Summary
Background
The aim of this study was to describe the gray-scale and color Doppler ultrasonography (US) and magnetic resonance (MR) imaging features of testicular adrenal rest tumors (TART) in patients with congenital adrenal hyperplasia.
Material/Methods
Forty-one patients with congenital adrenal hyperplasia were evaluated by gray-scale and color Doppler ultrasonography. Totally eighteen adrenal rest tumors in 9 patients were diagnosed TART on US and MR imaging. Gray-scale and color Doppler US and MR findings of the patients were documented.
Results
A total of eighteen masses were evaluated in nine patients. The mean age of these patients was 14.3±4.5 (range 10.1–23.3) years. US revealed hypoechoic lesions around the mediastinum testis with hypervascularity dispersing in ten patients and hypovascularity in two patients. In six patients, the lesions were hyperechoic with poor vascularity. Lesions exhibited homogeneous (n=8) and heterogeneous (n=10). Testicular microlithiasis was present in 4 of 9 patients with TART. Doppler ultrasound showed normal testicular vessels passing through the mass which were undisturbed, not displaced and not change in caliber. MRI features were the following: all lesions were hypointense on T2- and hyperintense (n=12) and isointense (n=6) on T1-weighted images. All masses revealed homogeneous contrast enhancement on postcontrast T1-weighted images.
Conclusions
Ultrasonography and MRI are good methods for detecting and monitoring TART. US is the first preferable modality because it is quick and cheap than MRI. Bilateral mostly hypoechoic lesions depicted around the mediastinum testis with no mass effect is highly suggestive for the diagnosis of testicular adrenal rest tissues on ultrasonography. Normal testicular vessels coursing through the lesions undisturbed and not change in caliber is described specific for this kind of tumors.
Keywords: Magnetic Resonance Imaging; Testicular Diseases; Ultrasonography, Doppler, Color
Background
Congenital adrenal hyperplasia (CAH) is an autosomal recessive disorder that affects steroid synthesis in the adrenal gland. More than 90% of CAH cases results from deficiency of 21-Hydroxylase (21-OHD) [1,2]. One of the most important and frequently detected complications in male patients with CAH are testicular tumors [3–5]. Testicular adrenal rest tumors (TART) are thought to arise from aberrant adrenal cells in the testes; TARTs are always benign and mostly bilateral [1–5]. A careful microscopic examination shows that adrenal rest cells are present in the testicles of all male patients with CAH [6]. To date, the exact etiology of the tumors is not completely understood. However, the main accepted hypothesis for the development of TART points towards chronically elevated adrenocorticotropic hormone (ACTH) concentrations [7]. The presence of TART may lead to obstruction of the seminiferous tubules, with irreversible damage to the testicular tissue leading to infertility in adult male patients [7]. Other ectopic adrenocortical tissue locations due to hyperplasia of adrenal remnants in patients with CAH include the spermatic cord, celiac plexus, broad ligaments, liver, normal fetal ovaries, and spinal canal [7,8].
The reported prevalence of TARTs varies between 0 and 94%, depending on the selection of patients (age, hormonal control) and the method of tumor detection (palpation, ultrasound or magnetic resonance imaging) [9]. Usually, only tumors larger than 2 cm are detectable by palpation because of their location within the rete testis. The tumors can be easily missed, when imaging techniques are not performed.
Testicular ultrasonography (US) is accepted as the best modality for imaging of adrenal rest tumors in patients with CAH. However, magnetic resonance (MR) imaging has been increasingly used to detect adrenal rest tumors as well. Therefore, we wanted to describe the gray-scale and color Doppler US and MR imaging characteristics of TARTs in patients with CAH.
Material and Methods
Forty-one patients with CAH were analyzed retrospectively in this study (between 2010 and 2015). The diagnosis of CAH was based on clinical and laboratory data. Thirty-one of these patients were diagnosed as 21-OHD, and the remaining 10 patients had 11-hydroxylase deficiency. Mutation analysis was performed in only 21 patients. The mean age of all patients was 12.1±4.7 years (range3.5 to 23.3 years). Twenty-eight patients (68.3%) were pubertal. The metabolic control of the patients was classified as good or poor. Metabolic control was accepted as good when the serum 17-hydroxyprogesterone (17-OHP) level was <30.3nmol/l, serum androstenedione (AS) concentrations were in the normal range for pubertal stages, and plasma renin activity was within the normal range for age in most analyses throughout follow-up. The patients with poor control were noncompliant; their clinic visits were irregular, and they had high hormone levels (17-OHP >30.3nmol/l, high AS for pubertal stage) at most follow-up analyses.
Testicular ultrasonography was performed as part of the endocrine control protocol that is used in our Pediatric Endocrinology Clinic for patients with CAH or to follow up patients with known TARTs. MRI was performed in nine patients who were diagnosed with TART on gray-scale and color Doppler US.
Ultrasonography was performed by two experienced radiologists, with a 12-MHz linear-array transducer (Logiq 9 RTD5 system; GE Healthcare). After obtaining standard images, testicles were evaluated for size and echogenicity. Masses in testicles were assessed for size, echotexture, definition of shape and margins, and presence of microcalcification. The lesions were classified as hyperechoic/ hypoechoic and homogenous/heterogeneous. Color Doppler US was performed if a testicular mass was detected by gray-scale US. The vascularity of the normal testicular tissue was compared with that of the mass. The vascularization of the mass was classified as hypo- or hypervascular, by comparing it with vascularity of the normal testes. MR imaging studies were performed on a 1.5-T scanner (Symphony; Siemens Healthcare), with the use of single surface coils. All images were obtained in 3-mm section thickness. The image matrix was 180×256 mm, and the FOV was 240-mm. In all 9 patients, we obtained sagittal and axial spin-echo T1-weighted images, and sagittal, coronal, and axial fast spin-echo T2-weighted images with and without fat suppression. T1-weighted images were obtained after an injection of gadolinium (0.1 mmol/kg).
Eight out of nine patients who were diagnosed with TART on MR imaging and US were followed-up with ultrasound annually; controls were evaluated and compared with initial findings after four years. One patient was lost to follow-up. Follow-up gray-scale and color Doppler US examinations were performed again, using the same device and methods, by the same two radiologists. This study was approved by the local ethical committee.
Statistical analyses
Statistical analyses were carried out with SPSS (version 12.0). The results are presented as medians (minimummaximum) or as percentages. The Mann-Whitney U tests were used for the statistical analyses. P value lower than 0.05 was accepted as significant.
Results
Thirty patients (73.2%) had good clinical control, and 11 patients (26.8%) had poor clinical control. Of the nine patients (21.9%), who had a diagnosis of TART, six had good metabolic control and three had poor metabolic control. The mean age of these patients was 14.3±4.5 (range 10.1–23.3) years and was not different from that of the patients without TARTs (11.4±4.5 years; p=0.09). Seven patients with TART were younger than 18 years. Six patients were 21-OHD, and 3 patients were 11-OHD.
All 9 cases were bilateral, and there were 18 lesions in total. The following US features of tumors were observed: twelve masses were hypoechoic, six were hyperechoic, eight were homogenous, and ten were heterogeneous. All lesions were sharply delineated from the normal parenchyma. The shapes of the lesions were round to oval (oval in five and round in four patients), ranging in diameter from 3 mm to 1.5 cm. The location was near the mediastinum testis or testicular hilum. The mass effect was excluded by revealing that the vessel traversing through the lesions was not displaced by the lesions (Figure 1). Color Doppler US revealed that ten masses were hypervascular and eight were hypovascular. Doppler ultrasound depicted slight hypervascularity dispersing homogeneously within the lesions, and showed normal testicular vessels passing through the mass that were undisturbed, not displaced, and with no change in caliber (Figure 2). On scrotal US, varicoceles were detected in 6 pubertal patients (5 unilateral left-sided, 1 bilateral). One patient had both varicocele and TART. Four patients with TARTs also had testicular microlithiasis.
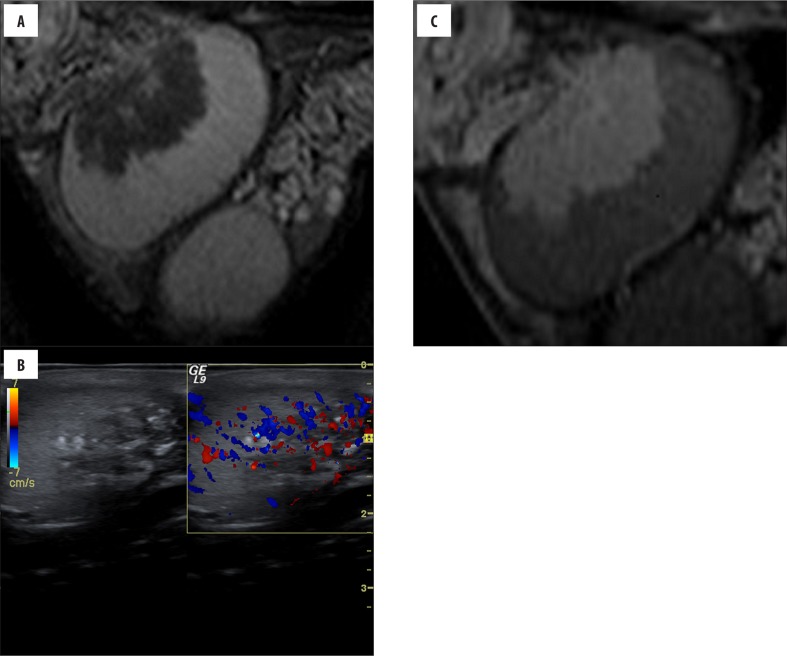
Figure 1.
(Case 4) Asymptomatic, 23-year-old boy with CAH, ultrasound performed 2 years before was normal. Ultrasonography (A) shows a hypervascular heterogeneous lesion located around the mediastinum testis. Also, coarse calcifications and fibrous septation are seen. Sagittal (B) T2-weighted image shows an intra-testicular mass that is hypointense, as compared with normal testicular tissue. Sagittal postcontrast (C) T1-weighted image reveals a marked enhancement that is greater than that of normal parenchyma.
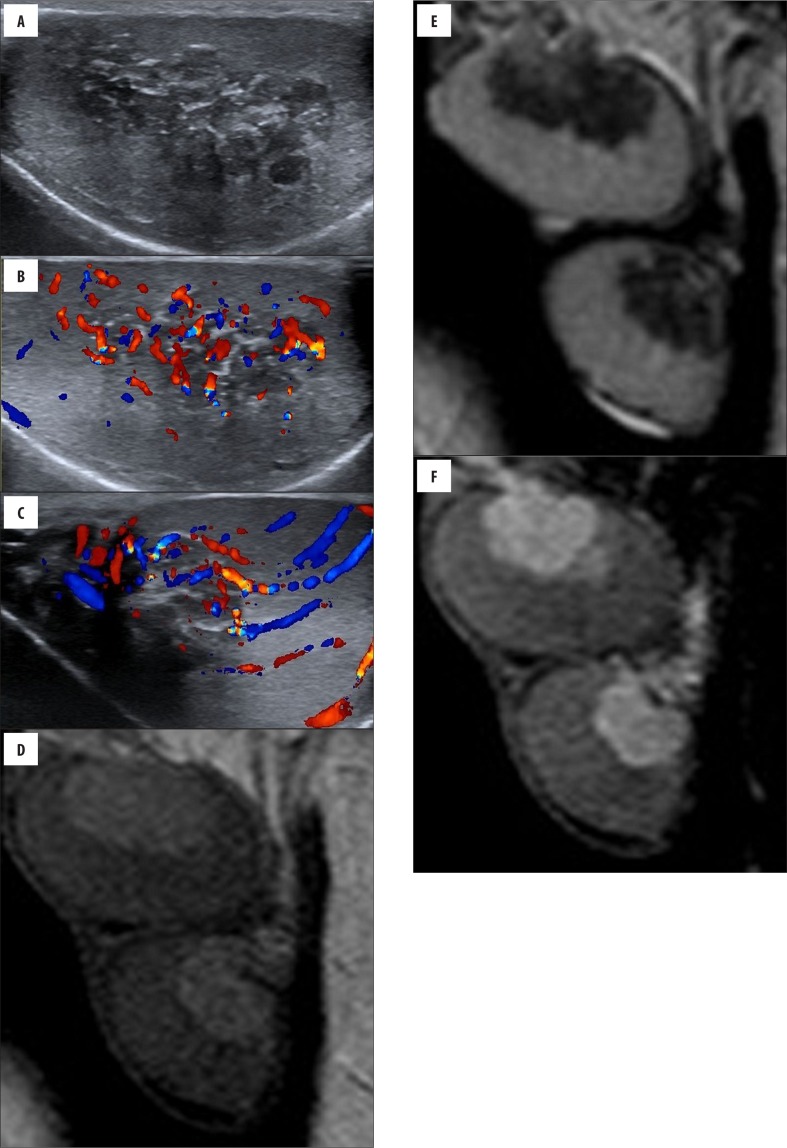
Figure 2.
(Case 9) Bilateral palpable testicular masses in a 13-year-old boy with CAH. Longitudinal (A, B) gray-scale and color Doppler sonogram images of the left testicle show hypervascular heterogeneous hypoechoic lesions within mediastinum testis. Color Doppler US (C) of the right testicle shows normal testicular vessels coursing undisturbed through the mass; the vessels are not displaced and have a normal caliber. Sagittal (D) T2-weighted MRI shows intra-testicular masses that are hypointense, as compared with normal testicular tissue. Sagittal (E) pre-contrast T1-weighted image shows bilateral testicular lesions that are slightly hyperintense. Sagittal (F) postcontrast T1-weighted image reveals marked enhancement in the masses that is greater than that of normal parenchyma.
At the end of US follow-up, after four years, the masses disappeared in 3 patients, TARTs had smaller sizes in 2 patients, and there was no change in size in 3 patients. US features of adrenal rest tissue and the number of lesions were similar to baseline evaluations in five patients. The treating endocrinologists considered as stable, over the period of four years, 2 patients with smaller size TARTs and 3 patients with stable-size TARTs. Regression of tumors in three patients, as compared to baseline US, was related to the normalization of hormone levels.
The following MR imaging features of adrenal rest tissue were observed: the lesions were isointense (n=6) or slightly hyperintense (n=12) in comparison to the testicular parenchyma on T1-weighted images. All lesions were slightly hypointense relative to the testicular parenchyma, without evidence of a capsule or pseudocapsule formation on T2-weighted images. In all lesions, contrast-enhanced images revealed homogeneous contrast enhancement that was more pronounced than that of normal testicular parenchyma (Figure 3). The imaging findings of TARTs are shown in Table 1.
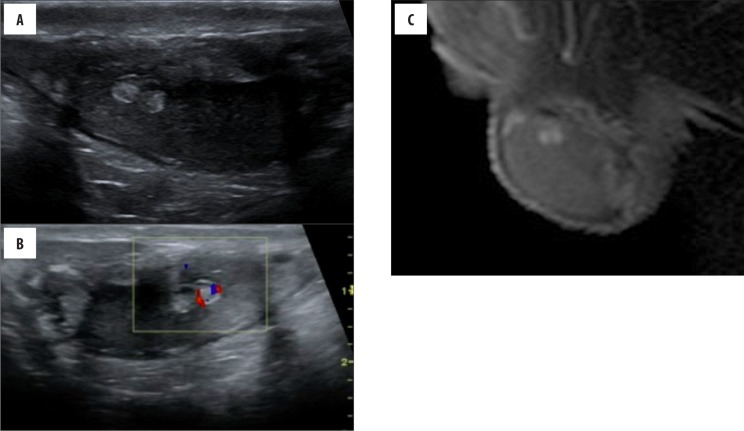
Figure 3.
(Case 2) A 12-year-old boy with CAH. Longitudinal sonogram (A, B) of the testis reveals echogenic lesions in the superior part of the parenchyma, with a relatively low vascularity. Sagittal (C) postcontrast T1-weighted images show marked contrast enhancement of TART.
Table 1.
The imaging findings of TART.
| Cases | Age Years |
Type | Metabolic control | Size, length. (mm) | MarginShape | US, appearance | Presence of calcification | Doppler US | Control US, size | T1-weighted | T2-weighted |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 21-OHD | Good | 8 | Sharp round | Hyperechoic Heterogeneous |
Positive | Hypervascular | No lesion | Hyperintense | Hypointense |
| 2 | 12.5 | 21-OHD | Good | 7 | Sharp oval | Hyperechoic Heterogeneous |
Positive | Hypovascular | Smaller, 4 mm | Isointense | Hypointense |
| 3 | 11.2 | 11-OHD | Good | 3 | Sharp round | Hyperechoic Homogenous |
Positive | Hypervascular | No lesion | Hyperintense | Hypointense |
| 4 | 23.3 | 11-OHD | Poor | 15 | Sharp round | Hyperechoic Heterogeneous |
Positive | Hypervascular | No change | Hyperintense | Hypointense |
| 5 | 10.5 | 21-OHD | Poor | 9 | Sharp oval | Hyperechoic Heterogeneous |
Negative | Hypovascular | No change | Isointense | Hypointense |
| 6 | 16.6 | 21-OHD | Poor | 12 | Sharp oval | Hyperechoic Heterogeneous |
Negative | Hypovascular | Smaller, 6 mm | Hyperintense | Hypointense |
| 7 | 14.8 | 11-OHD | Good | 9 | Sharp round | Hyperechoic Heterogeneous |
Negative | Hypervascular | No change | Hyperintense | Hypointense |
| 8 | 10.1 | 21-OHD | Good | 6 | Sharp oval | Hyperechoic Heterogeneous |
Negative | Hypovascular | No lesion | Hypointense | Hypointense |
| 9 | 13 | 21-OHD | Good | 13 | Sharp oval | Hyperechoic Heterogeneous |
Negative | Hypervascular | No control | Hyperintense | Hypointense |
Discussion
In patients with CAH, TARTs are thought to arise from residual nests of cells hypertrophy in the testes [1–5]. Studies have indicated that TARTs may be present in childhood, with an increasing prevalence after puberty [10]. In children, TARTs have a reported prevalence ranging from 18.3% to 29% [11–13]. In our study, the prevalence of TART (21.9%) was similar to the earlier reports. In patients with CAH, aberrant adrenal tissue may grow due to chronically elevated levels of ACTH or other unknown growth promoting factors. However, suppression of ACTH secretion with glucocorticoid treatment is not always successful in reducing tumor size, and TARTs may also be found in well-controlled patients [11]. In our study, metabolic control was good in 6 of 9 patients with TARTs. There is no pathological information for our patients, because a pathological examination is not required for the diagnosis of TART.
On ultrasonography, testicular adrenal rest tissue tumors (n=18) were hypoechoic or hyperechoic, were sharply delineated from testicular parenchyma, frequently had a heterogeneous echotexture, were located around the mediastinum testis without mass effect. Most of the masses were heterogeneous (n=10) in our study; but heterogeneous echogenicity might be caused by surgery, as reported in the literature [14]. Color Doppler US revealed that ten masses were hypervascular and showed normal testicular vessels passing through the mass (undisturbed, not displaced, with normal caliber). These findings are very important to differentiate adrenal rest tumors from other testicular masses that damage the surrounding vessels. In our study, six masses were hyperechoic with poor vascularity; however, a hypoechoic pattern is present in all such lesions, as reported in the literature [15]. Masses with a heterogeneous echotexture on Color Doppler US, as described above, should be evaluated as potential TARTs. In our study, on T2-weighted images, all lesions (n=18) were slightly hypointense relative to the testicular parenchyma, without evidence of capsule or pseudocapsule formation. Contrast-enhanced images revealed homogeneous contrast enhancement in all lesions that was more pronounced than that of the normal testicular parenchyma. These observations were similar to other reports [8,15–19]. We did not detect additional TARTs or any differences for the identified lesions on standard-protocol MRI. However, diffusion weighted imaging and apparent diffusion coefficient (ADC) values can be helpful when differentiating between normal, benign, and malignant scrotal lesions. Also, these sequences can improve detection and characterization of scrotal diseases, and hence can reduce unnecessary radical surgical procedures [20]. When planning the study, we did not include DWI and ADC, because we did not have enough patients diagnosed with malignant testicular masses or other benign diseases for comparison with TARTs.
Although not malignant, when longstanding, TARTs can lead to infertility via many mechanisms including impaired spermatogenesis, reduced gonadotrophin levels, and obstruction of the seminiferous tubules [9]. In our study, all patients, except for one, had normal gonadotropin and testosterone levels. That patient was non-compliant and had poor metabolic control. Four of our patients had testicular microlithiasis. Therefore, TARTs may increase the risk of testicular microlithiasis, but there is yet no evidence to support this supposition.
Conclusions
In conclusion, ultrasonography and MRI are good methods for detection and monitoring of TARTs. US is preferable, because it is quick, cheap, and can easily detect even very small adrenal rests that are a few millimeters in diameter As only basic MRI sequences were used to evaluate TARTs, we conclude that basic MRI does not add any value to US in the diagnosis of TARTs. In patients with a history of congenital adrenal hyperplasia, bilateral heterogeneous and hypoechoic lesions depicted on US around the mediastinum testis with no mass effect are highly suggestive of testicular adrenal rest tissues. In addition, normal testicular vessels coursing undisturbed through the lesions and with normal calibers are detected only in TARTs.
References
- 1.Speiser PW, White PC. Congenital adrenal hyperplasia. New Engl J Med. 2003;349:776–88. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 2.Forest MG. Recent advances in the diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Reprod Update. 2004;10:469–85. doi: 10.1093/humupd/dmh047. [DOI] [PubMed] [Google Scholar]
- 3.Howlett DC, Jones AJ, Saunders AJ. Painless testicular nodularity in a young man. Br J Radiol. 1997;70:1195–96. doi: 10.1259/bjr.70.839.9536916. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan JG, Gomel M, Kinder RB. Ectopic adrenocortical tissue found at groin exploration in children: Incidence in relation to diagnosis, age and sex. BJU Int. 2005;95:407–10. doi: 10.1111/j.1464-410X.2005.05310.x. [DOI] [PubMed] [Google Scholar]
- 5.Souverijns G, Peene P, Keuleers H, Vanbockrijck M. Ectopic localisation of adrenal cortex. Eur Radiol. 2000;10:1165–68. doi: 10.1007/s003309900263. [DOI] [PubMed] [Google Scholar]
- 6.Shanklin DR, Richardson AP Jr, Rothstein G. Testicular hilar nodules in adrenogenital syndrome. Am J Dis Child. 1963;106:243–50. doi: 10.1001/archpedi.1963.02080050245001. [DOI] [PubMed] [Google Scholar]
- 7.Claahsen-van der Grinten HL, Duthoi K, Otten BJ, et al. An adrenal rest tumour in the perirenal region in a patient with congenital adrenal hyperplasia due to congenital 3b-hydroxysteroid dehydrogenase. Eur J Endocrinol. 2008;159:489–91. doi: 10.1530/EJE-08-0311. [DOI] [PubMed] [Google Scholar]
- 8.Avila NA, Premkumar A, Merke DB. Testicular adrenal rest tissue in congenital adrenal hyperplasia: Comparison of MR imaging and sonographic findings. Am J Roentgenol. 1999;172:1003–6. doi: 10.2214/ajr.172.4.10587136. [DOI] [PubMed] [Google Scholar]
- 9.Claahsen-van der Grinten HL, Hermus AR, Otten BJ. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Int J Pediatric Endocrinology. 2009;2009:624823. doi: 10.1155/2009/624823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aycan Z, Bas VN, Cetinkaya S, et al. Prevalence and long-term follow-up outcomes of testicular adrenal rest tumours in children and adolescent males with congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 2013;78:667–72. doi: 10.1111/cen.12033. [DOI] [PubMed] [Google Scholar]
- 11.Stikkelbroeck NMML, Otten BJ, Pasic A, et al. High prevalence of testicular adrenal rest tumours, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86:5721–28. doi: 10.1210/jcem.86.12.8090. [DOI] [PubMed] [Google Scholar]
- 12.Cakir ED, Mutlu FS, Eren E, et al. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia. J Clin Res Pediatr Endocrinol. 2012;4:94–100. doi: 10.4274/jcrpe.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claahsen-van der Grinten HL, Sweep F, Blickman JG, et al. Prevalence of testicular adrenal rest tumors in male children with congenital adrenal hyperplasiadue to 21-hydroxylase deficiency. Eur J Endocrinol. 2007;157:339–44. doi: 10.1530/EJE-07-0201. [DOI] [PubMed] [Google Scholar]
- 14.Delfino M, Elia J, Imbrogno N, et al. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia: prevalence and sonographic, hormonal, and seminal characteristics. J Ultrasound Med. 2012;31:383–88. doi: 10.7863/jum.2012.31.3.383. [DOI] [PubMed] [Google Scholar]
- 15.Proto G, Di Donna A, Grimaldi F, et al. Bilateral testicular adrenal rest tissue in congenital adrenal hyperplasia: US and MR features. J Endocrinol Invest. 2001;24:529–31. doi: 10.1007/BF03343887. [DOI] [PubMed] [Google Scholar]
- 16.Wang ZI, Yang Z, Wang W, et al. Diagnosis of testicular adrenal rest tumors on ultrasound: A retrospective study of 15 cases report. Medicine. 2015;94:1471. doi: 10.1097/MD.0000000000001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitoz S, Atasoy C, Adiyaman P, et al. Testicular adrenal rests in a patient with congenital adrenal hyperplasia: US and MRI features. Comput Med Imaging Graph. 2006;30:465–68. doi: 10.1016/j.compmedimag.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad IC, Yilmaz TF, Kocakoç E. Doppler ultrasonography and magnetic resonance imaging findings of testicular adrenal rest tissue in a patient with 11 β hydroxilase deficiency. Medical Ultrasonography. 2014;16:3835. [PubMed] [Google Scholar]
- 19.Nagamine WH, Mehta SV, Vade A. Testicular adrenal rest tumors in a patient with congenital adrenal hyperplasia: Sonographic and magnetic resonance imaging findings. J Ultrasound Med. 2005;24:1717–20. doi: 10.7863/jum.2005.24.12.1717. [DOI] [PubMed] [Google Scholar]
- 20.Algebally AM, Tantawy HI, Yousef RR, et al. Advantage of adding diffusion weighted imaging to routine MRI examinations in the diagnostics of scrotal lesions. Pol J Radiol. 2015;80:442–49. doi: 10.12659/PJR.894399. [DOI] [PMC free article] [PubMed] [Google Scholar]