Abstract
Comfrey has been consumed by humans as a vegetable and a tea and used as an herbal medicine for more than 2000 years. Comfrey, however, produces hepatotoxicity in livestock and humans and carcinogenicity in experimental animals. Comfrey contains as many as 14 pyrrolizidine alkaloids (PA), including 7-acetylintermedine, 7-acetyllycopsamine, echimidine, intermedine, lasiocarpine, lycopsamine, myoscorpine, symlandine, symphytine, and symviridine. The mechanisms underlying comfrey-induced genotoxicity and carcinogenicity are still not fully understood. The available evidence suggests that the active metabolites of PA in comfrey interact with DNA in liver endothelial cells and hepatocytes, resulting in DNA damage, mutation induction, and cancer development. Genotoxicities attributed to comfrey and riddelliine (a representative genotoxic PA and a proven rodent mutagen and carcinogen) are discussed in this review. Both of these compounds induced similar profiles of 6,7-dihydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine (DHP)-derived DNA adducts and similar mutation spectra. Further, the two agents share common mechanisms of drug metabolism and carcinogenesis. Overall, comfrey is mutagenic in liver, and PA contained in comfrey appear to be responsible for comfrey-induced toxicity and tumor induction.
Herbal medicines have a huge global market, and Americans spend between $4 billion and $12 billion per year on herbal products (Willett et al., 2004). Recently, medicinal plants gained increasing attention with regard to their therapeutic properties as well as their potential adverse health effects. One of these botanical remedies is commercially known as comfrey, which belongs to the plant family Boraginaceae (Brauchli et al., 1982) and is a common garden plant growing globally. There are several species in the genus Symphytum that are referred to as comfrey (Roeder, 1995), including S. asperum Lepech (prickly comfrey), S. officinale L. (common comfrey; the major comfrey species), S. x uplandicum Nyman (Russian comfrey, or blue comfrey; a natural hybrid of S. officinale L. and S. asperum Lepech), S. tuberosum L. (tuberous comfrey), and S. caucasicum Bieb. (Caucasian comfrey). The term comfrey is used most often for Symphytum officinale L., which is a tall perennial with large hairy leaves and small purple flowers (Figure 1A) (Betz et al., 1994; Winship, 1991).
FIGURE 1.
The pictures of comfrey (Symphytum officinale): (A) whole plant; (B) comfrey roots.
Comfrey has been used as an herbal medicine for more than 2000 years (Rode, 2002). Comfrey is also consumed as a vegetable and is one of the most popular herbal teas in many countries. The frequent use of comfrey, generally as fresh or dried leaves and dried roots (Figure 1B), is a potential health risk owing to the presence of pyrrolizidine alkaloids (PA). The PA in comfrey are retronecine mono- and diesters, a class with lower toxicity than heliotridine monoester and macrocyclic diesters found in other plants (Rode, 2002). The PA content of comfrey is less than 1% and variably dependent on the plant part (Roeder, 1995; Stickel & Seitz, 2000). Higher PA concentrations occur in comfrey roots than leaves, and commercially available comfrey tablets containing high levels of PA are likely to be derived from comfrey roots (Couet et al., 1996; Huxtable et al., 1986). Abbott (1988) estimated that 5 comfrey leaves contain approximately 5 mg PA and 1 cup of comfrey root-tea contains 8–26 mg PA. Although the popularity of comfrey has declined due to knowledge of its dangers, this medicinal compound is still available commercially in several forms.
Comfrey produces hepatotoxicity in livestock and humans, and carcinogenesis in experimental animals. Comfrey induces hepatic veno-occlusive disease (VOD) in humans (Ridker & McDermont, 1989) and hepatocellular adenomas and hemangioendothelial sarcomas of the liver in rats (Hirono et al., 1978). Although there are no epidemiological data regarding the carcinogenicity of comfrey, these adverse effects have raised questions of its potential carcinogenicity in humans. Therefore, many countries have restricted its availability. In Canada, the distribution of comfrey has been banned in herbal medicinal products intended for ingestion (Snider, 1991). In Germany, the use of comfrey is limited to external products and the maximal permissible dose of PA is 100 μg/d with a duration of treatment limited to between 4 and 6 wk per year (Koll et al., 2004). In the United Kingdom, comfrey is one of the herbs under consideration for restriction (ILS, 1997). In 1993, the American Herbal Products Association recommended that comfrey be used only externally (ILS, 1997). In 2001, the U.S. Food and Drug Administration advised dietary supplement manufacturers to remove comfrey products from the market (FDA, 2001). In many parts of the world, however, there are presently no restrictions on the use of comfrey.
Pyrrolizidine alkaloids that are found in the human diet and associated with genotoxicity are the subject of several reviews (Chen et al., 2010; Fu et al., 2001; 2004; Prakash et al., 1999); however, there are few studies reporting on the mechanisms underlying comfrey-induced mutagenicity and carcinogenicity. Recently, the mutagenicity of comfrey was investigated and toxicogenomic studies were performed in rat liver, a target tissue for carcinogenesis (Guo et al., 2006, 2007; Mei et al., 2005, 2006; Mei & Chen, 2007). This review presents updated information on extraction of PA constituents from the comfrey plant, as well as metabolism, genotoxicity, and carcinogenicity of comfrey.
THERAPEUTIC USE OF COMFREY
Since antiquity, comfrey has been used both internally and externally in different forms for the treatment of a variety of diseases (Rode, 2002; Stickel & Seitz, 2000). Comfrey was used by Roman soldiers to treat bone fractures more than 2000 years ago (Sakakura et al., 2008). Both comfrey leaves and roots in the form of extract, ointment, or compress paste are applied externally in the treatment of inflammatory disorders of joints, wounds, bone fractures, gout, distortions, hematomas, and thrombophlebitis. For internal applications as infusions and extracts, comfrey is used to treat gastritis, gastroduodenal ulcers, and lung congestion (Roeder, 1995). Humans have widely used comfrey poultices or ointments, and consumed comfrey formulations of tea or tablets. Various investigators demonstrated the efficacy of comfrey in the treatment of distortions, strains and sprains, and muscle and joint complaints in several clinical trials (Koll et al., 2004; Predel et al., 2005; Grube et al., 2007). Comfrey root ointment significantly improved short-term symptoms after ankle sprain (Bleakley et al., 2008). The therapeutic properties of comfrey are based on its anti-inflammatory, analgesic, granulation promoting, and antiexudative properties, because comfrey contains varying quantities of allantoin, rosmarinic acid, triterpene saponins, tannins, mucopolysaccharides, and other hydroxycinnamon acid derivatives, which are critically important for its pharmacodynamic characteristics (Stickel & Seitz, 2000). Comfrey is also rich in many nutrients including protein, antioxidant vitamins, and vitamin B12 (Rode, 2002).
Recently, Sakakura et al. (2008) evaluated the influence of homeopathic treatment with comfrey on radiographic bone density. Titanium micro-implants were placed in the tibia of rats and animals received drinking water with or without 10 drops of comfrey per day for 7, 14, or 28 d. It was found that comfrey administration promoted an increase in radiographic bone density around the titanium implants in the initial period of bone healing (Sakakura et al., 2008). This healing capacity was associated with allantoin, one of the components of comfrey. Gomes et al. (2007) also demonstrated that 10% comfrey alcoholic extract reduced early development of preneoplastic liver lesions by inhibiting cell proliferation and modulating atypical cellular phenotypes in a short-term rat hepatocarcinogenesis assay.
HUMAN TOXICITY OF COMFREY
Comfrey also contains PA that are constituents of more than 6000 plants. Many PA produce hepatotoxic and carcinogenic effects in humans and animals (Fu et al., 2004). The major hepatotoxic manifestation in humans ingesting comfrey is hepatic VOD (Ridker et al., 1985; Weston et al., 1987), also called sinusoidal obstruction syndrome (SOS) (DeLeve et al., 2002). Several cases of VOD/SOS associated with comfrey ingestion were reported in humans (Bach et al., 1989; Ridker et al., 1985; Weston et al., 1987; Yeong et al., 1990), as well as in experimental animals (Yeong et al., 1991). Comfrey-induced dose-dependent hepatic VOD was also found in rats that were gavaged with a single dose of 200 mg/kg of the mixed PA, or 50 and 100 mg/kg thrice a week for 3 wk (Yeong et al., 1991).
The first reported case of VOD in humans after use of comfrey was a 49-yr-old woman, who consumed a minimum of 85 mg PA during a 6-mo period and had portal hypertension associated with obliteration of the smaller hepatic venules (Ridker et al., 1985). The second published case was a 13-yr-old boy, who was treated for Crohn’s disease with acupuncture and comfrey tea containing unknown quantities of comfrey leaves, and diagnosed with hepatic VOD by liver biopsy when he was admitted for investigation of hepatomegaly and ascites (Weston et al., 1987). A third published case involved a 47-yr-old woman who consumed up to 10 cups of comfrey tea per day in addition to taking comfrey pills for more than 1 year. Four years after beginning the consumption of comfrey, her serum aminotransferase activities were found to be abnormally high. Four years later she developed ascites and a liver biopsy specimen revealed dense fibrosis of portal tracts containing proliferating bile ductules (Bach et al., 1989). The fourth documented patient was a 23-yr-old young man in New Zealand who ate a predominantly vegetarian diet including steamed young comfrey leaves. He presented with hepatic VOD and severe portal hypertension, and subsequently died from liver failure (Yeong et al., 1990). The temporal association between comfrey ingestion and disease, as well as the exclusion of other possible causes, suggested a possible causal relationship. More recently, a 66-yr-old woman with chronic diseases was diagnosed with severe pulmonary hypertension because of progressive dyspnea, possibly due to drinking 1–1.5 L a day of herbal tea containing comfrey for months prior to hospitalization (Gyorik & Stricker, 2009).
There are no well-established methods available for the measurement of PA and their metabolites in body fluids, although experimental applications of both in-source collision-induced dissociation high-performance liquid chromatography mass spectrometry (CID-HPLC/MS) and HPLC/MS/MS analytical methods were successful for the determination of PA in blood samples obtained from rats treated with PA and in PA-containing plant material (Lin et al., 1998). Therefore, the diagnosis of PA-poisoning can only be made with chemical confirmation of the presence of toxic PA constituents in the herbal preparation that the patient consumed or, without chemical confirmation, when the herb in question is a known source of toxic PA (Ridker & McDermott, 1989). Huxtable et al. (1986) reported that an individual consuming 2 capsules per meal for 6 mo would receive a total of 162 or 1740 mg PA from the herbal preparation of comfrey leaves or roots, respectively.
ANALYSIS OF PYRROLIZIDINE ALKALOIDS IN COMFREY
Pyrrolizidine alkaloid-mediated toxicity is determined by 1,2-unsaturation in the pyrrolizidine ring and an ester function on the side chain. Among the 660 identified PA and their N-oxide derivatives about half are genotoxic and many are tumorigenic (Fu et al., 2004). Comfrey contains as many as 14 PA (Table 1), including 7-acetylintermedine, 7-acetyllycopsamine, anadoline, asperumine, echimidine, echinatine, intermedine, lasiocarpine, lycopsamine, myoscorpine, symlandine, symphytine, symviridine, and uplandicine. The chemical structures of these PA are shown in Figure 2.
TABLE 1.
Pyrrolizidine Alkaloids in Comfrey¶
| Pyrrolizidine alkaloid | Comfrey
|
||||
|---|---|---|---|---|---|
| Common | Prickly | Russian | Caucasian | Tuberous | |
| 7-Acetylintermedine | + | + | + | ||
| 7-Acetyllycopsamine | + | + | + | ||
| Anadoline | + | ||||
| Asperumine | + | ||||
| Echimidine | +a | + | + | + | + |
| Echinatine | + | ||||
| Intermedine | + | + | + | ||
| Lasiocarpine | +b | + | |||
| Lycopsamine | + | + | + | ||
| Myoscorpine | + | + | + | ||
| Symlandine | + | + | + | + | |
| Symphytine | + | + | + | ||
| Symviridine | + | + | + | ||
| Uplandicine | + | ||||
| PA content | 0.02–0.29% | 0.13–0.37% | ~0.20% | ~0.48% | ~0.02% |
FIGURE 2.
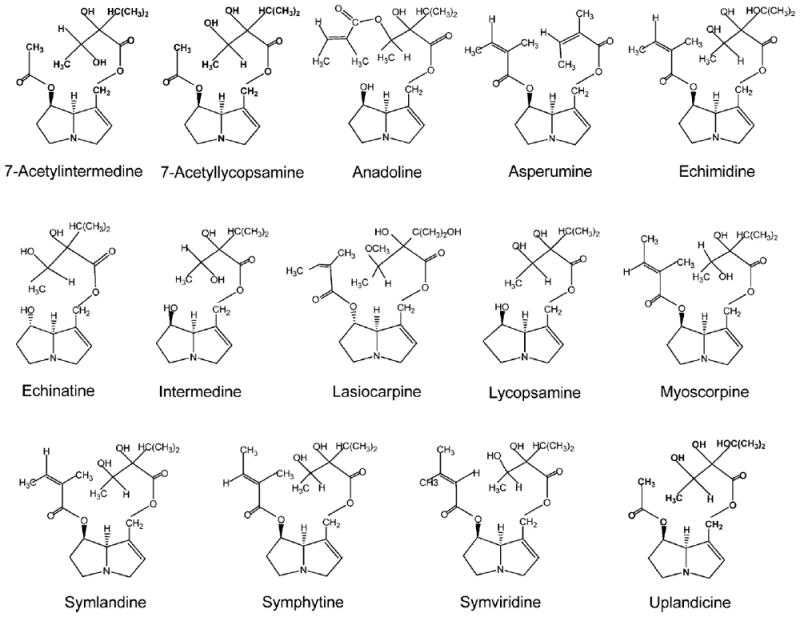
Structures of representative pyrrolizidine alkaloids found in comfrey.
Both PA and their N-oxide derivatives are present in plants. A number of extraction and analytical methodologies were developed to identify and quantify PA in plants and commercial plant products (Betz et al., 1994; Crews et al., 1997; Deinzer et al., 1977; Edgar et al., 2002; Fu et al., 2007; Mattocks, 1967; 1986; Roeder, 1995). After extraction, the N-oxides are converted to the parent PA by reduction with zinc (Vollmer et al., 1987). The concentration of PA in some plants is high. Roitman (1981) reported that a cup of tea made from comfrey roots might contain as much as 26 mg PA.
The PA in comfrey is easily extracted because PA are soluble in methanol and water. In the normal isolation procedure, PA are initially extracted from dried, ground plant material with methanol. Although liquid–liquid partitioning is a favored practice for sample preparation, a solid-phase extraction (SPE) method was developed using an Ergosil cleanup column that specifically binds PA (Gray et al., 2004). This sample preparation technique provides a rapid and reliable procedure for the isolation and concentration of PA from comfrey extracts.
Analytical methods for the determination of PA include thin-layer chromatography (TLC), HPLC, and capillary gas chromatography (GC), combined with ultraviolet (UV) detection, MS, or nuclear magnetic resonance (NMR) spectroscopy (Altamirano et al., 2005; Couet et al., 1996; Fu et al., 2007; Mattocks, 1986; Roeder, 1995). The most sensitive and specific methods used for the analysis of PA are HPLC/MS and GC/MS. It is worth noting that GC/MS methods are only suitable for the analysis of PA, and not for their N-oxides, because N-oxides are thermally unstable and typically require pre-reduction (Altamirano et al., 2005; Wuilloud et al., 2004).
Because HPLC with UV detection has a limited ability to detect those PA without a chromophore in their structure, Schaneberg et al. (2004) developed a reverse-phase HPLC method utilizing an evaporative light-scattering detector (ELSD) for the simultaneous detection of PA with and without chromophores. The limit of detection (LOD) for ELSD is approximately 40 μg/ml, and there are low cost and low maintenance requirements. Using HPLC with either UV or ELSD detection, lasiocarpine was detected in comfrey (Schaneberg et al., 2004).
Numerous studies reported identification and quantification of specific PA in comfrey. However, the quantity and the individual PA found in comfrey vary, mainly due to the differences in species, harvest time, age of the plant material, part of the plant extracted, and geographic region where the plants grew (Altamirano et al., 2005; Yeong et al., 1990). Couet et al. (1996) found that the major PA detected in comfrey roots were symphytine and symlandine, and the remaining PA comprised echimidine, lycopsamine, and 7-acetyllycopsamine. Altamirano et al. (2005) noted that comfrey contained higher amounts of intermedine and 7-acetylintermedine and lower amounts of uplandicine and symphytine. Kim et al. (2001) isolated symlandine, its stereoisomer symphytine, and echimidine from the comfrey roots. Recently, Liu et al. (2009) determined that lycopsamine, lasiocarpine, and echimidine are predominant PA in comfrey. Using LC/ESI-MS to analyze 10 herbal remedies that were labeled to contain comfrey, measurable quantities of PA including 7-acetyllycopsamine, echimidine, intermedine, symphytine, and uplandicine were found in the products (Altamirano et al., 2005).
Since comfrey is often consumed as an herbal tea, Betz et al. (1994) prepared hot-water infusions of comfrey roots and leaves. The resulting solution was extracted with chloroform–ammonium hydroxide, evaporated to dryness at 45°C, and the residue was resuspended in methanol for GC and GC/MS determination. By comparison to standards, intermedine, lycopsamine, 7-acetylintermedine, 7-acetyllycopsamine, and two other PA, presumed to be symphytine and symlandine, were identified in ranges from 0.1 to 400 ppm (Betz et al., 1994).
Oberlies et al. (2004) also prepared herbal teas by steeping comfrey leaves (10 g) in 1 L hot water. The resulting solution was extracted thrice with 1 L chloroform–ammonium hydroxide. The chloroform extract was concentrated in vacuo and PA were visualized via TLC analysis. Although the concentrations of symphytine and echimidine varied, 1 L comfrey tea contained up to 13.7 μg symphytine and 14.5 μg echimidine (Oberlies et al., 2004). Both symphytine and echimidine in comfrey were also isolated in an earlier study (Furuya & Araki, 1968). Vollmer et al. (1987) extracted PA from 10 g comfrey roots with 300 ml methanol. The extracted PA were detected selectively by TLC and quantitatively by NMR spectroscopic analysis and about 130–830 ppm of PA including symphytine, 7-acetylintermedine, 7-acetyllycopsamine, intermedine, and lycopsamine were detected.
METABOLISM OF COMFREY
Pyrrolizidine alkaloids are biologically and toxicologically inactive and require metabolic activation to exert these effects. These compounds undergo metabolic activation to yield the corresponding pyrrolic metabolites that react with cellular macromolecules, including proteins and DNA, to exert toxicity (Prakash et al., 1999). Metabolism and determination of metabolic activation pathways leading to toxicity, particularly genotoxicity, were extensively studied (Dueker et al., 1992; Fu et al., 2004; Lame et al., 1991; Lin et al., 1998, 1999, 2000, 2002; Mattocks, 1968, 1986; Reid et al., 1998; Yang et al., 2001). Hepatic cytochrome P-450 (CYP), specifically the CYP3A and CYP2B isoforms, are the major metabolizing enzymes involved in PA metabolism in rodents and humans (Fu et al., 2004; Reid et al., 1998).
Metabolism of lasiocarpine, which is one of the PA constituents in comfrey, by liver microsomes of male F344 rats was studied by Xia et al. (2006). 6,7-Dihydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine (DHP) was identified as a predominant and reactive metabolite (Figure 3).
FIGURE 3.
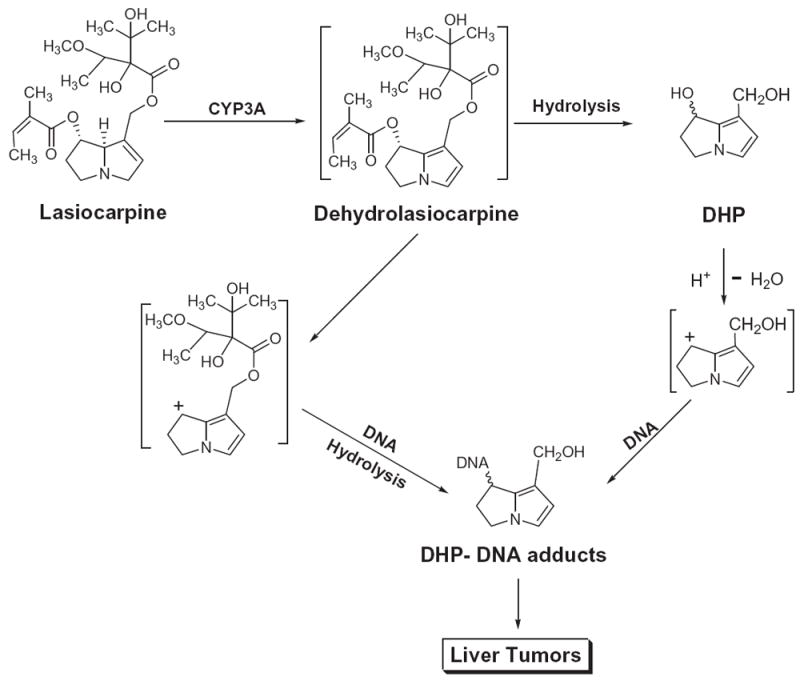
Metabolism of lasiocarpine. Lasiocarpine, a pyrrolizidine alkaloid found in comfrey, undergoes metabolic activation to its metabolites including 6,7-dihydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine (DHP) that reacts with DNA, leading to DNA adduct formation and liver tumor induction.
Metabolism of lasiocarpine by liver microsomes of untreated male F344 rats was examined in the presence of the specific CYP3A isozyme inhibitors, ketoconazole, or triacetyloleandomycin (TAO). Compared with metabolism produced by liver microsomes of untreated rats, DHP formation was reduced by 85–92% by ketoconazole and TAO. These results indicate that the metabolic formation of DHP from lasiocarpine was mediated predominantly by CYP3A isozyme (Xia et al., 2006).
The effects of dietary comfrey on drug metabolizing enzymes in rats have also been examined. Dietary comfrey was found to stimulate the activity of aminopyrine N-demethylase, while there were no marked effects on glutathione S-transferase or epoxide hydrolase activities in Long-Evans male rats (Garrett et al., 1982). The activity of the hepatic drug-metabolizing enzymes reflects the ability of animals to metabolize and conjugate the foreign compounds generated from comfrey.
The liver is the major organ for biotransformation of xenobiotics and drugs. The metabolic function in liver is primarily responsible for the detoxification of xenobiotics, usually by Phase II and Phase III enzymes, and the metabolites are readily excreted from the body. However, many xenobiotics may be metabolically activated to reactive toxic metabolites by enzymes, particularly by Phase I enzymes. Examination of genes that code for drug-metabolizing enzymes provides important pharmacological information with potential pharmacokinetic and toxicological implications for one or more given chemicals, and the changes in gene expression levels often reflect alterations of enzyme levels (Fuhr, 2000; Martignoni et al., 2006).
In our laboratory, Mei et al. (2006) investigated the changes in expression of genes involved in hepatic drug metabolism in rats fed 8% comfrey in the diet for 3 mo. A large number of metabolizing genes had altered expression (Table 2), including 20 Cyp genes, 3 glutathione S-transferase (Gst) genes, 6 ATP-binding cassette transporters, 6 solute carrier family transporters, carboxylesterase 2 (Ces2), flavin-containing monooxygenase 5 (Fmo5), and NAD(P)H dehydrogenase (Nqo1). Among these genes, Ces2, Cyp2c12, Cyp7a1, Cyp26, Gsta3, Abcc1, and Abcc3 were increased 6- to 21-fold, whereas Cyp2c, Cyp39a1, Gstm3, inmt, Abcc8, Slc22a8, and others were reduced. It is known that the Cyp superfamily plays a critical role in the Phase I metabolism of a variety of xenobiotics including drugs, carcinogens, steroids, and eicosanoids. The resultant metabolites of herbal or dietary constituents may lead to Cyp activation or inactivation by chemical modification of the heme, the apoprotein, or both, as a result of covalent binding of modified heme to the apoprotein (Zhou et al., 2004).
TABLE 2.
Genes Involved in Drug Metabolism Altered by 8% Comfrey Roots and 1 mg/kg Riddelliine in Rat Liver
| Gene symbol | Gene description | Fold changea
|
|
|---|---|---|---|
| Comfreyb | Riddelliinec | ||
| Phase I metabolism | |||
| Ces2 | Carboxylesterase 2 (intestine, liver) | 10.4 | 2.4 |
| Cyp2c | Cytochrome P-450, family 2, subfamily c | −33.3 | −270.3 |
| Cyp2c12 | Cytochrome P-450, family 2, subfamily c | 6.5 | 42.3 |
| Cyp2c39 | Cytochrome P-450, family 2, subfamily c | −2.9 | |
| Cyp2d1 | Cytochrome P-450, family 2, subfamily d | −2.6 | |
| Cyp2d2 | Cytochrome P-450, family 2, subfamily d | −2.3 | |
| Cyp2d3 | Cytochrome P-450, family 2, subfamily d | −2.6 | |
| Cyp2d5 | Cytochrome P-450, family 2, subfamily d | −2.3 | |
| Cyp3a18 | Cytochrome P-450, family 3, subfamily a | −2.6 | |
| Cyp2e1 | Cytochrome P-450, family 2, subfamily e | 2.2 | 2.1 |
| Cyp4a3 | Cytochrome P-450, family 4, subfamily a | −2.2 | |
| Cyp4a12 | Cytochrome P-450, family 4, subfamily a | −2.8 | |
| Cyp4b1 | Cytochrome P-450, family 4, subfamily b | −3.1 | |
| Cyp4f5 | Cytochrome P-450, family 4, subfamily f | 4.7 | |
| Cyp4f14 | Cytochrome P-450, family 4, subfamily f | −2.1 | |
| Cyp4f18 | Cytochrome P-450, family 4, subfamily f | −2.1 | |
| Cyp7a1 | Cytochrome P-450, family 7, subfamily a | 6.2 | |
| Cyp7b1 | Cytochrome P-450, family 7, subfamily b | 2.1 | |
| Cyp11b3 | Cytochrome P-450, family 11, subfamily b | −2.3 | |
| Cyp26 | Cytochrome P-450, family 26 | 18.5 | |
| Cyp39a1 | Cytochrome P-450, family 39, subfamily a | −3.4 | |
| Fmo5 | Flavin-containing monooxygenase 5 | 4.1 | 4.2 |
| Phase II metabolism | |||
| Gsta3 | Glutathione S-transferase, alpha 3 | 14.5 | 13.4 |
| Gstm3 | Glutathione S-transferase, mu 3 | −2.6 | |
| Gstp1 | Glutathione S-transferase, pi 1 | 3.2 | |
| Inmt | Indolethylamine N-methyltransferase | −29.3 | −11.7 |
| Nqo1 | NAD(P)H dehydrogenase, quinone 1 | 5.0 | 2.6 |
| Sult1c1 | Sulfotransferase family, cytosolic,1C, member1 | −2.7 | −3.2 |
| Phase III metabolism | |||
| Abcb1 | ATP-binding cassette, subfamily B | 97.0 | 11.3 |
| Abcb9 | ATP-binding cassette, subfamily b (MDR/TAP) | −4.3 | −2.7 |
| Abcc1 | ATP-binding cassette, subfamily c (CFTR/MRP) | 2.6 | |
| Abcc3 | ATP-binding cassette, subfamily c (CFTR/MRP) | 21.2 | 10.8 |
| Abcc6 | ATP-binding cassette, subfamily c (CFTR/MRP) | −2.4 | |
| Abcc8 | ATP-binding cassette, subfamily c (CFTR/MRP) | −10.0 | −2.6 |
| Atp13a5 | Atpase type 13A5 | −3.6 | −4.1 |
| Slc13a5 | Solute carrier family 13, member 5 | −2.9 | −2.4 |
| Slc16a4 | Solute carrier family 16, member 4 | −2.8 | −4.9 |
| Slc22a6 | Solute carrier family 22, member 6 | −3.7 | −2.4 |
| Slc22a8 | Solute carrier family 22, member 8 | −12.2 | −833.3 |
| Slc25a21 | Solute carrier family 25, member 21 | 4.4 | 2.7 |
| Slc25a30 | Solute carrier family 25, member 30 | −4.5 | −2.7 |
The p-value for all genes is less than .01 and the symbol of minus (−) means downregulation.
Data from Mei et al. (2006).
Data from Guo et al. (2007)
Alterations of gene expression by comfrey (Table 2) may be attributed partly to the PA constituents. To investigate which of these gene expression changes may be due to PA, the comfrey-induced drug-metabolizing genes were compared with those induced by riddelliine, a prototype of carcinogenic PA (Guo et al., 2007). Among the comfrey-regulated drug metabolizing genes, 22 genes were also altered by riddelliine treatment in the livers of exposed rats, with changes in the same direction (Table 2). The similarity of gene expression changes among these 22 genes regulated by two treatments was further assessed by calculating the correlation coefficient of the log2 fold changes. The correlation coefficient (R2) was .72, indicating reliable agreement between the two treatments and suggesting that there is a common mechanism of drug metabolism involved in these two chemical treatments (Guo et al., 2007).
There are three general Phase I pathways for the metabolism of PA: (1) oxidation of PA catalyzed by cytochrome P-450 (CYP) to the reactive pyrrolic metabolites, (2) N-oxidation catalyzed by CYP and flavin-containing monooxygenases (Fmo) to the corresponding PA N-oxide, and (3) hydrolysis of PA catalyzed by carboxylesterases to the necine base (Fu et al., 2004; Williams et al., 1989). Comfrey and riddelliine treatments increased the expression of Cyp2c12 (6.5- and 42.3-fold, respectively) and Cyp2e1 (both 2-fold), and decreased the expression of Cyp2c by 33-and 270-fold, respectively (Table 2). In addition, both comfrey and riddelliine treatments induced Fmo5 about 4-fold and Ces2 about 10- and 2-fold, respectively.
Phase II and Phase III enzymes usually function for detoxification. Phase II enzymes are involved in conjugation of the polar functional groups of Phase I metabolites and Phase III enzymes are transporters for these metabolites. Comfrey and riddelliine treatments also altered the expression of a number of Phases II and III genes (Table 2), such as Gsta3 and Nqo1 (increased 2- to 14-fold) and Abcb1 and Abcc3 (elevated more than 10-fold). Glutathione (GSH) conjugation with the toxic pyrrolic ester metabolites formed is generally recognized as one of the detoxification pathways, and the elevation of the GSH content in the liver is coupled with increased activity of GST in the early stage of non-severe PA-poisoning (Fu et al., 2004). When male Sprague-Dawley rats were administered a toxic dose of PA, such as monocrotaline (Lame et al., 1990; Yan & Huxtable, 1996) and retrorsine (Lin et al., 1999), hepatic GSH levels were significantly elevated. Furthermore, the rise in GSH content in liver and lung of rats correlated with enhanced formation of toxic pyrrolic metabolites (Yan & Huxtable, 1996). Our study on the comparison of the changes of drug metabolizing gene expression by comfrey and riddelline (Guo et al., 2007) suggests that modulations of drug-metabolizing genes by comfrey are attributed partly to PA constituents, and comfrey-induced toxicity may be associated with modulation of these drug-metabolizing enzymes by PA present in comfrey.
CARCINOGENICITY OF COMFREY
The toxicity of PA in humans has been recognized for a long time as evidenced by “Senecio disease” in South Africa in 1920 and PA-induced hepatic VOD in Jamaica in 1954 and in Afghanistan and India in 1976 (Stickel & Seitz, 2000). Using animal models, Hirono et al. (1978) reported that hepatocellular adenomas were induced in rats fed diets containing comfrey roots and leaves. In their study, ACI rats were fed comfrey leaves at 8, 16, or 33% of the diet or comfrey roots at 1, 2, 4, or 8% of the diet for up to 600 d. Liver tumors were induced in all experimental groups and the first hepatocellular carcinoma appearance was in the group dosed with 8% comfrey roots 7 mo after treatment (Figure 4). While no liver tumors were observed in the control group, the liver tumor incidence in the rats treated with comfrey leaves at 8, 16, and 33% of the diet were 4, 33, and 55%, respectively, in a dose-response relationship. However, the liver tumor incidence in the rats treated with comfrey roots at 1, 2, 4, and 8% of the diet were 80, 42, 33, and 79%, respectively. The non-dose-related tumor incidence suggested that the amount of tumorigenic PA contained in the comfrey roots varied, even though the material was collected in the same area at the same time (Hirono et al., 1978). The feeding of comfrey roots was more tumorigenic than ingestion of comfrey leaves. Although comfrey-induced neoplasms in the rats were found mostly in liver, sporadic urinary papillomas and carcinomas were also observed.
FIGURE 4.
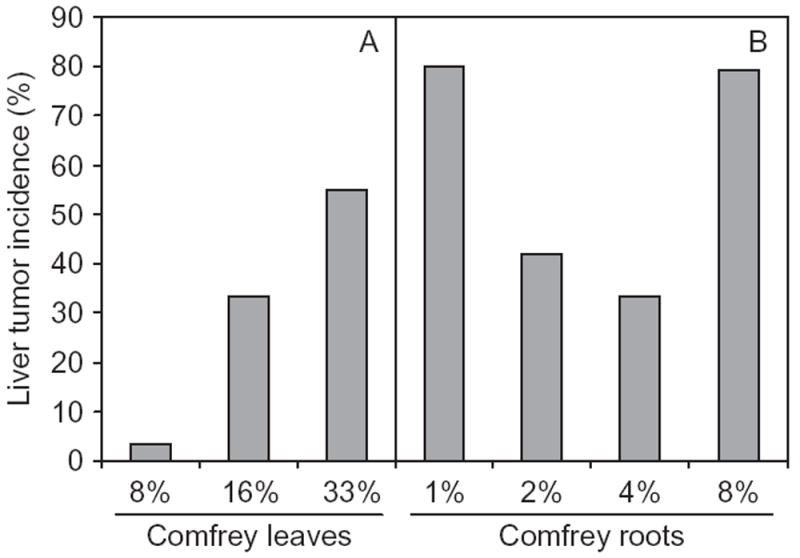
Tumor incidence in the livers of rats treated with comfrey. Male and female ACI rats were fed a diet containing comfrey leaves (A) or comfrey roots (B) at different percentages for up to 600 d. All liver tissues were autopsied at the termination of experiment. No liver tumors were observed in the control group. Data from Hirono et al. (1978).
Fu et al. (2001, 2004) reported that 17 PA induced tumors in rodents. Comfrey contains four of these PA: intermedine, lasiocarpine, lycopsamine, and symphytine. By the same injection schedule, i.e., intraperitoneal (ip) administration of a dose (10% of the LD50) twice a week for 4 wk and then once a week for 52 wk, Hirono et al. (1979) treated 20 ACI rats with symphytine at a dose of 13 mg/kg body weight, and Svoboda and Reddy (1972) treated F344 rats with lasiocarpine at a dose of 7.8 mg/kg body weight. The results showed that symphytine induced 4 liver tumors (3 hemangioendothelial sarcomas and 1 liver cell adenoma; 4/20, 20%), with the hemangioendothelial sarcomas showing metastasis in the lungs of 2 rats (Hirono et al., 1979). Lasiocarpine treatment resulted in 61% (11/18) of the rats with hepatocellular carcinomas, 33% with squamous-cell carcinoma of skin, and 28% with lung adenomas (Svoboda & Reddy, 1972). In another study, when 20 F344 rats were fed lasiocarpine at a dietary concentration of 50 ppm for 55 wk, 45% of the rats (9/20) developed angiosarcomas of the liver and 35% (7/20) had hepatocellular carcinomas (Rao & Reddy, 1978). The U.S. National Cancer Institute also reported that lasiocarpine was carcinogenic in F344 rats, producing hepatocellular tumors and angiosarcomas of liver in both genders and hematopoietic tumors in females, after the groups of rats were administered lasiocarpine in the diet at doses of 7, 15, or 30 ppm for 104 wk (NTP, 1978). When a mixture of PA, intermedine and lycopsamine, from seeds of Amsinckia intermedia (in the plant family Boraginaceae) was administered as a single dose of 500 or 1500 mg/kg to weanling rats, pancreatic tumors (particularly of the islet cells) were found (Schoental et al., 1970). These results demonstrated that four of the PA in comfrey, i.e., lasiocarpine, symphytine, intermedine, and lycopsamine, are carcinogenic in rats.
The mechanisms underlying lasiocarpine-induced tumors in rodents were studied by Xia et al. (2006). Prior to this study, it was demonstrated that the metabolism of riddelliine, a prototypical PA, generated DHP as a reactive metabolite, which bound to DNA in vitro and in vivo to form DHP-derived DNA adducts detected by 32P-postlabeling/HPLC analysis (Chou et al., 2003a, 2003b, 2003c; Yang et al., 2001). These DHP-derived DNA adducts were postulated to be potential biomarkers of PA exposure and PA tumori-genicity (Fu et al., 2004). Xia et al. (2006) examined the metabolism of lasiocarpine and riddelliine by rat liver microsomes in the presence of calf thymus DNA. The same set of DHP-derived DNA adducts was formed by these two PA as determined by 32P-postlabeling/HPLC analysis. Data indicated that metabolism of lasiocarpine produced DHP, which subsequently bound DNA and generated DHP-derived DNA adducts leading to tumor formation (Figure 3).
When female F344 rats were treated by gavage for 3 consecutive days with comfrey root extract at a dose of 120 μl per day or comfrey compound oil at 200 μl per day, the liver of rats contained DHP-derived DNA adducts similar to the positive control rats treated with riddelliine at 1 mg/kg per day (Figure 5). The average levels of DHP-derived DNA adducts after exposure to the comfrey root extract and comfrey compound oil were 22 adducts/108 nucleotides and 32 adducts/108 nucleotides, respectively, while the riddelliine exposure induced 1350 adducts/108 nucleotides (Chou & Fu, 2006). The DHP-derived DNA adducts (shown in Figure 5) include a pair of epimeric DHP-3′-dGMP adducts and 6 DHP-derived dinucleotides (Chou et al., 2003c).
FIGURE 5.
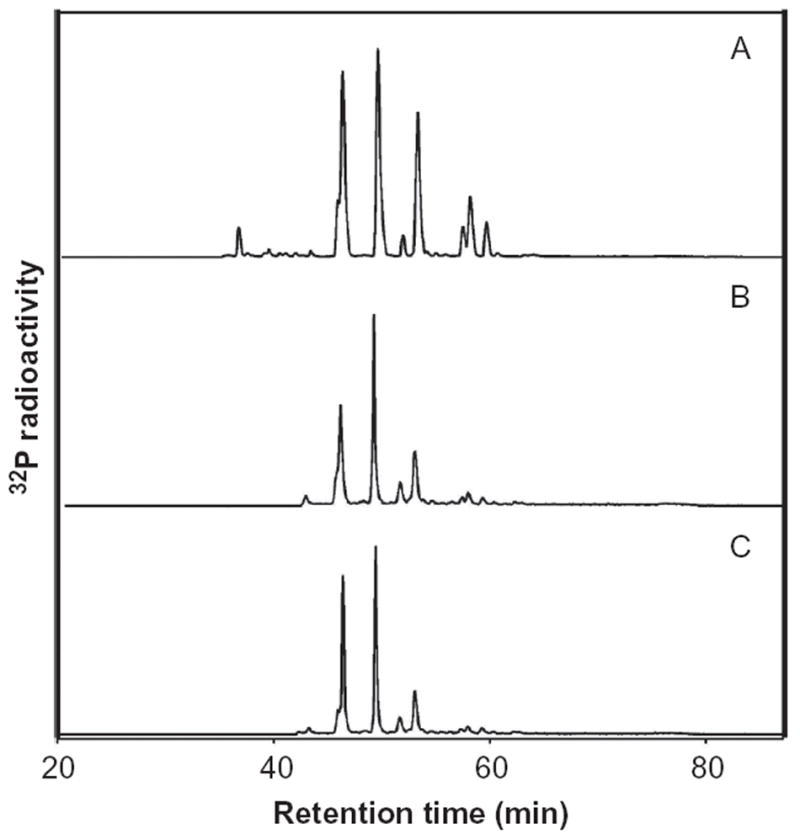
32P-Postlabeling/HPLC analysis of DHP-derived DNA adducts formed in the liver. Female F344 rats were treated with 1 mg/kg of riddelliine (A), 120 μl per day comfrey root extract (B), and 200 μl per day comfrey compound oil (C) by gavage for 3 consecutive days, and sacrificed 24 h after last treatment for DNA adduct analysis. Data from Chou and Fu (2006).
Alterations in the expression of genes and the implied biological functions in the liver of rats exposed to comfrey (mixture of the plant with PAs) and riddelliine (a prototypical carcinogenic PA) were compared (Guo et al., 2007). Using the Ingenuity Pathway Analysis Knowledge database, 46 functional processes were altered by both comfrey and riddelliine, which were about half of the functional processes altered by riddelliine and one-third of the functional processes altered by comfrey, with the top categories including cancer, cell death, cell morphology, cell-to-cell signaling and interaction, and tissue development (Guo et al., 2007). The expression of 387 cancer-related genes was significantly altered after comfrey exposure and the expression of 84 cancer-related genes was significantly changed by riddelliine treatment. Among them, there were 42 genes whose expressions were significantly altered by both comfrey and riddelliine treatments, with a strong correlation between the log2 fold changes of these genes. These common cancer-related genes include those involved in apoptosis and cell death, invasion, cell growth, cell morphology, and cell cycle (Guo et al., 2007). Data suggested that common mechanisms may be responsible for the toxicity and tumorigenicity of both comfrey and riddelliine, and that the carcinogenicity of comfrey is generated from PA.
GENOTOXICITY OF COMFREY
Using the Ames test strains Salmonella typhimurium TA98 and TA100, comfrey extracts (mainly PA) did not produce a positive genotoxic response in the presence and absence of S9, although adverse effects were found with TA98 (White et al., 1983). Using a plant test, Furmanowa et al. (1983) examined the mutagenic effects induced by comfrey extracts as well as lasiocarpine, which is a known carcinogen and served as a positive control in the study. Results showed that aqueous extracts of comfrey roots exerted antimitotic effects and induced chromosomal aberrations (CA). Frei et al. (1992) examined 16 PA, including 6 PA isolated from comfrey, for their genotoxic potency in the wing spot test of Drosophila melanogaster following oral application. All six PA isolated from comfrey were apparently genotoxic, with a rank order acetyl intermedine > acetyl lycopsamine > symphytine > symlandine > intermedine > lycopsamine (Frei et al., 1992). When a comfrey extract was investigated for its chromosome-damaging effect in human lymphocytes in vitro, the PA extract induced sister chromatid exchanges (SCE) and CA within the concentration range of 140–1400 μg/ml. The SCE-inducing capacity of comfrey was increased by simultaneous application of S9 mix (Behninger et al., 1989).
The mutagenicity of lasiocarpine, a PA in comfrey, was demonstrated in Salmonella (Haworth et al., 1983; Yamanaka et al., 1979) as well as in Drosophila (Yoon et al., 1985). Lasiocarpine was also tested for its effect in V79 Chinese hamster cells, and results demonstrated that lasiocarpine induced 8-azaguanine-resistant mutations in the presence of a metabolic activation system (Takanashi et al., 1980).
The mutagenicity of comfrey in rat liver was investigated in our laboratory using a transgenic rat mutational model (Mei et al., 2005). Groups of 6-wk-old male Big Blue rats were fed either a basal diet (NIH-31 pellets) or diets containing 2, 4, or 8% comfrey roots for 3 mo. This treatment schedule was based on the previous study that evaluated the carcinogenicity of comfrey (Hirono et al., 1978). The comfrey roots were ground and then blended with basal diet powder in a Hobart Mixer. Mutant frequencies (MF) were determined for the liver cII gene of rats treated with different concentrations of comfrey (Figure 6). Although the MF in the rats fed comfrey at different doses were about fourfold greater than the MF for control rats, there were no significant differences in the MF among the three comfrey-treated groups. These results were similar to the comfrey root-induced liver tumor incidence (Figure 4) and probably due to saturated mutagenic responses, even at the lowest dose used. Furthermore, in total, 200 and 99 independent mutations were identified from 2 and 8% comfrey-treated rats, respectively, and the types of mutations are summarized in Table 3. Statistical evaluation of these mutation spectra indicated that the spectra from comfrey-fed rats were significantly different from control, while there was no significant difference between the spectra induced by 2 and 8% comfrey-treated rats. G:C → T:A transversion was the major type of mutation in comfrey-fed rats, whereas G:C → A:T transition was the predominant mutation in the controls (Mei et al., 2005, 2006). In addition, an unusually high frequency of tandem base substitutions (13–17%) was observed among the mutations from comfrey-fed rats. The comfrey-induced mutation spectrum was compared with the spectrum induced by 1 mg/kg riddelliine, which also resulted in about a threefold increase in MF (Mei et al., 2004), and there was no significant difference between the spectra for comfrey and riddelliine (Mei et al., 2005). The unusual tandem base substitution mutations were suggested as a mutational signature for the genetic damage induced by PA (Mei et al., 2004). Overall, these results suggested that comfrey and riddelliine produced similar types of DNA adducts and mutational spectra, and that PA in comfrey are probably responsible for mutation induction and tumor initiation in rat liver.
FIGURE 6.
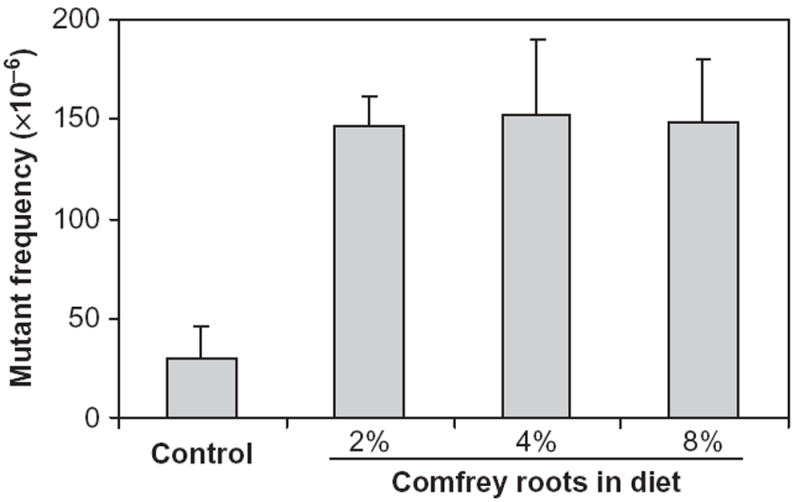
The cII mutant frequencies in the livers of rats treated with comfrey roots. Male Big Blue transgenic rats were fed a diet containing comfrey roots at different percentages (2, 4, and 8%) for 3 mo. Data represent means ± SD from six rats in each group. The comfrey-induced mutant frequencies were significantly higher than for controls (p < .05).
TABLE 3.
Summary of Independent Mutations in the Liver cII Gene From Rats Fed 2% and 8% Comfrey Roots
| Types of mutation | Control
|
2% Comfrey a
|
8% Comfrey b
|
|||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |
| Transitions | ||||||
| G:C → A:T | 20 | 43 | 24 | 12 | 11 | 11 |
| A:T → G:C | 1 | 2 | 9 | 4 | 6 | 6 |
| Transversions | ||||||
| G:C → T:A | 9 | 20 | 83 | 42 | 40 | 41 |
| G:C → C:G | 5 | 11 | 11 | 6 | 6 | 6 |
| A:T → T:A | 1 | 2 | 5 | 2 | 4 | 4 |
| A:T → C:G | 3 | 7 | 7 | 3 | 4 | 4 |
| Frameshifts | 7 | 15 | 26 | 13 | 14 | 14 |
| Complex | 0 | 0 | 2 | 1 | 1 | 1 |
| Tandem-base substitution | 0 | 0 | 33 | 17 | 13 | 13 |
| Total mutants screened | 46 | 200 | 99 | |||
Data from Mei et al. (2005).
Data from Mei et al. (2006).
The lungs, sequentially following liver, are the next most common site of PA-induced toxicity. Prakash et al. (1999) proposed that the biologically and toxicologically active pyrrolic metabolites formed in the liver are translocated to the lungs. The MF and mutational types in the lung cII gene from the rats fed 8% comfrey roots for 12 wk were also determined (Mei and Chen, 2007). In this study, the cII MF in the lungs after exposure to comfrey was 49 ± 9 × 10−6, which was significantly greater than that in the lungs of untreated control rats (34 ± 10 × 10−6). The mutational spectrum in lung from comfrey-fed rats was significantly different from the control, with 29% G:C → T:A and 16% A:T → T:A transversions. Data indicated that comfrey is mutagenic in rat lung and that mutagenicity of comfrey in rat lung results from the genotoxicity induced by PA present in the plant (Mei & Chen, 2007).
SUMMARY
Pyrrolizidine alkaloid-containing plants are probably the most common poisonous plants that affect livestock, wildlife, and humans. Comfrey is a PA-containing herbal plant that exhibits both therapeutic and toxicological activities. Although there are no epidemiological data regarding the carcinogenicity of comfrey, there are a number of cases that implicate human consumption of comfrey in the development of liver diseases. Comfrey and comfrey-containing PA are both genotoxic and tumorigenic in experimental animals, although the mechanisms are still not fully understood because comfrey contains multiple chemical constituents. Comparative studies showed that comfrey and riddelliine (a prototypical PA) induced similar profiles of DHP-derived DNA adducts as well as similar mutation spectrum profiles in the liver. In addition, there are strong correlations between gene expression fold change alterations produced by both agents, including drug-metabolizing genes and cancer-related genes. These results suggest that (1) comfrey induces liver tumors by a genotoxic mechanism, (2) comfrey and riddelliine may share common mechanisms of drug metabolism and carcinogenesis, and (3) PA contained in comfrey are the main active components responsible for rodent hepatic genotoxicity and tumor induction.
PA are common constituents of hundreds of plant species in many geographical regions globally, and are found as constituents or contaminants in many human food sources. Human exposure to PA typically occurs from contaminated foods, herbal preparations, and teas. Based on the studies reviewed here, there appears to be potential risk to human health posed by exposure to PA in the diet. Because of the concern about human exposure to genotoxic and tumorigenic PA, the International Programme on Chemical Safety (IPCS, 1989) considers PA a threat to human health and safety, and recently, the U.S. National Toxicology Program classified a PA (riddelliine) as “reasonably anticipated to be a human carcinogen” (NTP, 2008). Although many countries have restricted comfrey’s use based upon its carcinogenicity, for human health risk assessment it is anticipated that additional science-based mechanistic experimental data associated with comfrey and other PA may be useful to establish guidelines for regulatory decision making.
Acknowledgments
We thank Drs. Anane Aidoo and Fang Liu from NCTR for their helpful suggestions and comments. The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration.
References
- Abbott PJ. Comfrey: Assessing the low-dose health risk. Med J Aust. 1988;149:678–682. doi: 10.5694/j.1326-5377.1988.tb120821.x. [DOI] [PubMed] [Google Scholar]
- Altamirano JC, Gratz SR, Wolnik KA. Investigation of pyrrolizidine alkaloids and their N-oxides in commercial comfrey-containing products and botanical materials by liquid chromatography electrospray ionization mass spectrometry. J Am Off Assoc Chem Int. 2005;88:406–412. [PubMed] [Google Scholar]
- Bach N, Thung SN, Schaffner F. Comfrey herb tea-induced hepatic veno-occlusive disease. Am J Med. 1989;87:97–99. doi: 10.1016/s0002-9343(89)80492-9. [DOI] [PubMed] [Google Scholar]
- Behninger C, Abel G, Roder E, Neuberger V, Goggelmann W. Studies on the effect of an alkaloid extract of Symphytum officinale on human lymphocyte culturesetermination of pyrrolizidine alkaloids in commercial comfrey product. Planta Med. 1989;55:518–522. doi: 10.1055/s-2006-962084. [DOI] [PubMed] [Google Scholar]
- Betz JM, Eppley RM, Taylor WC, Andrzejewski D. Determination of pyrrolizidine alkaloids in commercial comfrey products (Symphytum sp.) J Pharm Sci. 1994;83:649–653. doi: 10.1002/jps.2600830511. [DOI] [PubMed] [Google Scholar]
- Bleakley CM, McDonough SM, MacAuley DC. Some conservative strategies are effective when added to controlled mobilisation with external support after acute ankle sprain: A systematic review. Aust J Physiother. 2008;54:7–20. doi: 10.1016/s0004-9514(08)70061-8. [DOI] [PubMed] [Google Scholar]
- Brauchli J, Luthy J, Zweifel U, Schlatter C. Pyrrolizidine alkaloids from Symphytum officinale L. and their percutaneous absorption in rats. Experientia. 1982;38:1085–1087. doi: 10.1007/BF01955382. [DOI] [PubMed] [Google Scholar]
- Chen T, Mei N, Fu PP. Genotoxicity of pyrrolizidine alkaloids. J Appl Toxicol. 2010;30:183–196. doi: 10.1002/jat.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MW, Fu PP. Formation of DHP-derived DNA adducts in vivo from dietary supplements and Chinese herbal plant extracts containing carcinogenic pyrrolizidine alkaloids. Toxicol Ind Health. 2006;22:321–327. doi: 10.1177/0748233706071765. [DOI] [PubMed] [Google Scholar]
- Chou MW, Jian Y, Williams LD, Xia Q, Churchwell M, Doerge DR, Fu PP. Identification of DNA adducts derived from riddelliine, a carcinogenic pyrrolizidine alkaloid. Chem Res Toxicol. 2003a;16:1130–1137. doi: 10.1021/tx030018y. [DOI] [PubMed] [Google Scholar]
- Chou MW, Wang YP, Yan J, Yang YC, Beger RD, Williams LD, Doerge DR, Fu PP. Riddelliine N-oxide is a phytochemical and mammalian metabolite with genotoxic activity that is comparable to the parent pyrrolizidine alkaloid riddelliine. Toxicol Lett. 2003b;145:239–247. doi: 10.1016/s0378-4274(03)00293-5. [DOI] [PubMed] [Google Scholar]
- Chou MW, Yan J, Nichols J, Xia Q, Beland FA, Chan PC, Fu PP. Correlation of DNA adduct formation and riddelliine-induced liver tumorigenesis in F344 rats and B6C3F(1) mice. Cancer Lett. 2003c;193:119–125. doi: 10.1016/s0304-3835(03)00045-4. [DOI] [PubMed] [Google Scholar]
- Couet CE, Crews C, Hanley AB. Analysis, separation, and bioassay of pyrrolizidine alkaloids from comfrey (Symphytum officinale) Nat Toxins. 1996;4:163–167. doi: 10.1002/19960404nt3. [DOI] [PubMed] [Google Scholar]
- Crews C, Startin JR, Clarke PA. Determination of pyrrolizidine alkaloids in honey from selected sites by solid phase extraction and HPLC-MS. Food Addit Contam. 1997;4:419–428. doi: 10.1080/02652039709374547. [DOI] [PubMed] [Google Scholar]
- Deinzer ML, Thomson PA, Burgett DM, Isaacson DL. Pyrrolizidine alkaloids: Their occurrence in honey from tansy ragwort (Senecio jacobaea L.) Science. 1977;195:497–499. doi: 10.1126/science.835011. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: Sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:27–42. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- Dueker SR, Lame MW, Morin D, Wilson DW, Segall HJ. Guinea pig and rat hepatic microsomal metabolism of monocrotaline. Drug Metab Dispos. 1992;20:275–280. [PubMed] [Google Scholar]
- Edgar JA, Roeder E, Molyneux RJ. Honey from plants containing pyrrolizidine alkaloids: A potential threat to health. J Agric Food Chem. 2002;50:2719–2730. doi: 10.1021/jf0114482. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. FDA advises dietary supplement manufacturers to remove comfrey products from the market. USFDA, Center for Food Safety and Applied Nutrition; 2001. http://www.fda.gov/Food/DietarySupplements/Alerts/ucm111219.htm. [Google Scholar]
- Frei H, Luthy J, Brauchli J, Zweifel U, Wurgler FE, Schlatter C. Structure/activity relationships of the genotoxic potencies of sixteen pyrrolizidine alkaloids assayed for the induction of somatic mutation and recombination in wing cells of Drosophila melanogaster. Chem Biol Interact. 1992;83:1–22. doi: 10.1016/0009-2797(92)90088-3. [DOI] [PubMed] [Google Scholar]
- Fu PP, Chou MW, Xia Q, Yang YC, Yan J, Doerge DR, Chan PC. Genotoxic pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides—Mechanisms leading to DNA adduct formation and tumorigenicity. J Environ Sci Health C Environ Carcinogen Ecotoxicol Rev. 2001;19:353–386. [Google Scholar]
- Fu PP, Xia Q, Lin G, Chou MW. Pyrrolizidine alkaloids—Genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab Rev. 2004;36:1–55. doi: 10.1081/dmr-120028426. [DOI] [PubMed] [Google Scholar]
- Fu PP, Xia Q, Chou MW, Lin G. Detection, hepatotoxicity, and tumorigenicity of pyrrolizidine alkaloids in Chinese herbal plants and herbal dietary supplements. J Food Drug Anal. 2007;15:400–415. [Google Scholar]
- Fuhr U. Induction of drug metabolising enzymes: Pharmacokinetic and toxicological consequences in humans. Clin Pharmacokinet. 2000;38:493–504. doi: 10.2165/00003088-200038060-00003. [DOI] [PubMed] [Google Scholar]
- Furmanowa M, Guzewska J, Beldowska B. Mutagenic effects of aqueous extracts of Symphytum officinale L. and of its alkaloidal fractions. J Appl Toxicol. 1983;3:127–130. doi: 10.1002/jat.2550030304. [DOI] [PubMed] [Google Scholar]
- Furuya T, Araki K. Studies on constituents of crude drugs. I. Alkaloids of Symphytum officinale Linn. Chem Pharm Bull (Tokyo) 1968;16:2512–2516. doi: 10.1248/cpb.16.2512. [DOI] [PubMed] [Google Scholar]
- Garrett BJ, Cheeke PR, Miranda CL, Goeger DE, Buhler DR. Consumption of poisonous plants (Senecio jacobaea, Symphytum officinale, Pteridium aquilinum, Hypericum perforatum) by rats: Chronic toxicity, mineral metabolism, and hepatic drug-metabolizing enzymes. Toxicol Lett. 1982;10:183–188. doi: 10.1016/0378-4274(82)90072-8. [DOI] [PubMed] [Google Scholar]
- Gomes MF, de Oliveira Massoco C, Xavier JG, Bonamin LV. Comfrey (Symphytum Officinale. L.) and experimental hepatic carcinogenesis: A short-term carcinogenesis model study. Evidence Based Complement Altern Med. 2007 doi: 10.1093/ecam/nem172.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DE, Porter A, O’Neill T, Harris RK, Rottinghaus GE. A rapid cleanup method for the isolation and concentration of pyrrolizidine alkaloids in comfrey root. J Am Off Assoc Chem Int. 2004;87:1049–1057. [PubMed] [Google Scholar]
- Guo L, Lobenhofer EK, Wang C, Shippy R, Harris SC, Zhang L, Mei N, Chen T, Herman D, Goodsaid FM, Hurban P, Phillips KL, Xu J, Deng X, Sun YA, Tong W, Dragan YP, Shi L. Rat toxicogenomic study reveals analytical consistency across microarray platforms. Nat Biotechnol. 2006;24:1162–1169. doi: 10.1038/nbt1238. [DOI] [PubMed] [Google Scholar]
- Guo L, Mei N, Dial S, Fuscoe J, Chen T. Comparison of gene expression profiles altered by comfrey and riddelliine in rat liver. BMC Bioinformatics. 2007;8(suppl. 7):S22. doi: 10.1186/1471-2105-8-S7-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorik S, Stricker H. Severe pulmonary hypertension possibly due to pyrrolizidine alkaloids in polyphytotherapy. Swiss Med Weekly. 2009;139:210–211. doi: 10.4414/smw.2009.12408. [DOI] [PubMed] [Google Scholar]
- Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E. Salmonella mutagenicity test results for 250 chemicals. Environ Mutagen. 1983;5(suppl. 1):1–142. [PubMed] [Google Scholar]
- Hirono I, Haga M, Fujii M, Matsuura S, Matsubara N, Nakayama M, Furuya T, Hikichi M, Takanashi H, Uchida E, Hosaka S, Ueno I. Induction of hepatic tumors in rats by senkirkine and symphytine. JNCI. 1979;63:469–472. [PubMed] [Google Scholar]
- Hirono I, Mori H, Haga M. Carcinogenic activity of Symphytum officinale. JNCI. 1978;61:865–869. [PubMed] [Google Scholar]
- Huxtable RJ, Luthy J, Zweifel U. Toxicity of comfrey–pepsin preparations. N Engl J Med. 1986;315:1095. [PubMed] [Google Scholar]
- Integrated Laboratory Systems. Comfrey and one of its constituent alkaloids symphytine. 1997 http://ntpniehsnihgov/ntp/htdocs/Chem_Background/ExSumPdf/Comfreypdf.
- International Programme on Chemical Safety. Health and Safety Criteria Guide 26. Geneva, Switzerland: WHO; 1989. Pyrrolizidine alkaloids health and safety guide. [Google Scholar]
- Kim NC, Oberlies NH, Brine DR, Handy RW, Wani MC, Wall ME. Isolation of symlandine from the roots of common comfrey (Symphytum officinale) using countercurrent chromatography. J Nat Prod. 2001;64:251–253. doi: 10.1021/np0004653. [DOI] [PubMed] [Google Scholar]
- Koll R, Buhr M, Dieter R, Pabst H, Predel HG, Petrowicz O, Giannetti B, Klingenburg S, Staiger C. Efficacy and tolerance of a comfrey root extract (Extr. Rad. Symphyti) in the treatment of ankle distorsions: Results of a multicenter, randomized, placebo-controlled, double-blind study. Phytomedicine. 2004;11:470–477. doi: 10.1016/j.phymed.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Lame MW, Morin D, Jones AD, Segall HJ, Wilson DW. Isolation and identification of a pyrrolic glutathione conjugate metabolite of the pyrrolizidine alkaloid monocrotaline. Toxicol Lett. 1990;51:321–329. doi: 10.1016/0378-4274(90)90075-w. [DOI] [PubMed] [Google Scholar]
- Lame MW, Jones AD, Morin D, Segall HJ. Metabolism of [14C]monocrotaline by isolated perfused rat liver. Drug Metab Dispos. 1991;19:516–524. [PubMed] [Google Scholar]
- Lin G, Cui YY, Hawes EM. Characterization of rat liver microsomal metabolites of clivorine, an hepatotoxic otonecine-type pyrrolizidine alkaloid. Drug Metab Dispos. 2000;28:1475–1483. [PubMed] [Google Scholar]
- Lin G, Cui YY, Liu XQ, Wang ZT. Species differences in the in vitro metabolic activation of the hepatotoxic pyrrolizidine alkaloid clivorine. Chem Res Toxicol. 2002;15:1421–1428. doi: 10.1021/tx0255370. [DOI] [PubMed] [Google Scholar]
- Lin G, Nnane IP, Cheng TY. The effects of pretreatment with glycyrrhizin and glycyrrhetinic acid on the retrorsine-induced hepatotoxicity in rats. Toxicon. 1999;37:1259–1270. doi: 10.1016/s0041-0101(98)00263-3. [DOI] [PubMed] [Google Scholar]
- Lin G, Zhou KY, Zhao XG, Wang ZT, But PP. Determination of hepatotoxic pyrrolizidine alkaloids by on-line high performance liquid chromatography mass spectrometry with an electrospray interface. Rapid Commun Mass Spectrom. 1998;12:1445–1456. doi: 10.1002/(SICI)1097-0231(19981030)12:20<1445::AID-RCM356>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Liu F, Wan SY, Jiang Z, Li SF, Ong ES, Osorio JC. Determination of pyrrolizidine alkaloids in comfrey by liquid chromatography–electrospray ionization mass spectrometry. Talanta. 2009;80:916–923. doi: 10.1016/j.talanta.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- Mattocks AR. Detection of pyrrolizidine alkaloids on thin-layer chromatograms. J Chromatogr. 1967;27:505–508. doi: 10.1016/s0021-9673(01)85914-8. [DOI] [PubMed] [Google Scholar]
- Mattocks AR. Chemistry and toxicology of pyrrolizidine alkaloids. New York: Academic Press; 1986. [Google Scholar]
- Mei N, Guo L, Fu PP, Heflich RH, Chen T. Mutagenicity of comfrey (Symphytum officinale) in rat liver. Br J Cancer. 2005;92:873–875. doi: 10.1038/sj.bjc.6602420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N, Guo L, Zhang L, Shi L, Sun YA, Fung C, Moland CL, Dial SL, Fuscoe JC, Chen T. Analysis of gene expression changes in relation to toxicity and tumorigenesis in the livers of Big Blue transgenic rats fed comfrey (Symphytum officinale) BMC Bioinformatics. 2006;7(suppl. 2):S16. doi: 10.1186/1471-2105-7-S2-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N, Heflich RH, Chou MW, Chen T. Mutations induced by the carcinogenic pyrrolizidine alkaloid riddelliine in the liver cII gene of transgenic Big Blue rats. Chem Res Toxicol. 2004;17:814–818. doi: 10.1021/tx049955b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei X, Chen T. The mutant frequency and types of mutations induced by comfrey in the lungs of transgenic Big Blue rats. J Food Drug Anal. 2007;15:458–465. [Google Scholar]
- National Toxicology Program. Bioassay of lasiocarpine for possible carcinogenicity (CAS No 303-34-4) 1978 http://ntpniehsnihgov/?objectid=07032341-E34A-AFA1-C61487B8222FC5A8.
- National Toxicology Program. Report on carcinogen. Fed Reg. 2008;73:23463–23465. http://ntp.niehs.nih.gov/files/23473_FR_23484_23430Apr22008_23508.pdf. [Google Scholar]
- Oberlies NH, Kim NC, Brine DR, Collins BJ, Handy RW, Sparacino CM, Wani MC, Wall ME. Analysis of herbal teas made from the leaves of comfrey (Symphytum officinale): reduction of N-oxides results in order of magnitude increases in the measurable concentration of pyrrolizidine alkaloids. Public Health Nutr. 2004;7:919–924. doi: 10.1079/phn2004624. [DOI] [PubMed] [Google Scholar]
- Prakash AS, Pereira TN, Reilly PE, Seawright AA. Pyrrolizidine alkaloids in human diet. Mutat Res. 1999;443:53–67. doi: 10.1016/s1383-5742(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Rao MS, Reddy JK. Malignant neoplasms in rats fed lasiocarpine. Br J Cancer. 1978;37:289–293. doi: 10.1038/bjc.1978.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MJ, Lame MW, Morin D, Wilson DW, Segall HJ. Involvement of cytochrome P450 3A in the metabolism and covalent binding of 14C-monocrotaline in rat liver microsomes. J Biochem Mol Toxicol. 1998;12:157–166. doi: 10.1002/(sici)1099-0461(1998)12:3<157::aid-jbt4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ridker PM, McDermott WV. Comfrey herb tea and hepatic veno-occlusive disease. Lancet. 1989;8639:657–658. doi: 10.1016/s0140-6736(89)92154-5. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Ohkuma S, McDermott WV, Trey C, Huxtable RJ. Hepatic venocclusive disease associated with the consumption of pyrrolizidine-containing dietary supplements. Gastroenterology. 1985;88:1050–1054. doi: 10.1016/s0016-5085(85)80027-5. [DOI] [PubMed] [Google Scholar]
- Ridker PN, McDermont WV. Hepatotoxicity due to comfrey herb tea. Am J Med. 1989;87:701. doi: 10.1016/s0002-9343(89)80414-0. [DOI] [PubMed] [Google Scholar]
- Rode D. Comfrey toxicity revisited. Trends Pharmacol Sci. 2002;23:497–499. doi: 10.1016/s0165-6147(02)02106-5. [DOI] [PubMed] [Google Scholar]
- Roeder E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie. 1995;50:83–98. [PubMed] [Google Scholar]
- Roitman JN. Comfrey and liver damage. Lancet. 1981;8226:944. doi: 10.1016/s0140-6736(81)91638-x. [DOI] [PubMed] [Google Scholar]
- Sakakura CE, Neto RS, Bellucci M, Wenzel A, Scaf G, Marcantonio E., Jr Influence of homeopathic treatment with comfrey on bone density around titanium implants: a digital subtraction radiography study in rats. Clin Oral Implants Res. 2008;19:624–628. doi: 10.1111/j.1600-0501.2007.01514.x. [DOI] [PubMed] [Google Scholar]
- Schaneberg BT, Molyneux RJ, Khan IA. Evaporative light scattering detection of pyrrolizidine alkaloids. Phytochem Anal. 2004;15:36–39. doi: 10.1002/pca.715. [DOI] [PubMed] [Google Scholar]
- Schoental R, Fowler ME, Coady A. Islet cell tumors of the pancreas found in rats given pyrrolizidine alkaloids from Amsinckia intermedia Fisch and Mey and from Heliotropium supinum L. Cancer Res. 1970;30:2127–2131. [PubMed] [Google Scholar]
- Snider S. Herbal teas and toxicity. FDA Consumer. 1991;25:30–33. [Google Scholar]
- Stickel F, Seitz HK. The efficacy and safety of comfrey. Public Health Nutr. 2000;3:501–508. doi: 10.1017/s1368980000000586. [DOI] [PubMed] [Google Scholar]
- Svoboda DJ, Reddy JK. Malignant tumors in rats given lasiocarpine. Cancer Res. 1972;32:908–913. [PubMed] [Google Scholar]
- Takanashi H, Umeda M, Hirono I. Chromosomal aberrations and mutation in cultured mammalian cells induced by pyrrolizidine alkaloids. Mutat Res. 1980;78:67–77. doi: 10.1016/0165-1218(80)90027-0. [DOI] [PubMed] [Google Scholar]
- Vollmer JJ, Steiner NC, Larsen GY, Muirhead KM, Molyneux RJ. Pyrrolizidine alkaloids: testing for toxic constituents of comfrey. J Chem Educ. 1987;64:1027–1030. [Google Scholar]
- Weston CF, Cooper BT, Davies JD, Levine DF. Veno-occlusive disease of the liver secondary to ingestion of comfrey. Br Med J (Clin Res Ed) 1987;295:183. doi: 10.1136/bmj.295.6591.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RD, Krumperman PH, Cheeke PR, Buhler DR. An evaluation of acetone extracts from six plants in the Ames mutagenicity test. Toxicol Lett. 1983;15:25–31. doi: 10.1016/0378-4274(83)90164-9. [DOI] [PubMed] [Google Scholar]
- Willett KL, Roth RA, Walker L. Workshop overview: Hepatotoxicity assessment for botanical dietary supplements. Toxicol Sci. 2004;79:4–9. doi: 10.1093/toxsci/kfh075. [DOI] [PubMed] [Google Scholar]
- Williams DE, Reed RL, Kedzierski B, Ziegler DM, Buhler DR. The role of flavin-containing monooxygenase in the N-oxidation of the pyrrolizidine alkaloid senecionine. Drug Metab Dispos. 1989;17:380–386. [PubMed] [Google Scholar]
- Winship KA. Toxicity of comfrey. Adverse Drug React Toxicol Rev. 1991;10:47–59. [PubMed] [Google Scholar]
- Wuilloud JC, Gratze SR, Gamble BM, Wolnik KA. Simultaneous analysis of hepatotoxic pyrrolizidine alkaloids and N-oxides in comfrey root by LC-ion trap mass spectrometry. Analyst. 2004;129:150–156. doi: 10.1039/b311030c. [DOI] [PubMed] [Google Scholar]
- Xia Q, Chou MW, Edgar JA, Doerge DR, Fu PP. Formation of DHP-derived DNA adducts from metabolic activation of the prototype heliotridine-type pyrrolizidine alkaloid, lasiocarpine. Cancer Lett. 2006;231:138–145. doi: 10.1016/j.canlet.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Nagao M, Sugimura T, Furuya T, Shirai A, Matsushima T. Mutagenicity of pyrrolizidine alkaloids in the Salmonella/mammalian-microsome test. Mutat Res. 1979;68:211–216. doi: 10.1016/0165-1218(79)90152-6. [DOI] [PubMed] [Google Scholar]
- Yan CC, Huxtable RJ. Effects of monocrotaline, a pyrrolizidine alkaloid, on glutathione metabolism in the rat. Biochem Pharmacol. 1996;51:375–379. doi: 10.1016/0006-2952(95)02189-2. [DOI] [PubMed] [Google Scholar]
- Yang YC, Yan J, Doerge DR, Chan PC, Fu PP, Chou MW. Metabolic activation of the tumorigenic pyrrolizidine alkaloid, riddelliine, leading to DNA adduct formation in vivo. Chem Res Toxicol. 2001;14:101–109. doi: 10.1021/tx000150n. [DOI] [PubMed] [Google Scholar]
- Yeong ML, Clark SP, Waring JM, Wilson RD, Wakefield SJ. The effects of comfrey derived pyrrolizidine alkaloids on rat liver. Pathology. 1991;23:35–38. doi: 10.3109/00313029109061438. [DOI] [PubMed] [Google Scholar]
- Yeong ML, Swinburn B, Kennedy M, Nicholson G. Hepatic veno-occlusive disease associated with comfrey ingestion. J Gastroenterol Hepatol. 1990;5:211–214. doi: 10.1111/j.1440-1746.1990.tb01827.x. [DOI] [PubMed] [Google Scholar]
- Yoon JS, Mason JM, Valencia R, Woodruff RC, Zimmering S. Chemical mutagenesis testing in Drosophila. IV. Results of 45 coded compounds tested for the National Toxicology Program. Environ Mutagen. 1985;7:349–367. doi: 10.1002/em.2860070310. [DOI] [PubMed] [Google Scholar]
- Zhou S, Koh HL, Gao Y, Gong ZY, Lee EJ. Herbal bioactivation: The good, the bad and the ugly. Life Sci. 2004;74:935–968. doi: 10.1016/j.lfs.2003.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]


