Abstract
Background
Antenatal exposure to parasites can affect infants’ subsequent responses to vaccination. The present study investigated how maternal prenatal infections and newborns’ antiparasite cytokine profiles relate to immunoglobulin G (IgG) responses to standard vaccination during infancy.
Methods
A total of 450 Kenyan women were tested for parasitic infections during pregnancy. Their newborns’ responses to Plasmodium falciparum, schistosome, and filaria antigens were assessed in cord blood lymphocytes. Following standard neonatal vaccination, this infant cohort was followed biannually to age 30 months for measurement of circulating IgG levels against Haemophilus influenzae b (Hib), diphtheria toxoid (DT), hepatitis B virus (HBV), and tetanus toxoid.
Results
Trajectories of postvaccination IgG levels were classified by functional principal component (PC) analysis to assess each child’s response profile. Two main components, PC1, reflecting height of response over time, and PC2, reflecting crossover from high to low responses or from low to high responses, were identified. Cord blood cytokine responses to schistosome and filarial antigens showed a significant association between augmented antihelminth interleukin 10 and reduced antibody levels, particularly to DT and HBV, and a more rapid postvaccination decline in circulating IgG levels against Hib.
Conclusion
Antenatal sensitization to schistosomiasis or filariasis and related production of antiparasite interleukin 10 at birth are associated with reduced antivaccine IgG levels in infancy, with possibly impaired protection.
Keywords: Parasitic infection, prenatal exposure, cytokine balance, Th1-Th2, host parasite interaction, immunogenicity, vaccine
This study shows that antenatal sensitization to filariasis and schistosomiasis and associated antiparasite interleukin 10 release by cord blood lymphocytes at birth is associated with reduced antivaccine immunoglobulin G levels in early childhood that could lead to impaired protection.
Fetal existence is typically infection free until birth, and transplacental transfer of maternal antibodies provides the bulk of life-sustaining infant immunity immediately after birth. However, evidence now suggests that the newborn infant’s T and B cells are already active at the time of delivery and that these responses can be appreciably matured and patterned by antigen exposures that were experienced before birth. Furthermore, this patterning results in significant alteration in responses to neoantigens during infancy, including changes in response to routine childhood vaccines [1–3]. Our previous studies have shown that, when mothers carry chronic infections during pregnancy (such as those due to malaria parasites, filariae, schistosomes, or soil-transmitted helminths), infant immune responsiveness to these pathogens can already be established at the time of birth [4–7]. Such parasites excel at manipulation of their human host’s immune system to perpetuate their residence within the body. Studies of antigen-driven cord blood T-cell proliferation and cytokine responses indicate that newborns can exhibit sensitization or even tolerization (via regulatory T-cell immunomodulation) to these pathogens at delivery [4, 8–10]. Prenatal experience influences the acquisition of cellular and humoral immune responses that can alter the course of infection later in childhood [11–13]. We have shown that prenatal parasite exposures are associated with reduced responses to early life receipt of live vaccine (eg, bacille Calmette-Guerin against tuberculosis) [14], peptide antigen vaccine (eg, diphtheria toxoid [DT] vaccine), or peptide-conjugate vaccine (eg, Haemophilus influenza b [Hib] vaccine) [15].
Whether and how the prenatal immune response to parasite antigens in utero influences the vaccine response profiles in early childhood remains poorly understood. The present study investigated how prenatal infections and antiparasite cytokine profiles at birth relate to profiles of antibody responses to standard vaccination during infancy.
METHODS
Study Design and Study Participants
Healthy pregnant women and their offspring born at the Msambweni District Hospital on the south coast of Kenya were enrolled in this mother-child cohort study from 2006 to 2009. Pregnant women provided venous blood, urine, and stool specimens at their first antenatal clinic visit and again at delivery. For the mother-infant pairs, maternal venous blood, placental intervillous blood, and umbilical cord blood specimens were collected at delivery, as previously described [16]. Infant venous blood and urine and stool samples were collected beginning at 6 months of age and every 6 months thereafter until age 30 months. Plasma was stored at −80°C until antibody assays were performed. The cellular immune response at birth was performed on fresh cells. Infants received standardized immunizations provided by the Ministry of Health following established Kenya National Health Service guidelines. Pentavalent vaccine (composed of DT, tetanus toxoid [TT], whole-cell Bordetella pertussis, hepatitis B virus [HBV], and Hib vaccines) was given at 6, 10, and 14 weeks of age; oral trivalent polio was given at birth and 6, 10, and 14 weeks of age; and 1 dose of measles vaccine was given at 9 months.
Ethics
Approval for the study was obtained from the Kenya Medical Research Institute National Ethical Review Committee and from the Institutional Review Board for Human Studies at University Hospitals of Cleveland Case Medical Center. Mothers provided written informed consent for their participation and that of their infants.
Maternal and Infant Infection Status
Maternal venous blood specimens collected at enrollment, usually during the second or early third trimester, and at delivery, along with intervillous placental blood, cord blood, and infant venous blood specimens, were examined for malaria parasite infection status by light microscopy, and then DNA was extracted from blood and tested for the presence of Plasmodium falciparum by real-time quantitative polymerase chain reaction analysis [17]. Stool and urine specimens were examined for the presence of intestinal helminths and Schistosoma haematobium ova as described previously [14, 18, 19]. S. haematobium infection status was also assessed by performing an enzyme-linked immunosorbent assay (ELISA) to detect soluble worm antigen of S. haematobium (SWAP)–specific immunoglobulin G4 (IgG4) antibodies in collected plasma samples. Positive results of an assay that detects circulating filarial antigen in plasma samples (the Og4C3 assay; TropBioMed, Townsville, Australia) and/or an ELISA that measures Brugia malayi antigen (BMA)–specific IgG4 antibodies indicated lymphatic filariasis (LF) [14, 18].
Cord Blood Lymphocyte Cultures
Cord blood mononuclear cells (CBMC) were isolated from fresh cord blood specimens and were cultured in the presence of parasite antigens as follows. First, for malaria parasites, recombinant P. falciparum 44-kb C-terminal fragment of merozoite surface protein, P. falciparum phosphoriboprotein P0, and peptides corresponding to previously identified T-cell epitopes in the 83-kDa C-terminal fragment of MSP-1, designated P2 (GYRKPLDNIKDNVGKMEDYIKK; codons 250–71) and P3 (KLNSLNNPHNVLQNFSVFFNK; codons 1101–21), were used. Second, for schistosomes, SWAP was used. Third, for filariae, saline extracts of adult BMA were used as previously described [16, 20, 21]. Antigen concentrations were adjusted to levels in which no detectable antigen-driven cytokine response was observed in CBMCs from healthy North American newborns. The endotoxin concentration in these preparations was 0.5 ng/mL, which is 5–50-fold less than that required for lipopolysaccharide stimulation of cytokines from human lymphocytes. CBMCs were either left unstimulated or stimulated with the individual parasite antigens listed above. All culture supernatants were collected at 72 hours and immediately frozen at −80°C for storage, pending cytokine assays. Quantification of interferon γ (IFN-γ), interleukin 5 (IL-5), interleukin 13 (IL-13), interleukin 10 (IL-10), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) was performed on culture supernatants by the Luminex assay (BioRad). A positive CBMC response to malaria parasites, Schistosoma organisms, or filariae was defined as a cytokine response level at least 2 times greater than that seen when CBMCs were cultured in medium alone (background).
Measurement of Plasma IgG Levels in Response to Hib, DT, HBV, and TT Vaccinations
Response to vaccination was determined by standard ELISAs for determining IgG levels against TT, DT, HBV, and Hib as previously described [15].
Statistical Analysis
We classified each child’s CBMC cytokine responses to parasite antigen as either positive (defined as a cytokine level in the presence of antigen ≥2 times that in medium alone) or negative (defined as a cytokine level in the presence of antigen <2 times that in medium alone). Because multiple antigens were tested for malaria parasites, we defined a positive antimalarial response as response to at least 2 malaria parasite antigens. To investigate the association between the different anti–parasite-specific cytokine responses, we calculated the tetrachoric correlation for all pair-wise measurements [22] as a summary measure of association specifically designed for binary data. Values range from −1 to 1, with a similar interpretation to Pearson correlation findings.
To find patterns of variation within the longitudinal vaccine response data, we used a functional principal component (FPC) approach implemented in the R package fpca [23]. Each FPC represented a different dimension of variation in the longitudinal data. Because few individuals had >3 timed observations, we only estimated 2 FPCs for the response to each of the 4 vaccines studied. The antivaccine IgG response was log transformed for all analyses.
To determine whether the functional FPC scores for the different vaccines were correlated, we used a multivariate longitudinal random effects model with a random effect for each FPC of each of the 4 vaccines (ie, 8 total random effects). Loadings from the fpca R package were treated as known in the analysis to determine how each random effect would influence an observed value. We estimated the unstructured covariance matrix for all 8 FPCs. In the random effects model, a 4-degree-of-freedom spline [24] was used to model the shape of the mean vaccine response across time for each vaccine. The nlme package in R [25] was used to fit the model.
To discover whether predictors such as cord blood cytokine responses to malaria parasite, schistosome, or filarial antigens were associated with the vaccine response profiles, we used a multivariate hierarchical random effects model similar to that described above. However, in this model, we assumed that the cytokine response could influence the longitudinal outcome by influencing the FPC scores (ie, the cytokine response was used as a predictor). Omnibus tests were performed using the Wald test with both the naive and robust sandwich-based standard errors. To be conservative, we reported P values to be the maximum of the naive and sandwich-based estimates.
RESULTS
Characteristics of the Study Population
A total of 510 mother-newborn pairs were recruited between 2006 and 2009; of those, 406 children underwent informative cord blood testing, returned at least twice after delivery, and were included in the present analysis. For assessment of postvaccination antibody responses, study children were followed every 6 months up to 30 months of age (Supplementary Figure 1). Overall, 78.3% of the mothers had ≥1 of the following infections: P. falciparum malaria (27.6%), lymphatic filariasis (44.7%), Schistosoma haematobium infection (32.4%), and intestinal helminth infection (33.8%). A total of 21.7% of mothers had none of these infections. Prenatal polyparasitism was common among our maternal cohort; 1 infection was detected in 29.6% of women, 2 infections in 27.6%, 3 infections in 15.6%, 4 infections in 4.2%, and 5 infections in 1.3% [15].
Malarial, Schistosomal, and Filarial Antigen-Induced Cytokine Production by CBMC
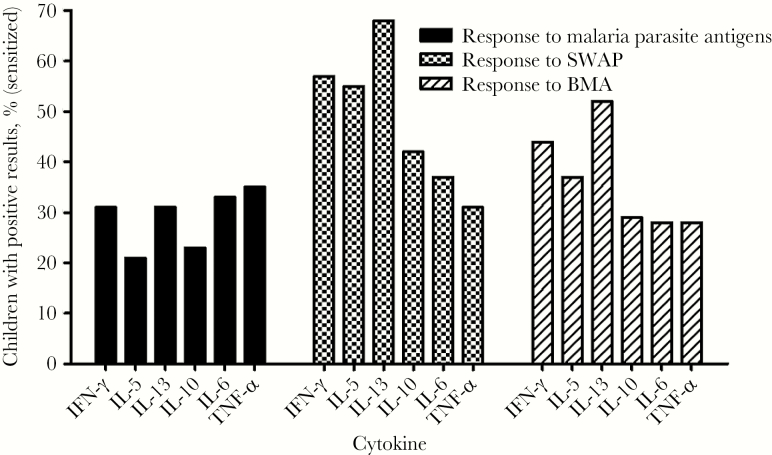
To determine the overall pattern of cytokine production to antigen-specific lymphocytes in newborns indicative of prenatal sensitization, CBMCs from Kenyan offspring of women with malaria, schistosomiasis, and/or filariasis were stimulated with malarial (n = 450), schistosomal (n = 426), and filarial antigens (n = 433) in vitro. As shown in Figure 1, the prevalence of newborn cytokine responses to the parasite antigens varied widely, indicating in utero immune exposure to the respective maternal infections. There was a strong correlation between schistosome (SWAP) and filaria (BMA) antigen- induced IL-5 and IFN-γ production by CBMCs, indicating a mixed T-helper type 1 (Th1) and Th2 cytokine response (Figure 2). Similarly, there was mixed Th1 and Th2 cytokine production in response to malaria parasite antigens, although the correlation was less pronounced.
Figure 1.
Frequency of significant cytokine production (interferon γ [IFN-γ], interleukin 10 [IL-10], interleukin 13 [IL-13], interleukin 5 [IL-5], tumor necrosis factor α [TNF-α], and interleukin 6 [IL-6]) by newborns’ cord blood mononuclear cells (CBMCs) induced by malaria parasites (black bars; n = 450), schistosomes (checked bars; n = 426), and filariae (striped bars; n = 433). CBMCs were cultured at 2 × 106 cells/mL and stimulated with the respective parasite antigens, then supernatants were collected after 72 hours for determination of cytokine levels as described in Methods. Cytokine responses were considered positive if the response was ≥2 times the background response of unstimulated cells. Values denote the percentage of children with a positive cytokine response after fetal exposure to each pathogen. For malaria parasites, the antigens were recombinant Plasmodium falciparum 44-kb C-terminal fragment of merozoite surface protein (MSP1-42), MSP1 peptide, and P. falciparum phosphoriboprotein P0 peptide; for schistosomes, soluble worm antigen of Schistosoma haematobium (SWAP); and for filariae, Brugia malayi antigen (BMA).
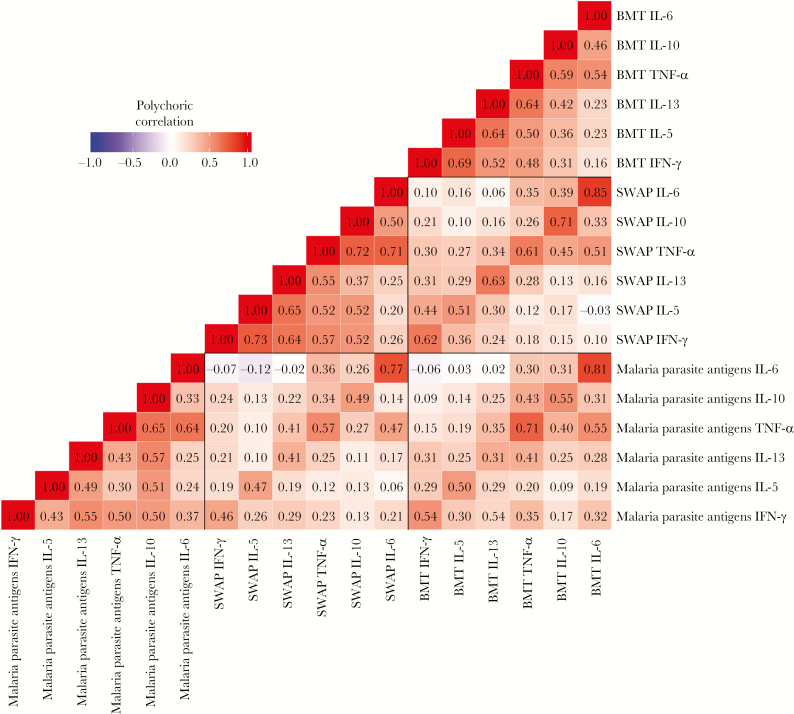
Figure 2.
Heat map of the polychoric correlation between net interferon γ (IFN-γ), interleukin 10 (IL-10), interleukin 13 (IL-13), interleukin 5 (IL-5), tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6) cytokine responses of cord blood mononuclear cells (CBMCs) to recombinant Plasmodium falciparum 44-kb C-terminal fragment of merozoite surface protein (MSP1-42), MSP1 peptide, and P. falciparum phosphoriboprotein P0 peptide; soluble worm antigen of Schistosoma haematobium (SWAP); and Brugia malayi antigen (BMA). CBMCs were cultured under conditions described in Figure 1. Values shown are correlation coefficients.
Correlation Analysis of Malarial, Schistosomal, and Filarial Antigen-Induced Cytokine Production by CBMCs
We further examined the intercorrelation of CBMC antigen-dependent augmentation in cytokine responses to parasite antigens (recombinant MSP142/peptides of MSP1, SWAP, and BMA). Although the different cytokines’ response rates varied widely, there was a moderate-to-strong correlation between responses to antigens of different parasites for the same cytokine, particularly IL-6 and TNF- α (Figure 2), and there were correlations between different cytokine responses to the same antigen. Correlation with the IL-10 response was noted for TNF-α, IFN-γ, IL5, and IL-13 for malaria parasite antigens, and correlation between IL10 and TNF-α, IFN-γ, and IL5 responses was observed for schistosome antigens.
We also observed significant levels of cytokine responses among unstimulated cells (medium controls). The concentration of cytokines spontaneously released by unstimulated cells was greatest for TNF-α and IL-6, followed by IL-10 and IL-13, whereas IFN-γ and IL-5 secretion in unstimulated cells was comparatively low. Pair-wise correlation of individual cytokine outputs was moderately strong: scatterplots of unstimulated and stimulated cytokine responses by CBMCs are shown in Supplementary Figure 2.
Pattern of Circulating Antivaccine IgG From 6 to 30 Months of Age
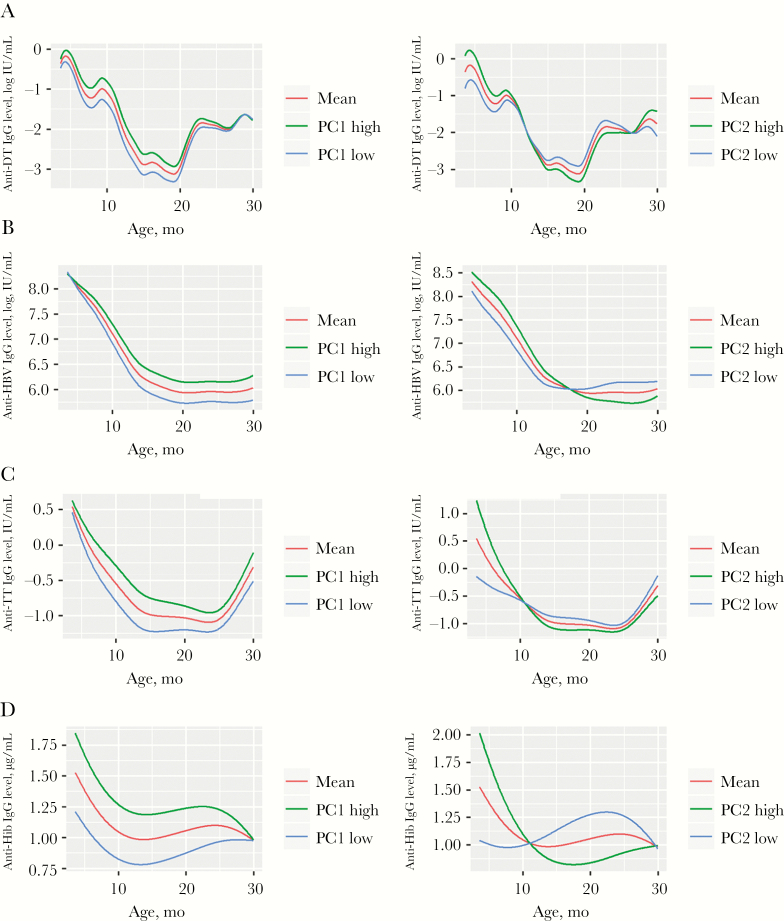
To summarize the complex individual dynamics of IgG from 6 to 30 months of age, the combined early life values over time were analyzed using functional PCA [26, 27], which assumes that each individual’s antivaccine IgG responses may have distinct trajectory patterns. This dimension reduction defined 2 quantitative components that could be used to evaluate each child’s pattern: PC1, reflecting the height of the response over time, and PC2, reflecting the tendency to crossover from a high to low response or from a low to high response (Figure 3). In a combination of PC1 and PC2 effects, antivaccine IgG levels could start out high after vaccination and remain high (high-high), they could start high and fall to much lower levels by age 30 months (high-low), they could start low and remain low (low-low), or they could start low and increase after 12 months of age.
Figure 3.
Patterns of circulating anti–diphtheria toxoid (DT; A), anti–hepatitis B virus (HBV; B), anti–tetanus toxoid (TT; C), and anti–Haemophilus influenzae (D) immunoglobulin G (IgG) in infants during ages 6–30 months. A functional principal component (PC) approach was used to find patterns of variation within the longitudinal vaccine responses. PC1 and PC2 represent functional principal components of the antivaccine IgG profile; PC1 (left panels) reflects the height of the response over time, and PC2 (right panels) reflects the tendency to crossover from high to low levels or from low to high levels of antivaccine antibody over time. IgG levels were measured by enzyme-linked immunosorbent assay. In each panel, the red line shows the mean value for all children; green and blue lines show the mean values for children with high (top quartile) and low (bottom quartile) PC values, respectively. Vaccinations were given at 6, 10, and 14 weeks of life, without further boosting, according to Kenya Ministry of Health guidelines.
Relationship of Prenatal Exposure to Schistosomiasis and Filariasis on the Trajectories of Individual Vaccine Responses
Table 1 shows the association between maternal schistosomiasis or filariasis during pregnancy and the dynamics of antibody responses to the different vaccines among infants during ages 6–30 months. These are associated with PC1 and PC2 values, using a multivariate longitudinal random effects model with a random effect for each FPC of each of the 4 vaccines (ie, 8 total random effects). Prenatal exposure to schistosomiasis significantly reduced PC1 to DT, corresponding to overall reduced anti-DT IgG response in affected children. Similarly, prenatal exposure to filariasis significantly decreased the antibody response pattern (PC1) to HBV and to DT. Prenatal exposure to filariasis was also significantly associated with greater PC2, indicating a greater tendency to crossover from high to low levels of IgG antibodies to Hib over time. No significant association was observed between prenatal malaria exposure and either the PC1 or PC2 components of vaccine responses.
Table 1.
Testing of the Association Between Prenatal Schistosoma haematobium or Filarial Exposure and the 2 Principal Components (PC1 and PC2) of the 6–30-Month Trajectories of Early Life Antivaccine Immunoglobulin G Responses
| Exposure, Outcome, PC | Estimate (95% CI) | P a |
|---|---|---|
| Schistosomiasis | ||
| H. influenzae | ||
| PC1 | 0.372 (−.88 to 1.63) | .5529 |
| PC2 | 0.776 (−.15 to 1.70) | .0945 |
| Tetanus toxoid | ||
| PC1 | −0.765 (−1.89 to .35) | .1718 |
| PC2 | −0.629 (−1.34 to .08) | .0781 |
| Hepatitis B virus | ||
| PC1 | 0.002 (−1.06 to 1.07) | .9965 |
| PC2 | 0.248 (−.28 to .78) | .3478 |
| Diphtheria toxoid | ||
| PC1 | −1.2 (−2.42 to .02) | .0485 |
| PC2 | 0.528 (−.22 to 1.27) | .1571 |
| Filariasis | ||
| H. influenzae | ||
| PC1 | −0.136 (−1.65 to 1.38) | .8574 |
| PC2 | 1.228 (.13 to 2.32) | .0251 |
| Tetanus toxoid | ||
| PC1 | −0.596 (−1.95 to .76) | .3799 |
| PC2 | −0.546 (−1.36 to .27) | .1819 |
| Hepatitis B virus | ||
| PC1 | −1.387 (−2.71 to −.06) | .0363 |
| PC2 | 0.389 (−.26 to 1.04) | .2339 |
| Diphtheria toxoid | ||
| PC1 | −3.597 (−5.00 to −2.19) | 3.1 × 10–7 |
| PC2 | 0.617 (−.24 to 1.47) | .1499 |
See Methods for a description of the PC analyses.
Abbreviations: CI, confidence interval; Hib, Haemophilus influenzae b.
aValues <.05 are considered statistically significant.
Cellular Immunological Responses at Birth as a Predictor of Overall Response to Vaccines in Childhood
The impact of maternal infection on vaccine responses will likely occur by in utero priming to parasite antigens that cross the placenta and create immune responses that persist into infancy, when children are first vaccinated. When we assessed the overall linkage of PC1 and PC2 to individual cytokine responsiveness to parasite antigens at birth, a highly significant association was observed for the neonatal presence of antischistosome and antifilaria IL-10 production (P = .007 and P < .0001, respectively; Table 2). By contrast, no association was observed for responses of other cytokine to helminth antigens or for any CBMC cytokine responses to malaria parasite antigens (Table 2).
Table 2.
Association Between Individual Cytokine Responses to Malaria Parasites, Schistosoma haematobium, or Filariae at Birth and the Principal Components of the 6–30-Month Trajectories of the Immunoglobulin G Response to Vaccines Given at Ages 6, 10, and 14 Weeks
| Antigen(s) | P a | |||
|---|---|---|---|---|
| IFN-γ | IL-5 | IL-13 | IL-10 | |
| Malaria parasite antigensb | .97 | .43 | .89 | .9 |
| SWAP | .36 | .57 | .17 | .007 |
| BMA | .9 | .05 | .57 | 8.93 × 10–6 |
Abbreviations: BMA, Brugia malayi antigen; IFN-γ, interferon γ; IL-5, interleukin 5; IL-10, interleukin 10; IL-13, interleukin 13; SWAP, soluble worm antigen of S. haematobium.
aValues <.05 are considered statistically significant.
bRecombinant Plasmodium falciparum 44-kb C-terminal fragment of merozoite surface protein peptide, MSP1, and P. falciparum phosphoriboprotein P0 peptide.
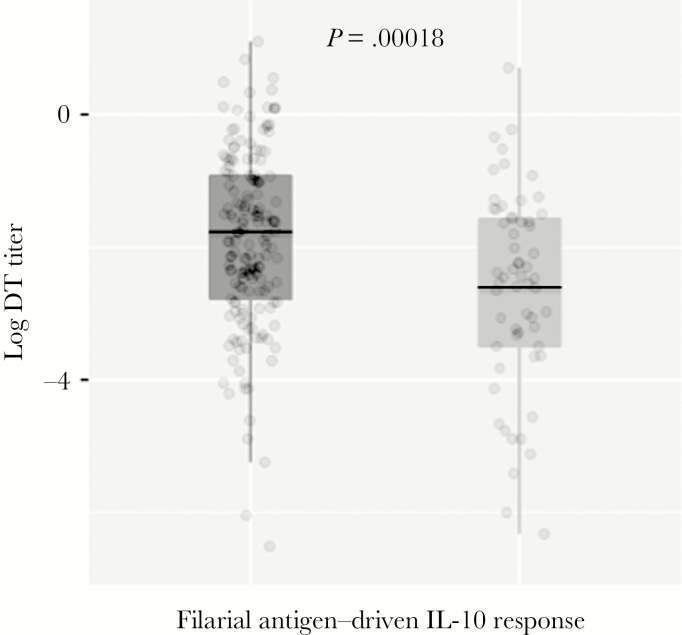
To illustrate how filarial or schistosome antigen-induced IL-10 responsiveness altered antivaccine IgG levels at a given age, Figure 4 shows the differences in anti-DT IgG levels at 12 months age between children who had filarial antigen–driven IL-10 release by CBMCs at birth and those who did not. Among newborns, those with filarial antigen–induced IL-10 release (n = 161) had significantly lower anti-DT levels at 12 months of age, compared with newborns who did not show CBMC filarial antigen–induced IL-10 production (n = 60; P = .00018).
Figure 4.
The association of filarial antigen–induced interleukin 10 (IL-10) release by cord blood mononuclear cells at birth with anti–diphtheria toxoid (DT) immunoglobulin G (IgG) levels at 12 months of age, by positivity (n = 161, right hand plot) or negativity (n = 60, left hand plot) for filarial antigen–driven IL-10 response by cord blood mononuclear cells (CBMCs) at birth. CBMCs were cultured and stimulated with filarial antigen, and IL-10 levels were measured as described in Figure 1. Dots represent values for individual children. Box plots reflect the median values and 25% and 75% quartiles, with whiskers denoting 95% confidence intervals. P = .00018, by the Student t test of log-transformed data.
DISCUSSION
In this study, we have observed that IL-10 production by CBMCs in response to helminth (schistosome and filarial) antigens is associated with downward shifts in the profiles of infant vaccine antibody responses, particularly to DT. This association was seen only with newborns’ antischistosome and antifilaria IL-10 responses and not with other cytokine responses or with any measured antimalarial cytokine responses. We observed a number of different patterns in vaccine response trajectories. Diminished overall vaccine response (PC1) was seen for DT with prenatal schistosome or filaria exposure and for HBV with filaria exposure. A late decline in anti-Hib IgG response (PC2) was noted after prenatal schistosome or filarial exposure.
Malaria parasite–induced IL-10 production by CBMCs was not associated with changes in vaccine response patterns, even though this antiprotozoal response was moderately correlated with individual antihelminth IL-10 responses. This could be due to several factors. First, the number of children with an IL-10 response to schistosome and filarial antigens was greater than the number with an IL-10 response to malaria parasite antigens, possibly because helminth infections persisted throughout pregnancy, resulting in a longer period of fetal exposure than that to malaria parasites, which is usually more transient. Second, schistosomal and filarial antigens are crude extracts and could have a more mitogenic effect on CBMCs, such that there is more of a global IL-10 response. Third, malaria parasite–induced IL-10 may be produced by a different CBMC subset, with a different bystander effect on subsequent vaccine immune responses.
We and others have found that the risk of early parasitic infections is heightened by an immune tolerization to parasite antigens that is detectable at birth in immune responder cells recovered from the newborn’s umbilical cord blood [5]. Such tolerization is a function of fetal exposure to maternal parasitic infections in utero, and we hypothesize that its impact spills over to impair infant responses to early childhood vaccination. It appears that sensitization to helminth antigens in utero is associated with an early switch to a type 2 response profile, which subsequently influences responses to standard vaccinations given at birth and 6, 10, and 14 weeks of age, as is done in Kenya [14]. It appears that chronic helminth infections, which foster immunological skewing toward a Th2 response combined with generation of regulatory T-cell responses [28, 29], can affect humoral responses to unrelated neoantigens, likely via inhibition of Th1 responses [30–33]. Previously, it has been shown that a high level of IL-10 generated by microfilaria-induced regulatory T cells [34, 35] effectively promotes parasite tolerance. We believe that the higher levels of IL-10 observed in neonates born to Schistosoma- and filaria-infected mothers are likely to be downregulating the Th1 immune responses needed for IgG responses to standard vaccines [36].
We observed that the newborn cytokine responses to malaria parasite, schistosome, and filarial antigens varied, often with a mixed Th1 and Th2 response for each parasite. Our study hypothesis was that an integrative assessment of multiple cytokines involved in the immune response in CBMCs would provide better insight into the patterns of antibody response to vaccines in parasite-exposed children. Although the response rates differed among cytokines, there was a moderate-to-strong correlation for the same cytokine between different antigens, and there were significant correlations between different cytokine responses to the same antigen. Because of the multiplicity of potential inputs and response data, our approach to analysis was to use dimension reduction via functional PCA to quantify overall patterns of IgG response between 6 and 30 months of age and to then test the links between in utero exposures and these major components of the subsequent antivaccine response. This approach also allowed us to identify the links between individual parasite–driven cytokine responses at birth and the overall patterns of vaccine responses during early life.
Previously, researchers conducting intervention studies of maternal anthelmintic treatment in Uganda have shown that maternal Mansonella perstans infection is associated with higher IL-10 responses to both bacille Calmette-Guerin and TT but without reduction in Th1 or Th2 immune responses; other maternal helminth infections showed little effect [1]. In our study, we did not examine cytokine responses to vaccine antigens but to malaria parasite, schistosome, and filaria antigens in CBMCs. In a recent study in Ecuador, researchers did not find any association of antenatal maternal helminth infections with antibody responses to infant vaccines (diphtheria, tetanus, pertussis, measles, rubella, and Hib), but modestly increased immunoglobulin A responses to oral vaccines were observed [31]. They did not examine the cytokine responses to parasite antigens, as we did in our study.
There were strengths and limitations to the present study. A strength of our study is the prospective cohort design from birth to 30 months of age, which allowed analysis of individual variation in vaccine response over time. We examined the cytokine responses to parasite antigens in cord blood lymphocytes to detect the presence of influential prenatal sensitization to malaria parasites, schistosomes, or filariae. We used recombinant blood-stage antigens and peptides for detection of sensitization to malaria parasite antigens, whereas we tested the cytokine responses only to crude schistosomal and filarial antigens, which may have influenced our rates of detectable CBMC stimulation. Of note, the outcomes of the present study were based solely on the IgG antibody responses to Hib, DT, HBV, and TT vaccines. We did not examine effects at the antibody subclass level, and it is possible that skewing of subclass distribution and individual subclass levels could affect vaccine-mediated protection. The children were vaccinated against Hib, DT, HBV, and TT at 6–14 weeks of age, but vaccine response was not measured until 6 months of age, and the children’s immediate responses to vaccination are not known. It is possible that children who were exposed to natural infection during the observation intervals may have had augmented antivaccine IgG responses, which could have masked differences between our groups in their postprimary and postsecondary responses. An in-depth assessment of maternal and child nutrition was not done in this study, and aspects of macronutrient and micronutrient intake may have had confounding effects on infection and response to vaccines [37]. The subset of CBMC-producing antigen-driven IL-10 was not determined. Despite these limitations, we feel that this study offers an accurate demonstration of a significant association between augmented antihelminth IL-10 response at birth and downward shifts in the profiles of antivaccine antibody response in early childhood.
In summary, maternal schistosomiasis and filariasis is associated with a suppressed fetal immune state at birth. Active suppression by IL-10 may lead to decreased responses to unrelated antigenic targets, such as standard childhood vaccine antigens. Further studies are warranted to document whether antenatal maternal antiparasitic treatment can mitigate the impact of prenatal parasitic exposures on early life vaccine responses.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study participants and the maternity nurses, clinical officers, laboratory technicians, and data managers at Msambweni District Hospital.
Financial support. This work was supported by the National Institutes of Health (grants HD058587 [to A. D. L. and I. M.] and AI064687 [to C. L. K.]), the Bill and Melinda Gates Foundation (award OPP1066685 to C. H. K.), and the Clinical and Translational Science Collaborative of Case Western Reserve University (support to I. M.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Elliott AM, Mawa PA, Webb EL et al. . Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunisation. Vaccine 2010; 29:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tweyongyere R, Mawa PA, Emojong NO et al. . Effect of praziquantel treatment of Schistosoma mansoni during pregnancy on intensity of infection and antibody responses to schistosome antigens: results of a randomised, placebo-controlled trial. BMC Infect Dis 2009; 9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riner DK, Ndombi EM, Carter JM et al. . Schistosoma mansoni infection can jeopardize the duration of protective levels of antibody responses to immunizations against hepatitis B and tetanus toxoid. PLoS Negl Trop Dis 2016; 10:e0005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. King CL, Malhotra I, Wamachi A et al. . Acquired immune responses to Plasmodium falciparum merozoite surface protein-1 in the human fetus. J Immunol 2002; 168:356–64. [DOI] [PubMed] [Google Scholar]
- 5. Malhotra I, Dent A, Mungai P et al. . Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med 2009; 6:e1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dent A, Malhotra I, Mungai P et al. . Prenatal malaria immune experience affects acquisition of Plasmodium falciparum merozoite surface protein-1 invasion inhibitory antibodies during infancy. J Immunol 2006; 177:7139–45. [DOI] [PubMed] [Google Scholar]
- 7. Malhotra I, Ouma JH, Wamachi A et al. . Influence of maternal filariasis on childhood infection and immunity to Wuchereria bancrofti in Kenya. Infect Immun 2003; 71:5231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. King CL, Malhotra I, Mungai P et al. . B cell sensitization to helminthic infection develops in utero in humans. J Immunol 1998; 160:3578–84. [PubMed] [Google Scholar]
- 9. Chêne A, Briand V, Ibitokou S et al. . Placental cytokine and chemokine profiles reflect pregnancy outcomes in women exposed to Plasmodium falciparum infection. Infect Immun 2014; 82:3783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ibitokou SA, Boström S, Brutus L et al. . Submicroscopic infections with Plasmodium falciparum during pregnancy and their association with circulating cytokine, chemokine, and cellular profiles. Clin Vaccine Immunol 2014; 21:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malhotra I, Mungai PL, Wamachi AN et al. . Prenatal T cell immunity to Wuchereria bancrofti and its effect on filarial immunity and infection susceptibility during childhood. J Infect Dis 2006; 193:1005–13. [DOI] [PubMed] [Google Scholar]
- 12. Rachas A, Le Port A, Cottrell G et al. . Placental malaria is associated with increased risk of nonmalaria infection during the first 18 months of life in a Beninese population. Clin Infect Dis 2012; 55:672–8. [DOI] [PubMed] [Google Scholar]
- 13. Borgella S, Fievet N, Huynh BT et al. . Impact of pregnancy-associated malaria on infant malaria infection in southern Benin. PLoS One 2013; 8:e80624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malhotra I, Mungai P, Wamachi A et al. . Helminth- and Bacillus Calmette-Guérin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol 1999; 162:6843–8. [PubMed] [Google Scholar]
- 15. Malhotra I, McKibben M, Mungai P et al. . Effect of antenatal parasitic infections on anti-vaccine IgG levels in children: a prospective birth cohort study in Kenya. PLoS Negl Trop Dis 2015; 9:e0003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malhotra I, Mungai P, Muchiri E et al. . Distinct Th1- and Th2-Type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect Immun 2005; 73:3462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malhotra I, Dent A, Mungai P, Muchiri E, King CL. Real-time quantitative PCR for determining the burden of Plasmodium falciparum parasites during pregnancy and infancy. J Clin Microbiol 2005; 43:3630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malhotra I, Ouma J, Wamachi A et al. . In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J Clin Invest 1997; 99:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Labeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination?PLoS Negl Trop Dis 2009; 3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malhotra I, Wamachi AN, Mungai PL et al. . Fine specificity of neonatal lymphocytes to an abundant malaria blood-stage antigen: epitope mapping of Plasmodium falciparum MSP1(33). J Immunol 2008; 180:3383–90. [DOI] [PubMed] [Google Scholar]
- 21. DuVall AS, Fairley JK, Sutherland L et al. . Development of a specimen-sparing multichannel bead assay to detect antiparasite IgG4 for the diagnosis of Schistosoma and Wuchereria infections on the coast of Kenya. Am J Trop Med Hyg 2014; 90:638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drasgow F. Polychoric and polyserial correlations, Encyclopedia of Statistical Sciences. New Jersey: John Wiley & Sons, Inc, 1988. [Google Scholar]
- 23. Peng J, Paul D. A geometric approach to maximum likelihood estimation of the functional principal components from sparse longitudinal data. J Comput Graph Stat 2009; 18:995–1015. [Google Scholar]
- 24. Hastie TJ, Tibshirani RJ. Generalized additive models. In: Monographs on Statistics and Applied Probability. London: Chapman & Hall, 1992. [Google Scholar]
- 25. Pinheiro J, Bates D, DebRoy S, Sarkar D; R Core Team nlme: Linear and Nonlinear Mixed Effects Models, 2016. [Google Scholar]
- 26. Hall P, Hosseini-Nasab M. On properties of functional principal components analysis. J Roy Stat Soc B 2006; 68:109–26. [Google Scholar]
- 27. Horvath L, Kokoszka P.. Inference for functional data with applications. New York: Springer, 2012. [Google Scholar]
- 28. van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 2007; 212:475–90. [DOI] [PubMed] [Google Scholar]
- 29. Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol 2011; 186:2780–91. [DOI] [PubMed] [Google Scholar]
- 30. Elias D, Akuffo H, Pawlowski A, Haile M, Schön T, Britton S. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine 2005; 23:1326–34. [DOI] [PubMed] [Google Scholar]
- 31. Elias D, Akuffo H, Thors C, Pawlowski A, Britton S. Low dose chronic Schistosoma mansoni infection increases susceptibility to Mycobacterium bovis BCG infection in mice. Clin Exp Immunol 2005; 139:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fletcher HA. Correlates of immune protection from tuberculosis. Curr Mol Med 2007; 7:319–25. [DOI] [PubMed] [Google Scholar]
- 33. Pearlman E, Kazura JW, Hazlett FE Jr, Boom WH. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J Immunol 1993; 151:4857–64. [PubMed] [Google Scholar]
- 34. Achary KG, Mandal NN, Mishra S et al. . In utero sensitization modulates IgG isotype, IFN-γ and IL-10 responses of neonates in bancroftian filariasis. Parasite Immunol 2014; 36:485–93. [DOI] [PubMed] [Google Scholar]
- 35. Satoguina JS, Adjobimey T, Arndts K et al. . Tr1 and naturally occurring regulatory T cells induce IgG4 in B cells through GITR/GITR-L interaction, IL-10 and TGF-beta. Eur J Immunol 2008; 38:3101–13. [DOI] [PubMed] [Google Scholar]
- 36. Ateba-Ngoa U, Adegnika AA, Zinsou JF et al. . Cytokine and chemokine profile of the innate and adaptive immune response of Schistosoma haematobium and Plasmodium falciparum single and co-infected school-aged children from an endemic area of Lambaréné, Gabon. Malar J 2015; 14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guerrant RL, Oriá RB, Moore SR, Oriá MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev 2008; 66:487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.