Radiation therapy is one of the oldest modalities for cancer treatment and is currently prescribed to more than 50% of all patients. It is based on delivering high doses of ionizing radiation to well-localized tumor targets in the body. The goal is to kill all the tumor cells with acceptable toxic effects to the surrounding normal tissue, which is unavoidably exposed. Indeed, radiotherapy success is limited by the toxicity in the normal tissue.
X-rays (photons) are used in most patients treated with conventional radiotherapy. As x-rays are delivered from an external source, they deposit most of their energy upstream of the tumor in healthy tissue. This energy deposition also occurs beyond the tumor, affecting additional healthy tissue. Special beams delivered from many directions and intensity modulation are used to increase the ratio of tumor to healthy tissue dose; however, the volume of irradiated healthy tissue increases (“dose bath”). Even with these technological advantages, x-rays are relatively inexpensive and serve as the biological response baseline for comparisons with other radiation treatment modalities.
A minority of patients are treated with proton therapy. Protons carry an electric charge, which accounts for their improved physical properties over x-rays. These physical properties result in the sparing of normal tissue because of a low energy deposition rate as they enter the body, followed by a steep increase (Bragg peak) at the end of their range (ie, when they reach the tumor), with essentially no energy delivered beyond the tumor. However, the scatter of protons in a lateral direction and the uncertainty in their physical range in tissue limit their precise delivery to a tumor target. Despite their physical advantage over x-rays, the biological effectiveness of protons is only marginally improved (by 10%). More than 120 000 patients have been treated with protons in 55 different centers worldwide, but facility costs are significantly higher than for x-rays. Indeed, the controversy over the cost-effectiveness of this treatment modality is ongoing.1
Ions heavier than protons, especially carbon ions, offer additional physical advantages over protons (Table).2 Due to their increased mass, heavier ions have limited lateral scattering and maintain their direction when aimed at a tumor. This results in sharp lateral dose deposition edges. Their physical range uncertainty mostly stems from patient imaging uncertainties and therefore is similar to that of protons; heavy ions exhibit a much sharper dose fall off than protons in the longitudinal direction. In addition, nuclear interactions activate the atomic nuclei of the irradiated tissue to induce localized radioactivity that can be externally imaged and used for in vivo tumor and normal tissue dose deposition verification. Heavy charged particles initially traversing normal tissues are only slightly more deleterious than x-rays in causing adverse effects in normal tissue. As they reach the end of their range and come to a stop in the tumor target, their biological effectiveness is greatly (up to 3 or 4 times) enhanced (Figure).2 This unique feature may allow heavy ions to effectively kill radioresistant tumors and potentially overcome therapeutic resistance due to hypoxia within the tumor, leading to an increase in the therapeutic ratio. The exact magnitude and quality of the biological effects of heavy ions along their path still carries uncertainties and is an active area of research. The uncertainty is caused by the special radiobiological properties of the densely ionizing heavy ion track, which can be a concern for normal tissue toxicity and an advantage for tumor ablation and for eliciting immune response. Only a few pediatric patients, typically treated with protons, have received carbon ion therapy so far, because of the potential risk of secondary cancers. Similarly to protons, irradiation of moving targets, as well as irradiation of static targets but through moving tissue, is technologically challenging with heavy ions because of the uncertainty and danger that the Bragg peak of incoming ions will be placed in healthy tissue. Robust treatment planning and motion management techniques were developed to mitigate the effect of these uncertainties, and clinical trials are being designed to study survival improvements while avoiding significant toxic effects. An example of an ongoing effort is a phase 3 randomized clinical trial comparing photons with carbon ions in unresectable locally advanced pancreatic cancer, sponsored by the US National Cancer Institute.
Table.
Comparison of X-rays, Protons, and Heavy Ions
| Parameter | X-rays | Protons | Carbon Ions |
|---|---|---|---|
| Volume of irradiated normal tissue | Large | Small | Smallest |
| Biological effectiveness | |||
| In normal tissue | Low | Low | Low |
| In tumor tissue | Low | Low | High |
| Targeting precision | Low | High | Highest |
| System cost | Low | High | Highest |
| Worldwide centers, No. | Thousands | 55 | 10 |
| Worldwide treated patients, estimate | Millions | 120 000 | 15 000 |
Figure. Biological Effectiveness vs Physical Dose Advantage.
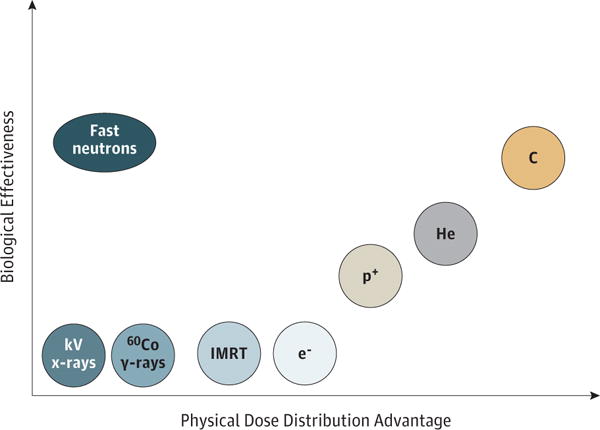
Heavy ions also have excellent biological properties because their DNA damage is difficult to repair. Carbon ions (C) have similar biological effectiveness to fast neutrons but better physics dose distribution thanks to the Bragg peak and the pencil-beam scanning. Protons (p+) have dose distribution similar to that of carbon, slightly worse because of the high lateral scattering, and a biological effectiveness similar to x-rays. 60Co indicates cobalt-60; e−, electrons; He, helium; IMRT, intensity-modulated radiotherapy.
This technology that precisely places a highly biologically effective dose of radiation specifically in a tumor target was originally pioneered between 1975 and 1992 at the Lawrence Berkeley National Laboratory.3 However, oncologists and clinical scientists in the United States have not had access to this cancer care tool for nearly a quarter century as this technology has been developed with national government support in Asia and Europe. The National Institute of Radiological Sciences (NIRS) in Chiba, Japan, has the greatest experience in heavy-ion therapy, specifically carbon ions, having treated more than 8000 cancer patients with carbon ions since 1994. Clinical experience at NIRS shows that carbon ions are effective in treating various solid-tumor histologic subtypes, including adenocarcinoma, adenoid cystic carcinoma, malignant melanoma, and various types of sarcomas, which are often resistant to conventional x-rays.4 Emerging clinical data have been reported for some gastrointestinal tumors, especially local recurrence of rectal cancer and pancreatic adenocarcinoma.4 The clinical success of the NIRS has led to the planning and establishment of 4 more carbon ion therapy centers in Japan. Other centers currently treating patients with carbon ions are located in Germany (Heidelberg and Marburg), Italy (Pavia), and China (Shanghai and Lanzhou). Several other centers are planned or are under construction in Europe and Asia.
The most serious impediment to developing heavy-ion therapy centers in the United States has been the high initial capital cost. The cost of a state-of-the-art heavy-ion system with the capacity to treat 1000 patients per year, while approximately twice as expensive as a similarly sized proton center, remains less than that of the development of a chemotherapeutic or biological agent. The elevated cost of a heavy-ion therapy system as compared with conventional x-rays is due to the complexity of the system needed to reach deeply seated tumors, which requires a particle accelerator, generally a synchrotron, measuring approximately 20 m in diameter. The circular beam with a diameter of approximately 5 mm needs to be magnetically steered both vertically and horizontally with submillimeter precision from a distance of approximately 2500 mm to “paint” the tumor in 3 dimensions with the prescribed therapeutic radiation dose.
Initiatives are already under way in the United States to bring heavy-ion therapy back. The National Cancer Institute has awarded 2 planning grants to establish the research components of a future US heavy-ion therapy facility.5 The Department of Energy has also awarded grants to fund technological developments of accelerator and delivery systems related to heavy-ion therapy. There is an immediate need to construct a heavy-particle therapy and research facility using existing, proven, and reliable technology to treat patients and conduct research, including rationally designed prospective clinical trials with well-defined and meaningful clinical end points. This US heavy-ion facility should be a national research and resource center. Concurrently, accelerator and beam delivery system research should continue to be supported by the Department of Energy and private industry. Defining the second phase of the US heavy-ion therapy effort would be the completion and commercialization of the next-generation accelerator and beam delivery systems that are more accurate and precise and, most importantly, less costly to build and maintain.
The appropriate implementation of heavy-ion therapy relies on an investment in comparative clinical trials aimed at understanding, optimizing, and personalizing the potential advantages of heavy ions using hypofractionated regimes alone or in combination with systemic and immunologic therapies. Such approaches should be complemented by use of radiogenomics and other biological assays to elucidate the biological response that would identify patients eligible for heavy-particle clinical trials. By necessity, this would require “big data” approaches for data analysis and mining to enhance the biological precision of this physically precise tool. The lack of comparative clinical trials is the main criticism against proton therapy.1 With appropriate oversight, stewardship, careful planning, and close collaboration of US and existing worldwide heavy-ion centers, the same fate will not be met with heavy-ion therapy.
Footnotes
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank Michael Story, PhD, Steve Jiang, PhD, Damiana Chiavolini, PhD, and Robert Timmerman, MD, for their fruitful discussions, revisions, and comments on this article. No compensation was received.
Contributor Information
Arnold Pompos, Department of Radiation Oncology, University of Texas Southwestern Medical Center, Dallas.
Marco Durante, Trento Institute for Fundamental Physics and Applications, National Institute of Nuclear Physics, Department of Physics, University of Trento, Trento, Italy.
Hak Choy, Department of Radiation Oncology, University of Texas Southwestern Medical Center, Dallas.
References
- 1.Mitin T, Zietman AL. Promise and pitfalls of heavy-particle therapy. J Clin Oncol. 2014;32(26):2855–2863. doi: 10.1200/JCO.2014.55.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeffler JS, Durante M. Charged particle therapy—optimization, challenges and future directions. Nat Rev Clin Oncol. 2013;10(7):411–424. doi: 10.1038/nrclinonc.2013.79. [DOI] [PubMed] [Google Scholar]
- 3.Castro JR, Saunders WM, Tobias CA, et al. Treatment of cancer with heavy charged particles. Int J Radiat Oncol Biol Phys. 1982;8(12):2191–2198. doi: 10.1016/0360-3016(82)90569-7. [DOI] [PubMed] [Google Scholar]
- 4.Kamada T, Tsujii H, Blakely EA, et al. Carbon ion radiotherapy in Japan. Lancet Oncol. 2015;16(2):e93–e100. doi: 10.1016/S1470-2045(14)70412-7. [DOI] [PubMed] [Google Scholar]
- 5.Laine A, Pompos A, Story M, Jiang S, Timmerman R, Choy H. International Symposium on Ion Therapy. Int J Part Ther. 2016;2(3):468–471. doi: 10.14338/IJPT-15-00028.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


