Abstract
Nipah virus is a zoonotic virus harbored by bats and lethal to humans. Bat-to-human spillovers occur every winter in Bangladesh. However, there is significant heterogeneity in the number of spillovers detected by district and year that remains unexplained. We analyzed data from all 57 spillovers during 2007–2013 and found that temperature differences explained 36% of the year-to-year variation in the total number of spillovers each winter and that distance to surveillance hospitals explained 45% of spatial heterogeneity. Interventions to prevent human infections may be most important during colder winters. Further work is needed to understand how dynamics of bat infections explains spillover risk.
Keywords: Nipah virus, Bangladesh, epidemiology, spatiotemporal spread
We explore the drivers of the spatiotemporal distribution of the highly lethal Nipah virus and demonstrate that year-to-year variability in the number of spillovers is linked to annual temperature differences and that spatial variability is associated with the distance to surveillance hospitals.
Nipah virus (NiV) is a bat-borne virus, lethal in humans, first identified in Malaysia in 1998 [1]. Pteropus bats are the natural reservoir host [2]. Since 2001, outbreaks have been observed nearly every year with mortality >70% [3]. In contrast to the Malaysian outbreak, where exposure to infected pigs was key, in Bangladesh the 2 major risk factors are person-to-person contact with a case and consumption of raw date palm sap [3–5]. Bats contaminate date palm sap by licking sap as it is collected in pots hanging in trees overnight [6]. Sap is typically collected early in the morning and sold and consumed within hours before it ferments [6]. Both human sap consumption and spillover events occur during the cooler and drier winter months [7].
Pteropus bats are found throughout Bangladesh [8]. However, spillovers are concentrated in the central and northwest region of the country. Bangladeshi villages where outbreaks have been observed are different from other communities in primarily one way: they have a higher proportion of residents who report drinking fresh date palm sap [8]. However, there are presumably many villages with relatively high date palm sap consumption, and we do not understand why outbreaks have been observed in some villages but not others. In addition, these community-level behaviors do not explain year-to-year variation in the number of outbreaks that are detected [3]. Weather, in particular, temperature and precipitation, may help determine some of these geographic and temporal differences. For example, we know that communities prefer to collect sap when the weather is coolest and the sap is sweet [7], so risk behavior could increase during colder winters. Since precipitation could spoil the sap being collected in open pots, increased precipitation could the reduce risk. In addition, NiV has been shown to survive longer in fruit juice at lower temperatures, suggesting that it may also survive longer in date palm sap at lower temperatures [9, 10]. Further, surveillance for NiV cases is centered on a network of 3 surveillance hospitals. Cases occurring farther away from surveillance hospitals may remain undetected [11]. The objective of this study was to describe the timing and location of observed spillover events in Bangladesh from a 7-year period (2007–2013) and to understand the factors associated with the risk of spillover over space and time.
METHODS
Spillover Events
In Bangladesh, cases have been identified through case-based surveillance at 3 tertiary care hospitals [12] since 2006 or through surveillance of media reports [13]. Most NiV infections cause severe disease with neurological symptoms [14]. All cases that present to surveillance hospitals with fever and signs of neurological illness during December through March are tested using an immunoglobulin M enzyme-linked immunosorbent assay to confirm NiV infection. Year-round surveillance of media reports for outbreaks covers all regions of the country. We defined a spillover event as the occurrence of the index case in every outbreak detected (ie, those presumably infected through contaminated date palm sap and not through person-to-person contact).
Our objective was to look at variation within the area where spillovers occur, so we restricted our analysis to districts where at least 1 spillover event was identified, as well as the districts located in between spillover districts, when the spillover districts were not contiguous (ie, the Nipah belt; Figure 1A). One case, in a resident of Dhaka, was excluded because it was linked to date palm sap acquired from elsewhere. Rural population estimates for each district were obtained from the 2011 census.
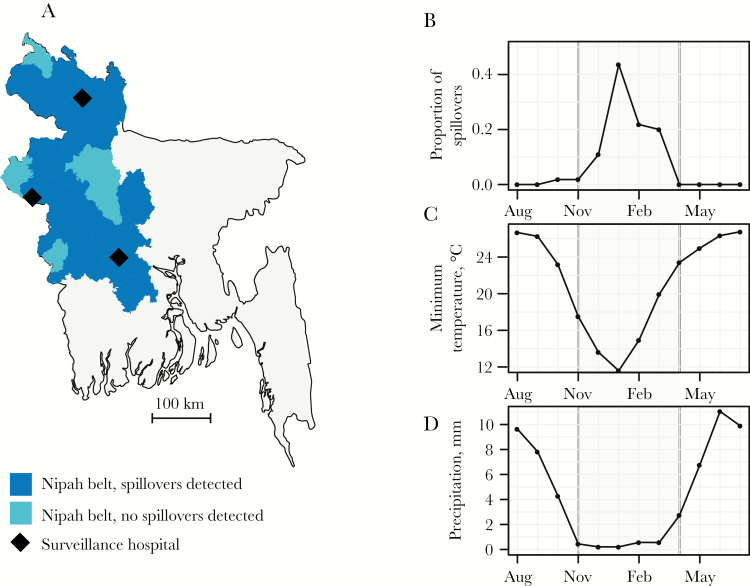
Figure 1.
A, District map of Bangladesh. B, Proportion of all observed spillover events by month. C and D, Monthly mean minimum temperature (C) and mean precipitation (D) for 10 districts, averaged for each month during 2007–2013.
Weather and Date Palm Tree Data
We obtained the mean temperature over 3-hour periods and daily rainfall data for 8 districts within the Nipah belt from 2007 to 2013 from the Bangladesh Meteorological Department. We estimated the mean temperature and precipitation for each week and month for each district. For each week, we also calculated the average daily minimum and maximum temperatures. For the 19 districts for which we did not have weather data available, we used data from the nearest district for which data were available. Because the number of date palm trees may also be an important driver of spillovers, we obtained counts of the number of date palm sap trees by district from the Bangladesh Bureau of Statistics.
Statistical Analysis
We used logistic regression to explore the factors associated with observing at least 1 spillover event for each district-week between 2007 and 2013. We fit a series of univariable logistic regression models in terms of explanatory factors that differed spatially (ie, rural population size, total number of date palm trees, and distance between the district’s centroid and the nearest surveillance hospital) and weather patterns that differed in both space and time (ie, temperature [mean, minimum, and maximum] and precipitation [mean, minimum, and maximum]). This approach allowed us to separately explore factors associated with the spatial heterogeneity and factors associated with temporal heterogeneity in spillover risk. Further, since the period of incubation for infection in humans is approximately 7–10 days [12, 14], for the explanatory variables that changed over time (ie, precipitation and temperature), we considered models that used values from the week before the illness onset of the index case, as well as models that used values from the same week of illness onset. We fitted polynomial splines for each model up to order 4 and selected the form with the lowest Akaike information criterion (AIC) as the best model.
Ethical Approval
Data used for this analysis were collected by studies approved by the ethical review board at icddr,b. All personal identifying information was delinked from the data before use.
RESULTS
Between January 2007 and December 2013, there were 57 spillovers detected in 20 different districts. Some districts only identified 1 spillover, while up to 7 spillovers were detected in others (Supplementary Figure 1). The number of spillover events detected each winter varied considerably. During the winter of 2008–2009, there were only 2 spillovers detected, but in the winter spanning 2012–2013 there were 18 (Supplementary Figure 1). January, when 40% of the spillover events occurred, was the month with the lowest mean temperature during every year of the study (Figure 1B and 1C).
Explaining Heterogeneities Across Districts
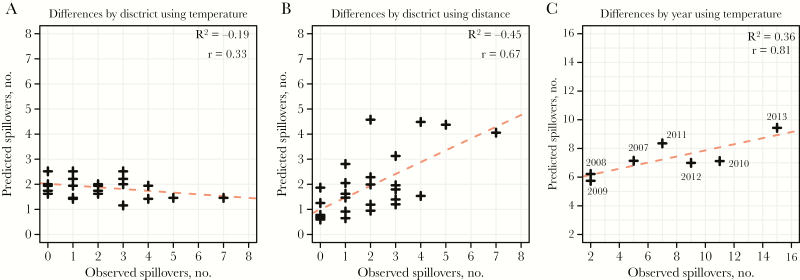
Temperature and precipitation were consistent across districts within any given month (Supplementary Figure 2). Across the 8 districts where climate data were available, there was an average standard deviation of only 0.6°C in mean weekly temperatures and an average standard deviation of 2.9 mm in weekly rainfall. These small differences in minimum temperature were not able to explain heterogeneities in the number of spillover events across districts (correlation, −0.33; P = .1; Figure 2A). Each 10-fold increase in the estimated number of date palm trees was associated with a 1.8-fold increase (95% confidence interval [CI], 1.1–2.9) in the odds of detecting a spillover event (Supplementary Table 1). The number of date palm trees explained only 8% of the variance in the number of spillover events (correlation, 0.28; P = .2). Each 10-km increase in the distance from a surveillance hospital was associated with a 0.78 reduction in the odds of detecting a spillover event (95% CI, .70–.87). The distance from the nearest surveillance hospital explained 45% of the variance in the number of spillover events per district (correlation, 0.67; P < .001; Figure 2B). Including the number of date palm trees per district in addition to the distance to a surveillance hospital in the same model did not improve model performance (P = .26, by the χ2 test). The size of the rural population in each district was not associated with the probability of a spillover (Supplementary Table 1).
Figure 2.
The number of spillover events predicted versus the number of spillover events observed. A, Per-district predictions from a univariate logistic regression model, based on the minimum temperature during the previous week . B, Per-district predictions from a univariate logistic regression model, based on distance to surveillance hospital. C, Per-year predictions from a univariate logistic regression model, using the minimum temperature during the previous week.
Explaining Heterogeneities in Spillover Risk Across Time
Low precipitation and low temperatures within any district-week were both strongly associated with an increased risk of a spillover event. Further, temperature and precipitation in the week prior to the spillover event were better predictors of events than temperature and precipitation during the week of the event, with AIC differences of 23 and 1, respectively (AIC differences of >10 are typically used as evidence of strong support of one model over another; Supplementary Table 1). Of all the weather factors considered, the minimum temperature in the week before a spillover event had the best model fit. We used this variable to predict the number of spillover events each year (Figure 2C). The observed and estimated number of spillover events were significantly correlated (correlation, 0.81; P = .03), and the model explained 36% of the variance in the number of spillover events per year.
Discussion
Understanding the drivers of heterogeneity in NiV spillovers in space and time may help optimize control efforts. We analyzed the spatiotemporal location of all spillovers over a 7-year period in Bangladesh. We found that small changes in temperature were highly correlated with differences in the probability of detecting a spillover. These temperature effects explained some of the differences in the number of outbreaks detected between years, with more outbreaks occurring in cooler years. These findings suggest that low temperatures are not simply a marker of winter, when date palm sap consumption occurs, but that small differences in temperature during this period result in differences in spillover risk. Mechanisms that could explain this observation include improved viral survivorship at colder temperatures [9] or increased sap harvest in colder winters; there is evidence of year-to-year differences in human sap consumption, although the cause is poorly understood [15]. Future studies that monitor changes in sap consumption over time are needed to help disentangle the contribution of these factors. While our models captured the rank order of the yearly number of spillover events (ie, they identified years with small numbers of spillovers versus years with large numbers; Figure 2C), the range of estimates (6–10 spillover events) was far smaller than that observed (3–15 spillovers). This suggests that other factors, such as epidemic dynamics in bat populations, or differences in human behaviors, such as sap consumption, may also help determine year-to-year variation.
As weekly temperatures were highly homogeneous across the districts at any time point, they could not explain spatial differences in spillover risk. However, the distance to the closest surveillance hospital was significantly correlated with where reported spillovers occurred and explained over half of the variability in the number of spillovers across districts. The NiV-endemic region represents a large area, with some communities >100 km from the nearest surveillance hospital. Infected individuals who reside far from surveillance hospitals may present to different hospitals or not make it to the surveillance hospitals before death, given that some patients die within 3–4 days after illness onset [14]. This suggests that many spillovers go undetected. Future efforts to quantify these would provide useful information for public health officials and could lead to insights about how to improve surveillance. Nevertheless, >40% of the spatial heterogeneity in the distribution of observed spillovers remained unexplained by the distance to the surveillance hospitals. Spatial differences in the frequency of sap consumption may contribute to these differences in risk [8].
Our analyses are aggregated at the district level, so we were unable to investigate variation at smaller spatial scales. We also only had weather data for a subset of all districts. However, both temperature and precipitation appeared highly homogeneous at any point across the districts where data was available. Also, owing to the nature of surveillance, we may have missed mild cases. However, the distribution of these cases is likely similar to that of severe cases, and therefore our estimates are probably not impacted by this potential limitation.
Understanding the risk for NiV spillover events is important for global public health, given the high case-fatality rates and the risk of more-successful human-to-human transmission following viral adaptations or after introductions of more highly transmissible strains. Our analysis shows that the risk of cross-species transmission from bats to humans across time and space in Bangladesh is partially affected by climate. NiV transmission dynamics in bats and the inability of surveillance to capture many spillover events may also explain some of spatio-temporal differences in where spillovers are observed. NiV cross-species transmission is complex, and a better understanding of transmission patterns in the reservoir host deserves further research. Policy makers could consider focusing prevention resources during the coldest weeks of the year, when the risk of NiV spillover is increased. Efforts to increase detection of spillover events could also be useful to reduce the risk of wider geographic spread of this lethal pathogen.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. icddr,b thanks the National Science Foundation, the National Institutes of Health, and the Centers for Disease Control and Prevention, for commitment to its research efforts; and the governments of Australia, Bangladesh, Canada, Sweden, and the United Kingdom, for providing core/unrestricted support.
Disclaimer. The donors had no role in preparation or review of this manuscript.
Financial support. This work was supported by the National Science Foundation/National Institute of Health (Ecology and Evolution of Infectious Diseases grant 2R01-TW005869) and the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
References
- 1. Chua KB, Bellini WJ, Rota PA et al. . Nipah virus: a recently emergent deadly paramyxovirus. Science 2000; 288:1432–5. [DOI] [PubMed] [Google Scholar]
- 2. Chua KB, Koh CL, Hooi PS et al. . Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect 2002; 4:145–51. [DOI] [PubMed] [Google Scholar]
- 3. Luby SP, Hossain MJ, Gurley ES et al. . Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg Infect Dis.2009; 15:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rahman MA, Hossain MJ, Sultana S et al. . Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis 2012; 12:65–72. [DOI] [PubMed] [Google Scholar]
- 5. Chakraborty A, Sazzad HMS, Hossain MJ et al. . Evolving epidemiology of Nipah virus infection in Bangladesh: evidence from outbreaks during 2010–2011. Epidemiol Infect 2016; 144:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan MS, Hossain J, Gurley ES, Nahar N, Sultana R, Luby SP. Use of infrared camera to understand bats’ access to date palm sap: implications for preventing Nipah virus transmission. Ecohealth 2010; 7:517–25. [DOI] [PubMed] [Google Scholar]
- 7. Nahar N, Sultana R, Gurley ES, Hossain MJ, Luby SP. Date palm sap collection: exploring opportunities to prevent Nipah transmission. Ecohealth 2010; 23:196–203. [DOI] [PubMed] [Google Scholar]
- 8. Gurley ES, Hegde S, Hossain K et al. . Convergence of humans, bats, trees. and culture in Nipah virus transmission, Bangladesh. Emerg Infect Dis 2017; 14:1446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fogarty R, Halpin K, Hyatt AD, Daszak P, Mungall BA. Henipavirus susceptibility to environmental variables. Virus Res 2008; 132:140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Wit E, Prescott J, Falzarano D et al. . Foodborne transmission of nipah virus in Syrian hamsters. PLoS Pathog 2014; 10:e1004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nikolay B, Salje H, Sturm-Ramirez K et al. . Evaluating hospital-based surveillance for outbreak detection in bangladesh: analysis of healthcare utilization data. PLoS Med 2017; 14:e1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naser AM, Hossain MJ, Sazzad HM et al. . Integrated cluster- and case-based surveillance for detecting stage III zoonotic pathogens: an example of Nipah virus surveillance in Bangladesh. Epidemiol Infect 2015; 143:1922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ao TT, Rahman M, Haque F et al. . Low-cost national media-based surveillance system for public health events, Bangladesh. Emerg Infect Dis 2016; 22:720–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hossain MJ, Gurley ES, Montgomery JM et al. . Clinical presentation of nipah virus infection in Bangladesh. Clin Infect Dis 2008; 46:977–84. [DOI] [PubMed] [Google Scholar]
- 15. Nahar N, Paul RC, Sultana R et al. . A controlled trial to reduce the risk of human nipah virus exposure in Bangladesh. Ecohealth 2017; 14:501–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.