Summary
Fahr syndrome is a rare neurodegenerative disorder characterized by symmetrical, bilateral calcifications in the basal ganglia, nucleus gyrus and cerebral cortex. The continuous advancement as well as widespread use of brain imaging have contributed to the increasing detection rates of such changes. Nevertheless, their etiology is understood only partially and the methods of causative treatment are limited. Due to various symptoms, Fahr syndrome may resemble diseases from the field of neurology, psychiatry, cardiology and even urology. This article provides an up-to-date review of the literature concerning Fahr syndrome in terms of clinical practice.
Keywords: Basal Ganglia Diseases, Calcinosis, Tomography, X-Ray Computed
Background
In 1930, a German pathologist, Karl Theodor Fahr, described a case of a man with symmetrical calcifications of the basal ganglia and cerebral cortex [1]. The syndrome, named after this author, manifests with a wide range of neurological as well as psychiatric symptoms. The pathogenesis and clinical presentation of Fahr syndrome, known also as bilateral striatopallidodentate calcinosis (BSPDC) or Chavany-Brunhes syndrome, are partially understood, but there is still a lot to be discovered [2].
Etiology
Fahr syndrome is characterized histologically by foci of symmetrical, non-atheromatic compounds embedded in a protein-polysaccharide complex that are located within the globus pallidus, striatum, dentate nucleus, basal ganglia as well as within white and gray matter of the brain and cerebellum [2]. The deposits consist of calcium, zinc, iron, aluminum, magnesium, silicon, copper and phosphorus [3]. There are significant differences in the distribution of calcifications between individual patients. This may indicate the different mechanisms of their formation. The location within the brain structures as well as contact with blood vessel are the most important factors determining the chemical composition of the deposits. On this basis, two types of mineralization were described, non-vascular and perivascular [4].
Some reports describe the inheritance of Fahr syndrome, mainly in an autosomal dominant way. So far, four genes have been proved to be related to primary familial brain calcification; namely, SLC20A2, PDGFRB, PDGFB and XPR1 [5–12]. However, in the majority of patients, the syndrome does not have a genetic background. Bilateral basal ganglia calcifications can be observed in disorders of calcium and phosphorus metabolism, especially in hypoparathyroidism. However, the frequency of their occurrence is low [13,14]. Ku1czycki et al. distinguished the following three types of biochemical abnormalities of calcium-phosphate homeostasis:
True hypoparathyroidism (low calcium and high phosphate serum concentration, increased cAMP and phosphate excretion in urine after stimulation with parathyroid hormone);
Pseudohypoparathyroidism (low calcium and high phosphate serum concentration, no increase in the excretion of cAMP and phosphate in urine after stimulation with parathyroid hormone);
Calcifications co-existing with normal serum calcium and phosphate levels, a significant increase in the excretion of cAMP after stimulation with parathyroid hormone and its relatively low phosphaturic effect [15,16].
According to Pronicka et al., resistance to parathyroid hormone may play a key role in some individuals with Fahr’s syndrome. It is manifested by a decreased phosphaturic effect of this hormone, while cAMP excretion in urine remains within a normal range. Interestingly, this response can be improved by beta-blockers, suggesting that Fahr’s syndrome may be a result of a defect of adrenergic receptors and their impaired relationship with parathyroid hormone receptors [17]. Another biochemical abnormality observed in Fahr’s syndrome is an increased alkaline phosphatase activity in the basal ganglia of the brain, while its level in serum remains normal. These changes may promote a precipitation of insoluble calcium phosphate salts in the nervous tissue [18]. In up to one-third of patients with Fahr’s syndrome, a close relationship between calcifications and impaired cerebral blood flow can be demonstrated [19]. Such patients present cerebral circulatory disturbances in the form of transient ischemic attacks or strokes. Perivascular positioning of calcifications may suggest aberrant function of adventitia that could predispose to blood-brain barrier damage and deposition of calcium compounds. A major argument in favor of a vascular theory was given by the studies using brain flow scintigraphy that provided evidence of an impaired blood flow in calcification sites [20]. Moreover, inflammatory conditions, e.g. meningitis or encephalitis, are taken into account when considering the pathogenesis of Fahr’s syndrome [21]. Coexistence of Fahr’s syndrome with lupus erythematosus, monoclonal gammopathy and brain tumors, such as astrocytoma or pineal body gangliocytoma, was described in some case reports [22–25]. A significant role in the discussed processes may be attributed to homocarnosine, a specific antioxidant dipeptide found in the central nervous system. It is believed that the inhibition of calcification formation within subcortical structures is a task of homocarnosine [2].
The presented data do not encompass all clinical situations leading to the formation of intracranial calcification. Calcifications occur more often in elderly patients and may be symptoms of many diseases such as toxoplasmosis, cytomegalovirus, syphilis, tuberculosis, torulopsis, cysticercosis, tuberous sclerosis and others [26,27]. It is worth mentioning that calcifications are quite commonly found in clinical practice and may be encountered as physiological calcifications in the pineal gland or choroid plexuses as well as on the walls of blood vessels.
Clinical Presentation
Fahr’s syndrome has an insidious, slow and progressive course. The symptoms usually occur between the fourth and sixth decade of life, but they have been also reported in children as well as in young adults [28]. The clinical presentation varies depending on the time of onset. Thus, three forms of the syndrome were distinguished:
Early childhood onset – characterized by inhibition of mental development and early mortality;
Early onset (approximately at the age of 30) – characterized by psychiatric symptoms;
Late onset (approximately at the age of 50) – characterized by progressive dementia and movement disorders [29–32].
The most common symptoms of Fahr’s syndrome in adults include parkinsonism, dystonia, ataxia, chorea and extrapyramidal syndromes. Some patients may present with seizures and pyramidal disorders [33,34]. Psychiatric symptomatology typically includes cognitive impairment, schizophrenia-like psychosis, hallucinations, delusions, anxiety, mania, depression, hyposchemazia as well as personality changes [35–38]. Headache, vertigo, stroke-like events, orthostatic hypotension, dysarthria, paresis, overactive bladder and syncope of unknown etiology have also been reported [39–41]. In children, Fahr’s syndrome may take the form of severe encephalopathy with dwarfism, microcephaly and optic nerve atrophy [28]. The patients who are under our observation complained of memory impairment, dyskinesia, recurrent loss of consciousness and urinary incontinence. It should be emphasized that only a small percentage of people with intracranial calcifications are symptomatic. In most cases, it is an accidental discovery without any clinical implications.
Diagnosis
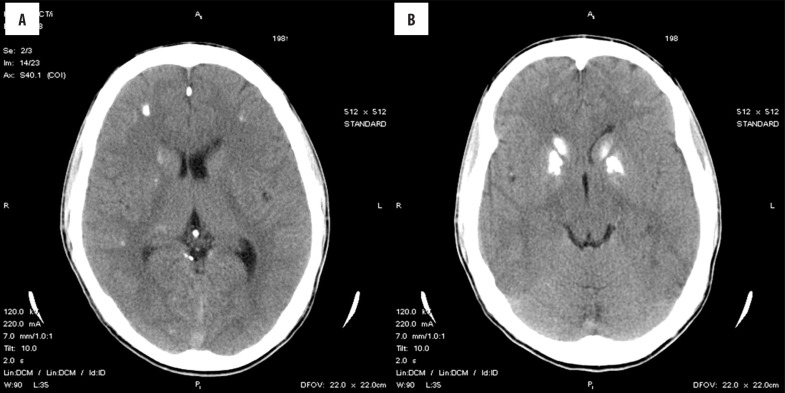
Although the first radiographic descriptions appeared more than 50 years ago, a broad availability of computed tomography (CT) was a cornerstone for the detection of intracranial calcifications and increased the number of diagnosed Fahr’s syndrome cases [42]. Currently, CT is the most valuable method that surpasses magnetic resonance imaging (MRI) (Figure 1A, 1B) [43,44]. Toscano et. al. suggested that transcranial sonography can be a useful tool in imaging basal ganglia calcinosis [45]. Differential diagnosis is hampered significantly by the lack of precise criteria. In that respect, it is important to detect calcification foci greater than 800 mm2 in surface area, regardless of their number [46]. Electroencephalography has a low diagnostic value, because all kinds of alterations in the central electrophysiological activity are possible [47]. Thus, neurological and psychological examinations as well as CT imaging remain the basic techniques for the diagnosis of Fahr’s syndrome.
Figure 1.
Bilateral calcifications in the basal ganglia and cerebellum found on computed tomography.
The following diagnostic criteria were established:
Bilateral calcifications of the basal ganglia or other areas of brain on neuroimaging (may not apply to patients from families with Fahr’s syndrome);
Progressive neurological dysfunction and/or psychiatric symptoms;
Onset between the ages of 40–50;
Absence of biochemical abnormalities and somatic states suggestive of a metabolic or mitochondrial disease;
Absence of toxic, infectious or traumatic causes of intracranial calcifications;
Positive family history of Fahr’ syndrome and/or proved genetic background.
If a patient meets the last criterion, the diagnosis can be made without the presence of one of the first two criteria. In cases of a negative or unknown family history, other five criteria must be fulfilled [48–51]. Not all intracranial calcifications point towards Fahr’s syndrome. Calcium deposits with a thin, linear or cloudy pattern have a high-specificity. On the other hand symmetric micronodular or asymmetric unilateral calcifications are not characteristic of this disease [51].
Treatment
In most cases, treatment is symptomatic and includes antipsychotics, antidepressants, antiepileptic and procognitive drugs. A distinctive feature of parkinsonian syndromes with concomitant calcifications is an outstanding resistance to the therapy with levodopa. This disorder more likely results from insensitivity of postsynaptic striatal structures rather than presynaptic damage observed in the primary parkinsonism [52].
Conclusions
Fahr’s syndrome has a rare radiological presentation of bilateral calcifications in the basal ganglia, nucleus gyrus and cerebral cortex. It may coexist with many symptoms resembling the diseases from the field of neurology, psychiatry, cardiology as well as urology. From a practical point of view, the most important issue is to differentiate the symptomatic and asymptomatic cases of Fahr’s syndrome and to detect abnormalities of calcium metabolism, primarily hypoparathyroidism, because their correction is the only causative treatment that can significantly improve symptoms and prognosis.
References
- 1.Fahr T. Idiopathische Verkalkung der Hirngefässe. Zentralbl Allg Pathol. 1930;50:129–33. [in German] [Google Scholar]
- 2.Manyam BV, Bhatt MH, Moore WD, et al. Bilateral striopallidodentate calcinosis: Cerebrospinal fluid, imaging and electrophysiological studies. Ann Neurol. 1992;31:379–84. doi: 10.1002/ana.410310406. [DOI] [PubMed] [Google Scholar]
- 3.Kuran W, Kozik M, Ku1czycki J. Analiza laserowa złogów rzekomowapniowych w zespole Fahra. Neurol Neurochir Pol. 1981;4:397–401. [in Polish] [PubMed] [Google Scholar]
- 4.Bouras C, Giannakopoulous P, Good PF, et al. A laser microprobe mass analysis of trace elements in brain mineralisations and capillaries in Fahr’s disease. Acta Neuropathol. 1996;92:351–57. doi: 10.1007/s004010050529. [DOI] [PubMed] [Google Scholar]
- 5.Arts FA, Velghe AI, Stevens M, et al. Idiopathic basal ganglia calcification-associated PDGFRB mutations impair the receptor signalling. J Cell Mol Med. 2015;19:239–48. doi: 10.1111/jcmm.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen WJ, Yao XP, Zhang QJ, et al. Novel SLC20A2 mutations identified in southern Chinese patients with idiopathic basal ganglia calcification. Gene. 2013;529:159–62. doi: 10.1016/j.gene.2013.07.071. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi T, Legati A, Nishikawa T, Coppola G. First Japanese family with primary familial brain calcification due to a mutation in the PDGFB gene: An exome analysis study. Psychiatry Clin Neurosci. 2015;69:77–83. doi: 10.1111/pcn.12238. [DOI] [PubMed] [Google Scholar]
- 8.Lemos RR, Ramos ME, Legati A, et al. Update and mutational analysis of SLC20A2: A major cause of primary familial brain calcification. Hum Mutat. 2015;36(5):489–95. doi: 10.1002/humu.22778. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira JR, Spiteri E, Sobrido MJ, et al. Genetic heterogeneity in familial idiopathic basal ganglia calcification (Fahr disease) Neurology. 2004;63:2165–67. doi: 10.1212/01.wnl.0000145601.88274.88. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Contreras M, Baker MC, Finch NA, et al. Genetic screening and functional characterization of PDGFRB mutations associated with basal ganglia calcification of unknown etiology. Hum Mutat. 2014;35:964–71. doi: 10.1002/humu.22582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taglia I, Mignarri A, Olgiati S, et al. Primary familial brain calcification: Genetic analysis and clinical spectrum. Mov Disord. 2014;29:1691–95. doi: 10.1002/mds.26053. [DOI] [PubMed] [Google Scholar]
- 12.Anheim M, López-Sánchez U, Giovannini D, et al. XPR1 mutations are a rare cause of primary familial brain calcification. J Neurol. 2016;263(8):1559–64. doi: 10.1007/s00415-016-8166-4. [DOI] [PubMed] [Google Scholar]
- 13.Koller WC, Cochran JW, Klawans HL. Calcification of the basal ganglia. Computerized tomography and c1inical correlation. Neurology. 1979;29:328–33. doi: 10.1212/wnl.29.3.328. [DOI] [PubMed] [Google Scholar]
- 14.Puvamendram K, Low CH, Boey HK, Tan KP. Basal ganglia calcification on computer tomographic scan. Acta Neurol Scand. 1982;66:309–15. doi: 10.1111/j.1600-0404.1982.tb06850.x. [DOI] [PubMed] [Google Scholar]
- 15.Kulczycki J, Pronicka E, Rowińska E, et al. Udział przytarczyc w patogenezie zwapnień śródmózgowiowych. Neurol Neurochir Pol. 1987;2:112–18. [in Polish] [PubMed] [Google Scholar]
- 16.el Maghraoui A, Birouk N, Zaim A, et al. Fahr syndrome and dysparathyroidism. 3 cases. Presse Med. 1995;24:1301–4. [PubMed] [Google Scholar]
- 17.Pronicka E, Kulczycki J, Rowińska E, Kuran W. Abolished phosphaturic response to parathormone in adult patients with Fahr’s disease and its restoration after propranolol administration. J Neurol. 1988;235:185–87. doi: 10.1007/BF00314315. [DOI] [PubMed] [Google Scholar]
- 18.Brannan TS, Burger AA, Chaudhary MY. Bilateral basal ganglia calcifications visualised on CT scan. J Neurol Neurosurg Psychiatry. 1980;43:403–6. doi: 10.1136/jnnp.43.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartecki BF, Kamieniowski J. Zwalniające niedokrwienie. Ogniskowe w przypadkach choroby Fahra. Neurol Neurochir Pol. 1979;4:443–47. [in Polish] [PubMed] [Google Scholar]
- 20.Uygur GA, Lin Y, Hellman RS, Tikofsky RS. Evaluation of regional cerebral blood flow in massive intracerebral calcification. J Nucl Med. 1995;36:610–12. [PubMed] [Google Scholar]
- 21.Bobek J, Nowak M. Familial form of Fahr syndrome (report of two cases) Neurol Neurochir Pol. 2000;34:167–75. [PubMed] [Google Scholar]
- 22.Ang LC, Rozdilsky B, Alport EC, Tchang S. Fahr’s disease associated with astrocytic proliferation and astrocytoma. Surg Neurol. 1993;39:365–69. doi: 10.1016/0090-3019(93)90201-b. [DOI] [PubMed] [Google Scholar]
- 23.Chang RS, Chun-Yin LW, Leong HS. Bilateral striatopallidodentate calcinosis associated with systemic lupus erythematosus: Case report and review of literature. J Neurol Sci. 2015:358(1–2): 518–19. doi: 10.1016/j.jns.2015.09.373. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama K. [A case of idiopathic, symmetrical naoarteriosc1erotic intracerebral calcification (Fahr’s disease) associated with M-proteinemia, followed by multiple myeloma.] Rinshko Shihkeigaku. 1991;31:781–89. [in Japanese] [PubMed] [Google Scholar]
- 25.Toroto K, Chiba Y, Ohtani T, et al. Pineal ganglioma in a patient with familial basal ganglia calcification and elevated serum alpha-fetoprotein; Case report. Neurosurgery. 1993;33:506–11. doi: 10.1227/00006123-199309000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Yamada M, Asano T, Okamoto K, et al. High frequency of calcification in basal ganglia on brain computed tomography images in Japanese older adults. Geriatr Gerontol Int. 2013;13:706–10. doi: 10.1111/ggi.12004. [DOI] [PubMed] [Google Scholar]
- 27.Ziąber J, Zientarski B, Bogusławska-Staniaszczyk R. Zwapnienia jąder podstawy i móżdżku. Neurol Neurochir Pol. 1993;27:721–28. [in Polish] [PubMed] [Google Scholar]
- 28.Billard C. Encephalopathy with calcifications of the basal ganglia in children. A reappraisal of Fahr syndrome with respect to 14 new cases. Neuropediatrics. 1989;20:12–15. doi: 10.1055/s-2008-1071258. [DOI] [PubMed] [Google Scholar]
- 29.Babbit DP, Tang T, Dobbs J. Idiopathic familiar cerebrovascular ferrocalcinosis (Fahr disease) and review of differential diagnosis of intracranial calcification in children. Am J Roentgenol Radium Ther Nuc Med. 1969;105:352–58. doi: 10.2214/ajr.105.2.352. [DOI] [PubMed] [Google Scholar]
- 30.Cummings JL, Gosendfeld LF, Houlihan JP. Neuropsychiatric disturbances associated with idiopathic calcification of the basal ganglia. Biol Psychiatry. 1983;18:591–601. [PubMed] [Google Scholar]
- 31.Francis AF. Familial basal ganglia calcification and schizophreniform psychosis. Br J Psychiatry. 1979;13:360–62. doi: 10.1192/bjp.135.4.360. [DOI] [PubMed] [Google Scholar]
- 32.Modrego PJ, Mojonero J, Serrano M, Fayed N. Fahr’s syndrome presenting with pure and progressive presenile dementia. Neurol Sci. 2005;26:367–69. doi: 10.1007/s10072-005-0493-7. [DOI] [PubMed] [Google Scholar]
- 33.Hoque MA, Siddiqui MR, Arafat Y, et al. Fahr’s disease: A very rare cause of epilepsy. Mymensingh Med J. 2010;19:127–29. [PubMed] [Google Scholar]
- 34.Matsui K, Yamada M, Kobayashi T, et al. [An autopsy case of Fahr disease (infantile form).] No To Hattatsu. 1992;24:358–63. [in Japanese] [PubMed] [Google Scholar]
- 35.Casamassima F, Lattanzi L, Perlis RH, et al. Efficacy of electroconvulsive therapy in Fahr disease associated with bipolar psychotic disorder: A case report. J ECT. 2009;25:213–15. doi: 10.1097/YCT.0b013e3181914d28. [DOI] [PubMed] [Google Scholar]
- 36.Munir K. The treatment of psychotic symptoms in Fahr’s disease with lithium carbonate. J Clin Psychopharmacol. 1986;1:35–38. [PubMed] [Google Scholar]
- 37.Wertele J, Rybakowski J. Przypadek Choroby Fahra u pacjentki z objawami depresji endogennej. Psychiatro Pol. 1988;4:341–43. [in Polish] [PubMed] [Google Scholar]
- 38.Seidler GH. Psychiatric and psychological aspects of Fahr syndrome. Psychiatr Prax. 1985;12:203–5. [PubMed] [Google Scholar]
- 39.Mufaddel AA, Al-Hassani GA. Familial idiopathic basal ganglia calcification (Fahr’s disease) Neurosciences (Riyadh) 2014;19:171–77. [PMC free article] [PubMed] [Google Scholar]
- 40.Simone O, Tortorella C, Antonaci G, Antonaci S. [An unusual case of transient loss of consciousness: the Fahr’s syndrome] Recenti Prog Med. 2008;99:93–96. [in Italian] [PubMed] [Google Scholar]
- 41.Tuglu D, Yuvanç E, Bal F, et al. Fahr syndrome unknown complication: Overactive bladder. Case Rep Urol. 2014;2014:939268. doi: 10.1155/2014/939268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virchow R. Kark-Metastasen. Virchows Arch Pathol Anat. 1955;8:103–13. [Google Scholar]
- 43.Kozic D, Todorovic-Djilas L, Semnic R, et al. MR imaging – an unreliable and potentially misleading diagnostic modality in patients with intracerebral calcium depositions. Case report. Neuro Endocrinol Lett. 2009;30:553–57. [PubMed] [Google Scholar]
- 44.Sahin N, Solak A, Genc B, Kulu U. Fahr disease: Use of susceptibility-weighted imaging for diagnostic dilemma with magnetic resonance imaging. Quant Imaging Med Surg. 2015;5(4):628–32. doi: 10.3978/j.issn.2223-4292.2015.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toscano M, Canevelli M, Giacomelli E, et al. Transcranial sonography of basal ganglia calcifications in Fahr disease. J Ultrasound Med. 2011;30:1032–33. doi: 10.7863/jum.2011.30.7.1032. [DOI] [PubMed] [Google Scholar]
- 46.Awrachami E, Cohn DF, Feibel M, Tadmor R. MRI demonstration and CT correlation of the brain in patients with idiopathic intracerebral calcification. J Neurol. 1994;241:381–84. doi: 10.1007/BF02033355. [DOI] [PubMed] [Google Scholar]
- 47.Schmid H, Haller R, König P. [Value of EEG in parathyroid gland disorders and/or symmetrical calcinosis of the basal ganglia (Fahr syndrome) Review of the literature with personal cases.] Wien Klin Wochenschr. 1986;98:486–90. [in German] [PubMed] [Google Scholar]
- 48.Moskowitz MA, Winickoff RN, Heinz ER. Familial calcification of the basal ganglions: a metabolic and genetic study. N Engl J Med. 1971;285(2):72–77. doi: 10.1056/NEJM197107082850202. [DOI] [PubMed] [Google Scholar]
- 49.Ellie E, Julien J, Ferrer X. Familial idiopathic striopallidodentate calcifications. Neurology. 1989;39(3):381–85. doi: 10.1212/wnl.39.3.381. [DOI] [PubMed] [Google Scholar]
- 50.Manyam BV. What is and what is not ‘Fahr’s disease’. Parkinsonism Relat Disord. 2005;11(2):73–80. doi: 10.1016/j.parkreldis.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Lazăr M, Ion DA, Streinu-Cercel A, Bădărău AI. Fahr’s syndrome: Diagnosis issues in patients with unknown family history of disease. Rom J Morphol Embryol. 2009;50(3):425–28. [PubMed] [Google Scholar]
- 52.Klawans HL, Lupton M, Simon L. Calcification of the basal ganglia as a cause of levodopa-resistant parkinsonism. Neurology. 1976;22:221–25. doi: 10.1212/wnl.26.3.221. [DOI] [PubMed] [Google Scholar]