Summary
The sternum is an uncommon site for neoplastic involvement and metastases are far commoner than primary neoplasms. Of the primary tumours, malignant lesions are more frequent than the benign lesions. Early diagnosis and treatment is prudent in such neoplasms not only to halt disease progression but also to prevent circulatory compromise resulting from the mass effect on the mediastinum. Sound knowledge of neoplasms affecting the sternum and their imaging appearance is essential to arrive at an early diagnosis and also to obviate biopsy in cases with classical imaging findings. Neoplastic involvement of the sternum is extremely unusual and should be considered malignant unless proven otherwise. Imaging may help in arriving at the diagnosis of these lesions, together with other factors such as patient's age, type of lesion (lytic/sclerotic or mixed), matrix mineralization, multiplicity and involvement of other sites.
Keywords: Sternum; Tomography, X-Ray Computed; X-Ray Film
Background
The sternum is a midline bone of the axial skeleton which anchors the rib cage. Its haematopoietic marrow provides a rich soil for seedling of various haematogenous diseases, including malignancies and infections. Virtually all bone tumours have predilection for the appendicular skeleton, flat bones being a relatively less favoured site. Of the flat bones, the sternum is an uncommon site of bone tumours with a meagre incidence of 0.65%, contributing to nearly 15% of chest wall tumours.
Metastases are the most common sternal neoplasms. Primary sternal neoplasms, whether benign or malignant, are quite rare; however, of these two, the latter are more common (Table 1). Not only neoplastic involvement of the sternum carries adverse prognostication, but other therapeutic implications associated soft tissue component can potentially cause mediastinal compression, which may prove detrimental due to the consequential SVC syndrome and circulatory arrest [1–3].
Table 1.
Classification of sternal neoplasms.
| Malignant | Benign |
|---|---|
|
Primary
• Chondrosarcoma • Osteosarcoma • Fibrosarcoma • Malignant fibrous histiocytoma • Plasmacytoma Secondary • Metastases (most common) • Multiple myeloma • Direct contiguous spread from adjacent malignancies-mediastinal masses, carcinoma lung, breast and thyroid |
• Osteochondroma • Osteoma • Hemangioma • Fibrous dysplasia • Langerhan’s cell histiocytosis |
Imaging Modalities
Traditionally, bone tumours are initially evaluated on plain radiographs for their location, pattern of bone destruction, matrix mineralization, periosteal reaction and associated soft tissue component. However, this modality has limitations in evaluating tumours of the flat bones, especially the sternum, as the lesion may not be projected in its entirety due to the superimposition by the surrounding lungs. Lateral radiographs may help, especially in distinguishing intra- and extra-thoracic lesions. However, plain radiography is of limited use in diagnosing small lesions as well as in evaluating the intrathoracic extent, which necessitates the use of cross-sectional imaging.
Computed tomography (CT) is the modality of choice for evaluating sternal lesions due to its inherent high spatial and contrast resolution, along with its multiplanar capabilities. It can resolve the limitations of radiography by providing excellent anatomical details to the surgeon pre-operatively and proves invaluable in determining the intrathoracic extent and mediastinal invasion. In certain bone tumours, including osteoid osteoma, demonstration of tiny radiolucent nidus on CT helps to arrive at the diagnosis non-invasively.
MRI promises to be a problem-solving modality in indeterminate cases, especially to delineate the exact extent of bone involvement and mediastinal invasion. It offers the advantage of being a radiation-free modality and may help in in-vivo characterization of the tumour in certain cases. However, failure to establish the pattern of matrix mineralization and type of bone marrow destruction, which are the essence of bone tumour diagnosis, limits its utility as the unequivocal modality for evaluation of sternal tumours. MRI is superior to CT in determining the mediastinal invasion.
Nuclear imaging is usually performed as part of skeletal survey in cases of suspected skeletal metastasis. It may help in ascertaining the malignant quotient non-invasively; however, it is of limited utility in tissue characterization.
This article highlights the imaging findings of sternal neoplasms. Knowledge of imaging features may help to narrow the differential diagnosis and may obviate biopsy in typical cases.
Primary Sternal Neoplasms
Chondrosarcoma
Chondrosarcoma is the most common malignant tumour of the chest wall, including the sternum. A cartilage containing tumour, it usually presents in the 4th–6th decade of life with a male preponderance (male-to-female ratio of 2: 1). It may arise de novo or de-differentiate from a pre-existing exostoses or enchondroma. Secondary chondrosarcomas may occur as a part of the spectrum of radiation-induced sarcomas (RIS) after previous therapeutic irradiation for thoracic, mediastinal and chest wall malignancies. Clinically, it presents as a slow-growing, large, midline mass which is usually painless.
On radiographs, the presence of stippled or “rings and arcs” pattern of calcifications hints to the chondroid matrix. The distribution of calcifications is peripheral (c.f. osteosarcoma, vide infra).
On CT, the tumour can be either intramedullary or juxta-cortical, causing cortical destruction along with the presence of a large, lobulated, soft tissue component having chondroid calcifications (Figure 1). Rapid enlargement and large soft tissue component favour malignancy; however, differentiation from malignant transformation in pre-existing exostoses and enchondroma continues to remain a dilemma to the radiologist. Myxoid chondrosarcoma, a rare variant consisting of only soft tissue matrix, may be indistinguishable from other soft tissue sarcomas on imaging [1,3–6].
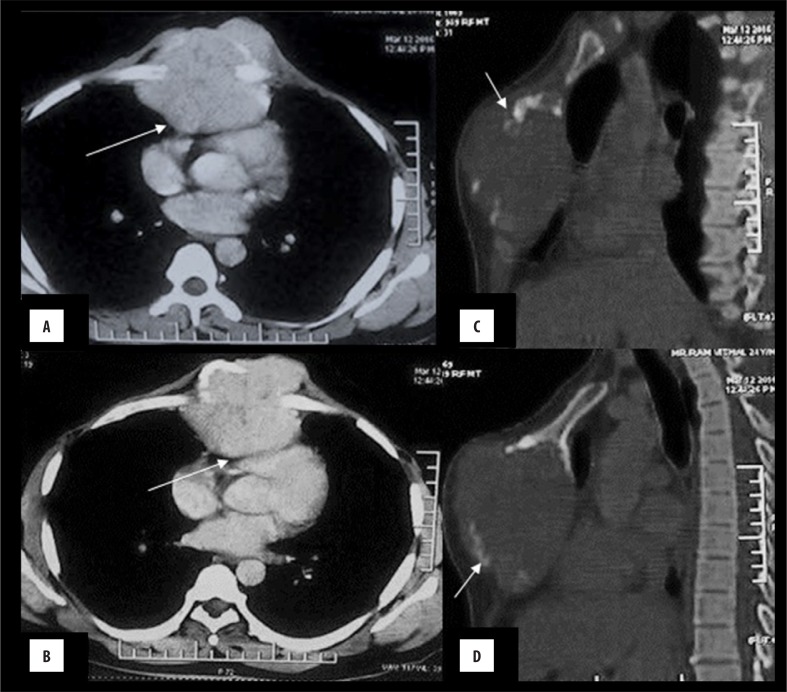
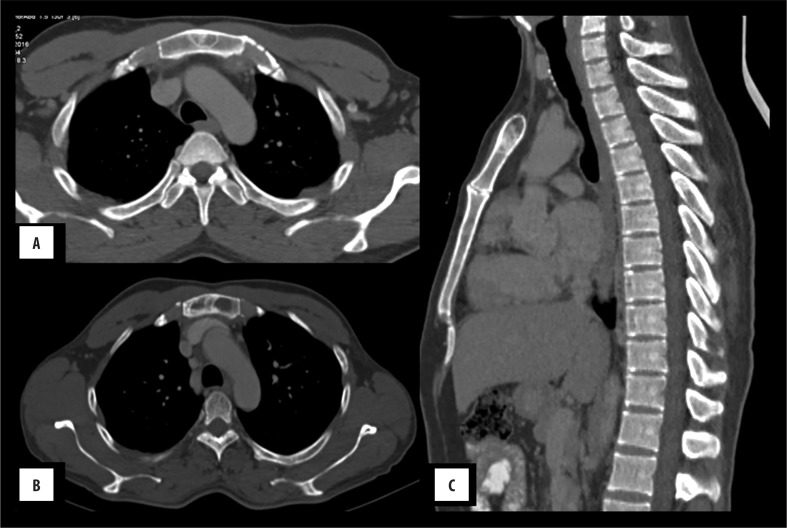
Figure 1.
Chondrosarcoma: A 55-year-old male with a midline chest swelling. (A, B): Axial CECT shows a lytic, destructive lesion of the sternum with heterogeneously enhancing, lobulated soft tissue causing compression of the right ventricle (arrow) with maintained fat planes. Arc-like calcification can be seen at its periphery, i.e. chondroid matrix (arrow), better appreciated in bone window (C, D).
Osteosarcomas (OS)
The sternum is an uncommon site for osteosarcomas which can be either primary or secondary to prior radiation exposure. As compared to the extremity osteosarcoma, sternal OS occurs in slightly older patients with a worse prognosis.
It presents with a rapidly enlarging, painful midline swelling. The lesion can be lytic, sclerotic or mixed (Figure 2). Classically, there is lytic permeative bone destruction with the presence of heterogeneous soft tissue having osteoid matrix characterized by amorphous, cloud-like calcifications in a central distribution [3,4,7.8].
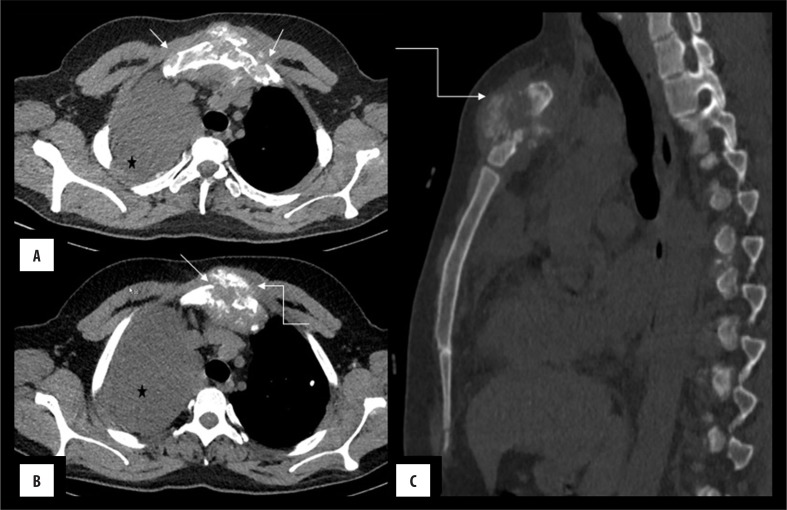
Figure 2.
(A–C) Sternal osteosarcoma: Lytic destruction (arrow) of the sternum with the presence of heterogeneously enhancing soft tissue showing amorphous calcification (curved arrow) predominantly in the central distribution. Associated soft tissue component is extending retro-sternally and invades the anterior mediastinum. Note is made of malignant right pleural effusion (*).
Ewing’s Sarcoma
Ewing’s sarcoma (EWS) is a malignant, round-cell tumour which is variously referred to as Askin’s or primitive neuroendocrine tumour. It usually presents in adolescence as a painful swelling. It has a high metastatic propensity leading to tumoural dissemination to the lungs, bones and lymph nodes in nearly one fourth of cases at the time of presentation. EWS occurs with a comparable frequency in both long (60%) and flat (40%) bones.
On CT, there is lytic permeative destruction of the involved bone which may show expansion (Figure 3). Cortical destruction and aggressive periosteal reaction may be present. Of all the sternal neoplasms, soft tissue component is disproportionately large for the magnitude of bone destruction [3,4].
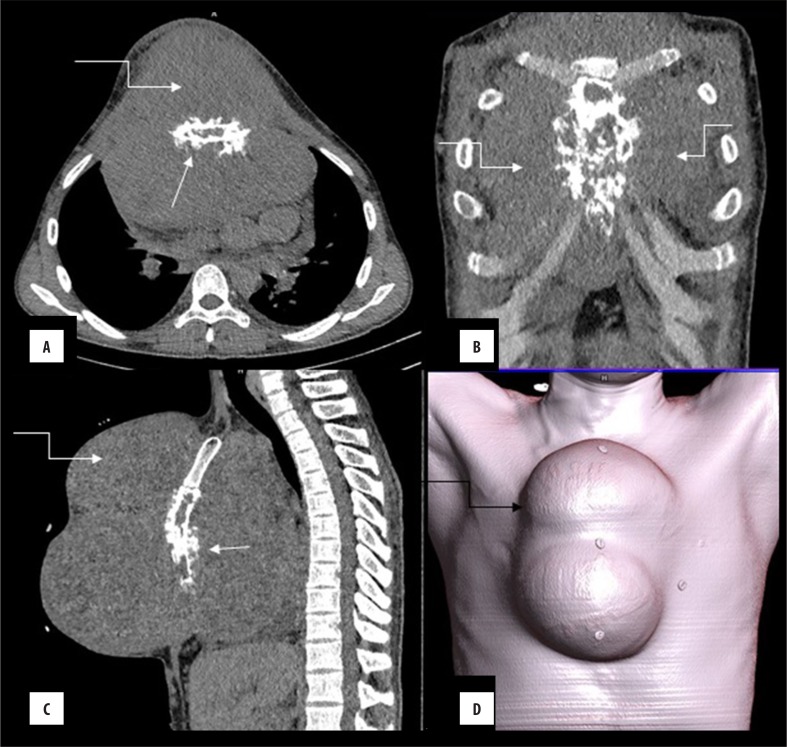
Figure 3.
(A–D) Ewing’s sarcoma: A 15-year-old male with a rapidly developing midline swelling and constitutional symptoms. CECT revealed lytic, permeative destruction of the sternum with spiculated (arrow) periosteal reaction. Note the presence of disproportionately large soft tissue component (curved arrow) for the bone destruction; quite characteristic of these tumours, better appreciated in volume-rendered image (D).
Plasmacytoma
Plasmacytoma and multiple myeloma (MM) refer to a continuum of clonal proliferations of myeloma cells. In plasmacytoma, the process is localized, as opposed to multiple myeloma in which there is a systemic involvement. Plasmacytoma is believed to be a precursor of MM, with an average time of conversion of 5 years. It presents in the 5th–7th decade of life.
Plasmacytoma and multiple myeloma have a predilection for the sternum due to the persistence of hematopoietic marrow until late adulthood. The lesions are lytic and may be solitary or multiple (Figure 4). Contrary to myeloma lesions occurring elsewhere, sternal myeloma may lack the characteristic sharply demarcated, non-sclerotic, punched-out margins. The lesions are usually expansile and cause cortical ballooning and destruction with the presence of lobulated, soft tissue component lacking any matrix mineralization. Distinguishing this imaging appearance from lytic expansile metastasis of renal and thyroid malignancies remains a dilemma to the radiologist, as cortical ballooning and destruction make imaging differentiation from the metastases difficult. Associated extraosseous, soft tissue component is usually scant, multilobulated and lacks any mineralization. It may show intrathoracic/mediastinal invasion [3,4,9,10].
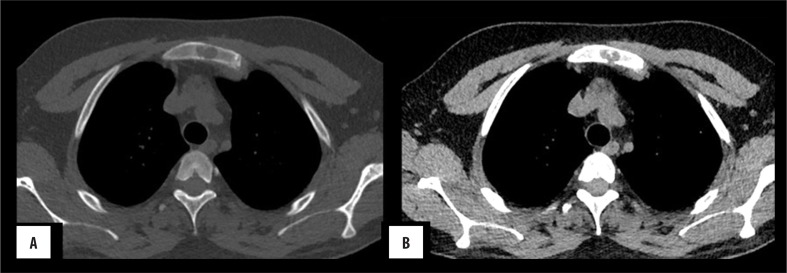
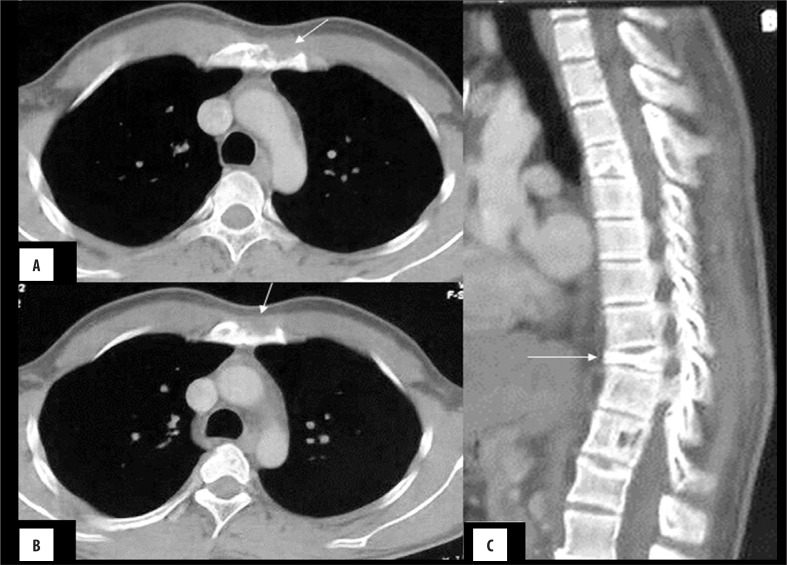
Figure 4.
Sternal plasmacytoma: (A) Axial bone window images showing a well-defined intramedullary lytic lesion in the body of the sternum. (B) Corresponding soft tissue window shows absence of soft tissue component.
Lymphoma
Haematological malignancies such as lymphoma may involve the sternum, usually secondary to the systemic involvement. They generally manifest in the 5th–8th decade of life. Sternal involvement is usually seen in Non-Hodgkin’s lymphoma with a very small number of cases of Hodgkin’s lymphoma reported so far.
There may be solitary or multiple lesions that may be lytic, sclerotic or mixed (Figure 5). Soft tissue component may be present [11].
Figure 5.
(A–C) Sternal lymphoma: Case of primary bone lymphoma with few mixed lytic-sclerotic lesions showing sclerotic margins (arrow) involving the manubrium.
Metastases
Metastases are the most common sternal neoplasms. Depending on the primary neoplastic site, both lytic and sclerotic deposits may occur, portending an unfavourable prognosis. Common primary tumours that may metastasize to the sternum include breast, lung, renal and thyroid malignancies. Sclerotic metastases, as in the prostatic carcinoma, need to be differentiated from enostosis (bone island). Bone island has sharply delineated margins. In dubious cases, uptake on scintigraphy favours metastases. Of the thyroid malignancies, follicular carcinoma has a propensity for the skeletal metastases in its haematogenous dissemination. Uncommonly, de-differentiated neoplasms or anaplastic thyroid carcinoma (Figures 6, 7) may metastasize to the sternum either haematogeneously or due to the contiguous spread.
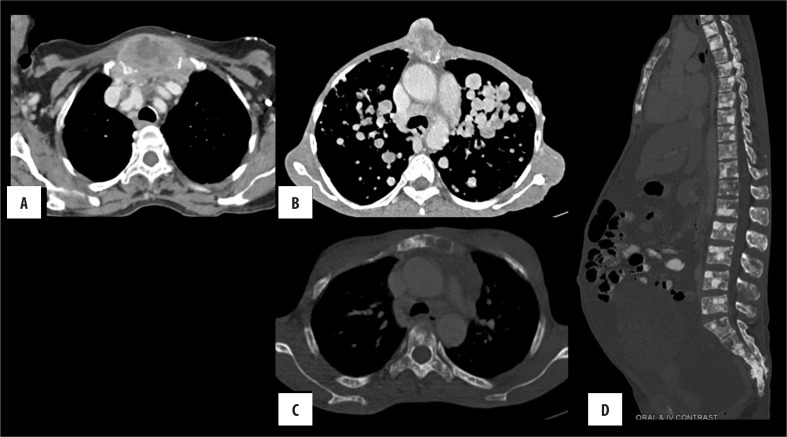
Figure 6.
Sternal metastasis (A) Anaplastic thyroid carcinoma (B) soft tissue sarcoma (C, D), carcinoma of the breast.
Figure 7.
(A, B) Neuroblastoma metastases: Left-sided thoracic neuroblastoma in a 5-year-old child. Bone window shows multiple sclerotic lesions in the sternum and vertebrae.
Chest wall is an important site for renal carcinoma metastases. Classically, renal and thyroid metastases present as pulsatile swellings. CT shows a lytic, expansile lesion causing cortical ballooning and thinning with associated enhancing soft tissue component attributed to its high vascularity. Other neoplasms, that may show direct sternal invasion, include mediastinal tumours such as thymic malignancies, lymphomas, etc. [1,2,4,12,13].
Benign tumours
The sternum is an uncommon location for benign tumours with a substantially lower incidence as compared to malignant tumours. Potentially, any benign tumour can occur, but common lesions include osteochondroma, enchondroma, haemangioma, haemangiopericytoma, fibrous dysplasia, osteoid osteoma and Langerhan’s cell histiocytosis (Figure 8).
Figure 8.
LCH (A, B): An 8-year-old male with a lytic lesion (arrow) in the sternum causing cortical breach with scant soft tissue lacking any matrix mineralization (C); Collapse of D10 vertebra was an ancillary finding suggesting the diagnosis along with skin lesions (not shown).
Haemangioma
Haemangioma is a hamartomatous vascular malformation occurring commonly in soft tissues. Osseous haemangioma is an extremely rare occurrence and usually involves the skull and vertebra.
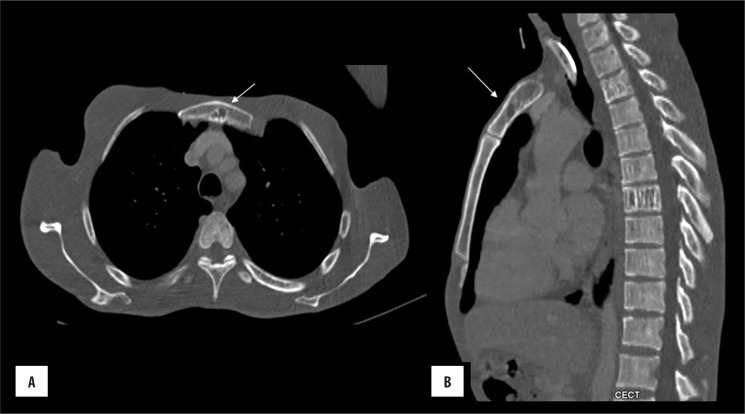
On CT, sternal haemangioma appears as a sharply demarcated, lucent lesion with thickened trabeculae coursing from its centre and giving rise to the “spoke wheel or honey comb appearance” (Figure 9). In the presence of characteristic imaging appearance, the lesion is otherwise a “don’t touch” skeletal lesion and hence, biopsy can be obviated [1,3,14].
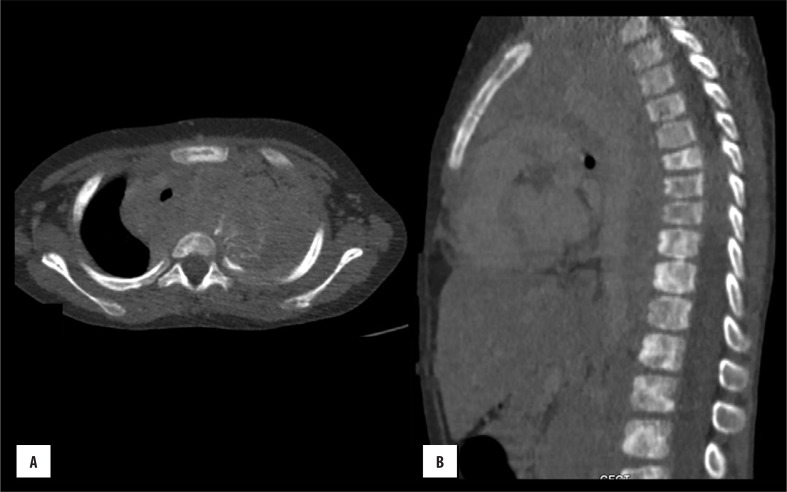
Figure 9.
Sternal haemangioma: (A) axial and (B) sagittal bone window images show lytic lesion in the sternum with a few coarse trabeculae within the centre of lesion.
Sternal Tumours – Perspective of the Surgeon
As for other bone malignancies, wide local excision is the treatment of choice for sternal malignancies in the absence of distant spread. However, because of the rib anchorage for cosmetic reasons, reconstruction is an important consideration. Even with widespread disease, tumour debulking is done to relieve the mediastinal compression and pain.
Conclusions
Sternal neoplasms are extremely rare; however, awareness of the imaging findings is of utmost importance. The neoplasms may be life-threatening not only due to their metastasizing potential, but also due to potential mediastinal compression that can result in sudden death. Imaging characteristics of these neoplasms parallel those of the lesions occurring in the extremities, with a certain distinction (Table 2). Imaging diagnosis is based on a multitude of factors including patient age, pattern of bone destruction, matrix mineralization all of which may help arrive at the diagnosis in certain cases.
Table 2.
Distinguishing features of sternal neoplasm.
| Neoplasm | Demography | Imaging findings | Difference from the extremity neoplasm |
|---|---|---|---|
| 1. Chondrosarcoma | > 40 years male predominance | Lobulated soft tissue mass with “ring and arc” calcification s/o chondroid matrix in peripheral distribution |
|
| 2. Osteosarcoma | Elderly | Lytic, sclerotic or mixed pattern |
|
| 3. Plasmacytoma | 5th–7th decade | Lytic expansile lesion | |
| 4. Ewing’s sarcoma | Adolescence |
|
|
| 5. Lymphoma | Permeative or moth eaten bone destruction with a large soft tissue component |
References
- 1.Restrepo CS, Martinez S, Lemos DF, et al. Imaging appearances of the sternum and sternoclavicular joints. Radiographics. 2009;29(3):839–59. doi: 10.1148/rg.293055136. [DOI] [PubMed] [Google Scholar]
- 2.Downey RJ, Huvos AG, Martini N. Primary andsecondary malignancies of the sternum. Semin Thorac Cardiovasc Surg. 1999;11:293–96. doi: 10.1016/s1043-0679(99)70071-7. [DOI] [PubMed] [Google Scholar]
- 3.Franquet T, Giménez A, Alegret X, et al. Imaging findings of sternal abnormalities. Eur Radiol. 1997;7(4):492–97. doi: 10.1007/s003300050190. [DOI] [PubMed] [Google Scholar]
- 4.O’Sullivan P, O’Dwyer H, Flint J, et al. Malignant chest wall neoplasms of bone and cartilage: a pictorial review of CT and MR findings. Br J Radiol. 2007;80(956):678–84. doi: 10.1259/bjr/82228585. [DOI] [PubMed] [Google Scholar]
- 5.Tateishi U, Gladish GW, Kusumoto M, et al. Chest wall tumors: Radiologic findings and pathologic correlation: part 2. Malignant tumors. Radiographics. 2003;23(6):1491–508. doi: 10.1148/rg.236015527. [DOI] [PubMed] [Google Scholar]
- 6.Capps E, Shiller SM, Cheek S, et al. Chest wall chondrosarcoma. Proc (Bayl Univ Med Cent) 2009;22(4):362–65. doi: 10.1080/08998280.2009.11928558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rad MP, Fattahi Masoum SH, Layegh P, Rad MS. Primary osteosarcoma of the sternum: A case report and review of the literature. Arch Bone Jt Surg. 2014;2(4):272–75. [PMC free article] [PubMed] [Google Scholar]
- 8.Pratt CB, Meyer WH, Rao BN, et al. Comparison of primary osteosarcoma of flat bones with secondary osteosarcoma of any site. Cancer. 1997;80(6):1171–77. [PubMed] [Google Scholar]
- 9.Lee JH, Lee WS, Kim YH, Kim JD. Solitary plasmacytoma of the sternum. Korean J Thorac Cardiovasc Surg. 2013;46(6):482–85. doi: 10.5090/kjtcs.2013.46.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J, Li Y, Wu W, et al. Solitary plasmacytoma of the sternum with a spiculated periosteal reaction: A case report. Oncol Lett. 2015;9(1):191–94. doi: 10.3892/ol.2014.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahin A, Meteroglu F, Eren S, Keles AN. A case of lymphoma simulating primary sternal tumour. APSP J Case Rep. 2014;5(1):2. [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhu M, Raju N, Kannan S, Vijayaraghavan RL. Sternal metastasis from dedifferentiated thyroid cancer: Value of 18F-fluorodeoxyglucose positron emission tomography – computed tomography imaging in thyroglobulin elevated negative iodine scan syndrome. Thyroid Res Pract. 2016;13:30–32. [Google Scholar]
- 13.Sabih Q, Spafford MF, Dietl CA. Poorly differentiated thyroid carcinoma with sternal invasion. A case report and review of the literature. Int J Surg Case Rep. 2014;5(11):816–20. doi: 10.1016/j.ijscr.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boker SM, Cullen GM, Swank M, Just JF. Hemangioma of sternum. Case report 593. Skeletal Radiol. 1990;19(1):77–78. doi: 10.1007/BF00197937. [DOI] [PubMed] [Google Scholar]