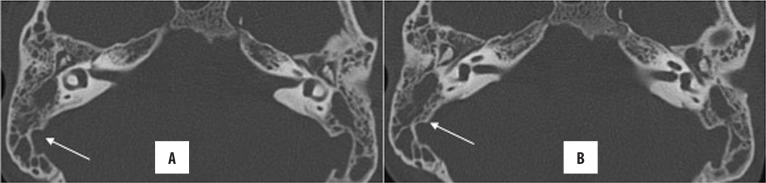
Right unsafe CSOM with skull base osteomyelitis. Axial HRCT images (A, B) showing soft tissue density in the right middle ear and mastoid cavity with destruction of mastoid septa and the medial wall of the right mastoid (arrow).
Bezold’s abscess occurs due to erosion of the mastoid tip with consequent spread of phlegmonous debris in the soft tissue of the neck. It is usually a complication of acute otomastoiditis but can occasionally be seen in CSOM [33].
Conclusions
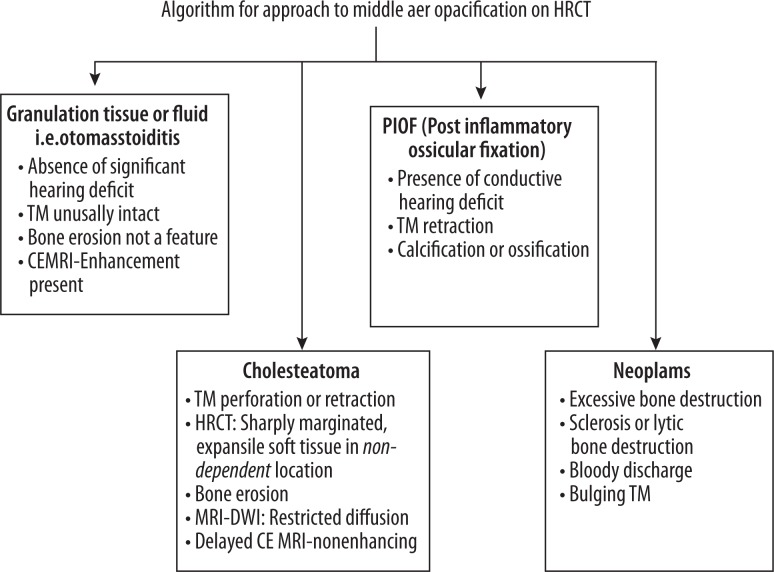
To conclude, apart from CSOM, a variety of conditions can present as middle ear opacification on HRCT. Also, a few other temporal complications of CSOM may appear as soft tissue attenuation, of which cholesteatoma is the main concern. Nevertheless, making a correct diagnosis of CSOM and mastoiditis is a multidisciplinary approach which requires clinical, radiological and histopathological correlation for successful treatment. The authors suggest a short, comprehensive clinico-radiological algorithm (Figure 18) for the differential diagnosis of middle ear opacifications seen on HRCT.
Figure 18.
Approach to middle ear opacifications found on HRCT.
Conflict-of-interest statement
No potential conflicts of interest.
References
- 1.The global burden of disease: 2004 update Geneva . World Health Organization. 2008. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full pdf, accessed 15 September 2012. [Google Scholar]
- 2.Platzek I, Kitzler HH, Gudziol V, et al. Magnetic resonance imaging in acute mastoiditis. Acta Radiol Short Rep. 2014;3(2):2047981614523415. doi: 10.1177/2047981614523415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephen AT, Leach AJ, Morris PS. Impact of swimming on chronic suppurative otitis media in Aboriginal children: A randomised controlled trial. Med J Aust. 2013;199(1):51–55. doi: 10.5694/mja13.10533. [DOI] [PubMed] [Google Scholar]
- 4.Ibekwe TS, Nwaorgu OG. Classification and management challenges of otitis media in a resource-poor country. Niger J Clin Pract. 2011;14(3):262–69. doi: 10.4103/1119-3077.86764. [DOI] [PubMed] [Google Scholar]
- 5.Juliano AF, Ginat DT, Moonis G. Imaging review of the temporal bone: Part I. Anatomy and inflammatory and neoplastic processes. Radiology. 2013;269(1):17–33. doi: 10.1148/radiol.13120733. [DOI] [PubMed] [Google Scholar]
- 6.Sreedhar S, Pujary K, Agarwal AC, Balakrishnan R. Role of high-resolution computed tomography scan in the evaluation of cholesteatoma: A correlation of high-resolution computed tomography with intra-operative findings. Indian J Otol. 2015;21:103–6. [Google Scholar]
- 7.Eshetu T, Aygun N. Imaging of the temporal bone: A symptom-based approach. Semin Roentgenol. 2013;48(1):52–64. doi: 10.1053/j.ro.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Barath K, Huber AM, Stampfli P, et al. Neuroradiology of cholesteatomas. Am J Neuroradiol. 2011;32(2):221–29. doi: 10.3174/ajnr.A2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosito LS, Netto LF, Teixeira AR, Da Costa SS. Classification of cholesteatoma according to growth patterns. JAMA Otolaryngol Head Neck Surg. 2016;142(2):168–72. doi: 10.1001/jamaoto.2015.3148. [DOI] [PubMed] [Google Scholar]
- 10.Corrales CE, Blevins NH. Imaging for evaluation of cholesteatoma: Current concepts and future directions. Curr Opin Otolaryngol Head Neck Surg. 2013;21:461–67. doi: 10.1097/MOO.0b013e328364b473. [DOI] [PubMed] [Google Scholar]
- 11.Bukurov B, Babić B, Dimitrijević M, et al. Congenital cholesteatoma of the middle ear – uncommon clinical presentation. Vojnosanit Pregl. 2014;71(5):503–5. [PubMed] [Google Scholar]
- 12.Anbarasu A, Chandrasekaran K, Balakrishnan S. Soft tissue attenuation in middle ear on HRCT: Pictorial review. Indian J Radiol Imaging. 2012;22:298–304. doi: 10.4103/0971-3026.111483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mcguire JK, Wasl H, Harris T, et al. Management of pediatric cholesteatoma based on presentations, complications, and outcomes. Int J Pediatr Otorhinolaryngol. 2016;80:69–73. doi: 10.1016/j.ijporl.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Maranhão A, Andrade J, Godofredo V, et al. Epidemiology of intratemporal complications of otitis media. Int Arch Otorhinolaryngol. 2014;18(2):178–83. doi: 10.1055/s-0033-1364172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo AC, Nemec SF. Opacification of the middle ear and mastoid: Imaging findings and clues to differential diagnosis. Clin Radiol. 2015;70(5):e1–13. doi: 10.1016/j.crad.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Rogha M, Hashemi SM, Mokhtarinejad F, et al. Comparison of preoperative temporal bone CT with Intraoperative findings in patients with cholesteatoma. Iran J Otorhinolaryngol. 2014;26(74):7–12. [PMC free article] [PubMed] [Google Scholar]
- 17.Trojanowska A, Drop A, Trojanowski P, et al. External and middle ear diseases: Radiological diagnosis based on clinical signs and symptoms. Insights Imaging. 2012;3(1):33–48. doi: 10.1007/s13244-011-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thukral CL, Singh A, Singh S, et al. Role of high resolution computed tomography in evaluation of pathologies of temporal bone. J Clin Diagn Res. 2015;9(9):TC07–10. doi: 10.7860/JCDR/2015/12268.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinha AK, Kumar A, Raushan EA, Kumar G. Bone resorption in chronic otitis media: An observational study. Int J Sci Stud. 2014;2(6):82–85. [Google Scholar]
- 20.Gomaa MA, Abdel Karim AR, Abdel Ghany HS, et al. Evaluation of temporal bone cholesteatoma and the correlation between high resolution computed tomography and surgical finding. Clin Med Insights Ear Nose Throat. 2013;6:21–28. doi: 10.4137/CMENT.S10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elefante A, Cavaliere M, Russo C, et al. Diffusion weighted MR imaging of primary and recurrent middle ear cholesteatoma: An assessment by readers with different expertise. Biomed Res Int. 2015;2015:597896. doi: 10.1155/2015/597896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juliano AF, Ginat DT, Moonis G. Imaging review of the temporal bone: Part I. Anatomy and inflammatory and neoplastic processes. Radiology. 2013;269(1):17–33. doi: 10.1148/radiol.13120733. [DOI] [PubMed] [Google Scholar]
- 23.Ayache D, Darrouzet V, Dubrulle F, et al. Imaging of non-operated cholesteatoma: Clinical practice guidelines. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129(3):148–52. doi: 10.1016/j.anorl.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Kumar SS, Thakar A. Spectrum of facial paralysis in chronic suppurative otitis media. Indian J Otol. 2012;18:92–94. [Google Scholar]
- 25.Kuo CL1, Shiao AS2, Yung M, et al. Updates and knowledge gaps in cholesteatoma research. Biomed Res Int. 2015;2015:854024. doi: 10.1155/2015/854024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rai T. Radiological study of the temporal bone in chronic otitis media: Prospective study of 50 cases. Indian J Otol. 2014;20:48–55. [Google Scholar]
- 27.Nevoux J, Lenoir M, Roger G, et al. Childhood cholesteatoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127(4):143–50. doi: 10.1016/j.anorl.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Groth A, Enoksson F, Hultcrantz M, et al. Acute mastoiditis in children aged 0–16 years – a national study of 678 cases in Sweden comparing different age groups. Int J Pediatr Otorhinolaryngol. 2012;76(10):1494–500. doi: 10.1016/j.ijporl.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Sharma N, Jaiswal AA, Banerjee PK, Garg AK. Complications of chronic suppurative otitis media and their management: A single institution 12 years’ experience. Indian J Otolaryngol Head Neck Surg. 2015;67(4):353–60. doi: 10.1007/s12070-015-0836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sennaroglu L, Sozeri B. Otogenic brain abscess: review of 41 cases. Otolaryngol Head Neck Surg. 2000;123(6):751–5. doi: 10.1067/mhn.2000.107887. [DOI] [PubMed] [Google Scholar]
- 31.Ryan JT, Pena M, Zalzal GH, Preciado DA. Otogenic lateral sinus thrombosis in children: A review of 7 cases. Ear Nose Throat J. 2016;95(3):108–12. [PubMed] [Google Scholar]
- 32.Gurung UA, Ullah KS, Shrivastav RP, Bhattarai H. Otitic hydrocephalus. Indian J Otol. 2011;17:167–69. [Google Scholar]
- 33.Pradhananga R. An unusual complication of chronic suppurative otitis media: Bezold abscess progressing to scapular abscess. Int Arch Otorhinolaryngol. 2014;18(4):412–14. doi: 10.1055/s-0034-1372511. [DOI] [PMC free article] [PubMed] [Google Scholar]