Abstract
Background
Hypertension (HTN) has long been associated with abdominal aortic aneurysm (AAA) development, and these cardiovascular pathologies are biochemically characterized by elevated plasma levels of angiotensin II (AngII) as well as interleukin-6 (IL-6). A biologic relationship between HTN and AAA has not been established, however. Accordingly, the objective of this study was to evaluate whether elevated tension may initiate IL-6 production to accumulate monocyte/macrophages and promote dilation of the abdominal aorta (AA).
Methods
An IL-6 infusion model (4.36 µg/kg/day) was created utilizing an osmotic infusion pump, and after 4 weeks, AA diameter was measured by digital microscopy. The AA was then excised for CD68 immunostaining and flow cytometric analysis with CD11b and F4/80 to identify macrophages. Aortic segments from wild-type mice were suspended on parallel wires in an ex vivo tissue myograph at experimentally derived optimal tension (1.2 g) and in the presence of elevated tension (ET, 1.7 g) for 3 hr, and expression of IL-6 and monocyte chemoattractant protein-1 (MCP-1) was evaluated by quantitative polymerase chain reaction (QPCR). Isolated aortic vascular smooth muscle cells (VSMCs) were subjected to 12% biaxial cyclic stretch or held static (control) for 3 hr (n = 7), and IL-6 and MCP-1 expressions were evaluated by QPCR.
Results
Four-week IL-6 infusion resulted in an AA outer diameter that was 72.5 ± 5.6% (P < 0.05) greater than that of control mice, and aortic dilation was accompanied by an accumulation of macrophages in the AA medial layer as defined by an increase in CD68 + staining as well as an increase by flow cytometric quantification of CD11b+/F4/80+ cells. Wild-type AA segments did not respond to ex vivo application of ET but cyclic stretch of isolated VSMCs increased IL-6 (2.03 ± 0.3 fold) and MCP-1 (1.51 ± 0.11 fold) expression compared to static control (P < 0.05). Pretreatment with the selective STAT3 inhibitor WP1066 blunted the response in both cases. Interestingly, AngII did not stimulate expression of IL-6 and MCP-1 above that initiated by tension and again, the response was inhibited by WP1066, supporting an integral role of STAT3 in this pathway.
Conclusions
An IL-6 infusion model can initiate macrophage accumulation as well as aortic dilation, and under conditions of elevated tension, this proinflammatory cytokine can be produced by aortic VSMCs. By activation of STAT3, MCP-1 is expressed to increase media macrophage abundance and create an environment susceptible to dilation. This biomechanical association between HTN and aortic dilation may allow for the identification of novel therapeutic strategies.
INTRODUCTION
Small asymptomatic abdominal aortic aneurysms (AAAs) are being increasingly diagnosed through the popular use of ultrasound screening, but there are currently no medical therapies directed at altering the underlying pathology as a means to attenuate growth. Development of AAA is a multifactorial process characterized by leukocyte infiltration, matrix degeneration, and vascular smooth muscle cell (VSMC) apoptosis, which have likewise been associated with several clinical risk factors.1–3 While hypertension (HTN) has been epidemiologically associated with AAA, a biomechanical relationship between increased wall tension and aneurysm formation has yet to be identified. Given that hypertensive as well as AAA patients have high serum levels of interleukin-6 (IL-6), systemic inflammation may provide this valuable link.4 For instance, monoclonal antibodies directed at proinflammatory cytokines can reverse aortic stiffening,5,6 suggesting that focusing on inflammation can meaningfully effect aortic matrix remodeling and potentially identify avenues to halt or reverse the degenerative changes leading to AAA. More specifically, the proinflammatory cytokine IL-6 can stimulate VSMC production of monocyte chemoattractant protein-1 (MCP-1) via the STAT3 transcription factor to contribute to accumulation and maturation of monocyte/macrophages, one cell population implicated in the production of proteases that drive matrix degradation and aortic dilation.7 The source of elevated IL-6 plasma levels has not been determined in HTN or AAA, but the potent vasoactive peptide angiotensin II (AngII) plays a role in both of these pathologies and can initiate IL-6 production in VSMCs.8 Considering the prevalence of vascular disease despite widespread use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, additional mechanisms must be studied. Previous work has demonstrated altered cell signaling due to increased aortic wall tension,9 and it has been hypothesized that elevated tension alone may initiate VSMC production of IL-6 to promote aortic remodeling. Therefore, the objectives of this study were to determine whether IL-6 treatment can accumulate monocyte/macrophages in the aortic media and initiate matrix degeneration to promote aortic dilation, and subsequently evaluate tension as a stimulus for IL-6 expression. Demonstrating the integral role of the IL-6-STAT3 pathway in aortic dilation has significant translational relevance and may allow development of targeted therapies to abrogate growth.
METHODS
In Vivo IL-6 Infusion
All animal care and surgical procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee (AR#3380). Following induction of anesthesia with 3% isoflurane and subcutaneous injection of 0.05 mg/kg buprenorphine, BPN3 mice (male and female, 14–16 weeks of age) underwent subcutaneous left flank implantation of an Alzet osmotic infusion pump (model 1004; Durect Corporation, Cupertino, CA) which delivered 4.36 µg/kg/day murine IL-6 (AF-216-16, Peprotech, Rocky Hill, NJ) for 4 weeks. Utilizing the dose-response curve generated by a murine colitis model,10 the current dose was calculated to approximate the plasma IL-6 levels of historical patients with small AAA.11 Mouse blood pressure was measured before and after IL-6 infusion utilizing the CODA8 tail-cuff method (Kent Scientific, Torrington, CT) as previously described.12 Following 4-week IL-6 infusion, the AA was exposed from the left renal vein to the bifurcation, and outer aortic diameter measurements were obtained by video microscopy methods previously described.13 The tissue was then harvested for histology, immunohistochemistry, or flow cytometric analysis.
Perfusion Fixation and Immunohistochemistry
Abdominal aortas from the left renal vein to the bifurcation were perfusion fixed in 10% formalin at approximately 100 mm Hg and subsequently soaked in 10% formalin for 48 hr. Aortic segments were then transferred to 70% ethanol for storage at 4°C. Fixed aortic segments were embedded in paraffin and sectioned at 5 µm thickness. In preparation for staining, tissue sections were deparaffinized, rehydrated, and treated with 1 % hydrogen peroxide in methanol for 30 min. A brief rinse in 0.2% phosphate-buffered saline and Tween-20 (PBST) immediately preceded a heat-induced epitope antigen retrieval phase performed in a sodium citrate buffer (pH 6.0). Following the antigen retrieval, blocking of nonspecific binding sites took place using 2% donkey serum (Abcam, Cambridge, UK) in a humidified chamber at 23°C. The CD68 primary antibody (Abcam, Cambridge, UK) incubation occurred in a humidified chamber at 23°C for 1 hr and 15 min, followed by Vectastain biotinylated secondary antibody exposure (Vector Laboratories, Burlingame, CA), PBST rinsing, and then Avidin-Biotin Complex coverage (Vector Laboratories, Burlingame, CA). A final DAB staining phase was conducted for 4 min before slide mounting and aortic section imaging with a Nikon Eclipse Ti Microscope with NIS imaging software. CD68 + macrophages were quantified in SigmaScan Pro 5 by averaging cell counts of 20 separate images that were sampled at 4 random, nonoverlapping, regions on each aortic section. Sampled segments of the aorta were taken from the beginning, middle, and end of the aortic serial sections to avoid potentially quantifying portions of the same macrophage present in directly adjacent sections. Additional infrarenal aortic sections from control and treated mice had luminal circumference measured with the SigmaScan software, and inner aortic (lumen) diameter was then calculated.
Flow Cytometry Analysis
Abdominal aortas from the left renal vein to the aortic bifurcation were collected in Accumax cell dissociation solution (AM105; Innovative Cell Technologies, San Diego, CA). Aortic tissue was then minced and placed in an enzyme digestion cocktail (1.25 mg/mL collagenase type1 (Gibco; 17100-017), 50 µg/mL porcine pancreatic elastase (Sigma-Aldrich; E1250-10MG), and 5 mM CaCl2 in base solution of Accumax) for 30 min at 37°C. Following digestion, cells were passed through a 70-µm cell strainer (252350; Falcon), rinsed with 4 mL of PBS, and centrifuged at 300 × g, for 10 min at 4°C. Cell pellet was collected and resuspended in PBS, then stained with live/dead near IR fixable dye (Thermo Fisher Scientific, Rochester, NY) according to the manufacturer’s instructions. Cells were blocked with mouse Fc receptor block (Miltenyi Biotec, San Diego, CA) and stained with conjugated primary antibodies: CD11b-FITC (clone M1/70, BD Biosciences, San Jose, CA) and F4/80-BV421 (clone BM8, Biolegends, San Diego, CA) for 20 min at 4°C. Before analysis, cells were washed and resuspended in PBS, and samples were then processed on a BD LSRFortessa X-20 cell analyzer (BD Bioscience) equipped with FACsDiva Software (BD Bioscience) with FlowJo V10 Software (Tree Star, Ashland, OR). The sequential gating strategy included selection by cell size, followed by a side-scatter technique to isolate single cells. Nonviable cells were then excluded and the remaining population was stained for the myeloid marker CD11b and the mature macrophage marker F4/80. Gates were set based on fluorescence minus one (FMO) controls.
SMC Culture and IL-6 Stimulation
Primary VSMC lines from murine aortic biopsies were established using an accepted outgrowth technique as previously described.14 The isolated VSMCs were maintained in SMC specific growth media with added supplement pack (SMC Growth Medium 2; C-22062, PromoCell, Heidelberg Germany) at 37°C in 5% CO2. Utilizing this approach, confluent VSMCs from culture passages 2–10 were treated with or without 10 ng/mL IL-6 (AF-216-16; Peprotech, Rocky Hill, NJ) for 3 hr and harvested for immunoblot analysis.
Immunoblot Analysis
Relative abundance of STAT3, pSTAT3 (Tyr705), and beta-actin were determined by immunoblotting. Briefly, 20 µg of each abdominal aortic tissue or VSMC homogenate, determined by the Pierce BCA total protein assay (23225; ThermoFisher Scientific, City, State), was fractionated on a 4% to 12% bis-tris gradient gel by electrophoresis. The proteins were transferred to nitrocellulose membranes (0.45 µm; Bio-Rad, Hercules, CA) and incubated in antisera specific for STAT3 (1:1,000; ab68153, Abcam, Cambridge, MA), pSTAT3 (Tyr705) (1:10,000; ab76315, Abcam), and betaactin (1:10,000; Biovision Inc.) in 5%BSA/PBS. A secondary peroxidase-conjugated antibody (primary antibody species specific) was applied (1:5,000; BSA/PBS), and signals were detected with a chemiluminescent substrate (Western Lighting Chemiluminescence Reagent Plus; PerkinElmer, San Jose, CA) and recorded on film. Band intensity was quantified using Gel-Pro Analyzer version 3.1.14 (Media Cybernetics, Silver Spring, MD).
Ex Vivo Tension Application
Following induction of anesthesia with 3% isoflurane and subcutaneous injection of 0.05 mg/kg buprenorphine, normotensive BPN3 mice (14–16 weeks of age; The Jackson Laboratory, Bar Harbor, ME) underwent laparotomy, and the abdominal aorta (AA) was harvested from the left renal vein to the bifurcation. Aortic segments were denuded of endothelium and suspended on parallel wires in an ex vivo tissue myograph in oxygenated physiologic salt solution. While maintained at experimentally derived optimal tension (OT, 1.2 g) or elevated tension (ET, 1.7 g) utilizing methods previously described,9,15 aortic segments were also treated with or without 100 nm AngII (A9525; Sigma). Following 3 hr of tension application, aortic segments were homogenized for gene expression analysis.
Biaxial Cyclic Tension and AngII Stimulation
VSMCs were seeded at a density of 5,000 cells per cm2 into amino coated Bioflex 6 well plates (BF-3001A; Flexcell International Corporation, Burlington, NC) and allowed to adhere overnight. Adherence culture media was then replaced with fresh complete VSMC media with or without a STAT3-specific inhibitor (WP1066; 2.5 µM, 573097; EMD Millipore Corp., Bellerica, MA) for 24 hr. Following 24-hr incubation, cell culture media was replaced with complete media with or without 100 nM AngII, and the plates were held under static conditions or subjected to 12% biaxial cyclic tension for 3 hr utilizing a Flexcell culture system (Flexcell International Corporation, Burlington, NC).
Quantitative Polymerase Chain Reaction
Total RNA was extracted using TRIzol Reagent (15596026, Thermo Fisher Scientific, Waltham, MA). One microgram of total RNA, determined by NanoDrop 2,000 (Thermo Fisher Scientific), was reverse transcribed and converted to complementary DNA (cDNA) using the iScript cDNA synthesis kit (1708891, Bio-Rad, Hercules, CA). Each cDNA sample was amplified with messenger RNA (mRNA) specific TaqMan Gene Expression Assays (IL-6, Mm00446190_m1; MCP-1, Mm00441242_m1; GAPDH, Mm99999915_g1; Thermo Fisher Scientific) on a CFX-96 real-time polymerase chain reaction machine (Bio-Rad). The relative expression of each mRNA was calculated and normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase. Expression values were calculated as 2−∆CT, in which the change in cycle threshold was defined as normalized gene expression.
Data Analysis
Comparisons between AA diameter measurements (inner and outer), CD68 + cell count, % CD11b+/F4/80 + cells, and pSTAT3/STAT3 ratio were made using the 2-sample t-test. mRNA levels were subjected to a one-way analysis of variance (prcomp: STATA) with post hoc analysis using pairwise comparison test. Data were then expressed as fold expression from baseline. Statistical tests were performed using STATA (Intercooled STATA 8, College Station, TX). Data are represented as mean ± standard error of the mean. Statistical significance was set at P < 0.05. Although the project utilized mice from both genders, it was not powered to detect sex-based differences in response to IL-6 infusion.
RESULTS
Interleukin-6 Infusion Induces AA Dilation and Infiltration of Macrophages into the Aortic Media
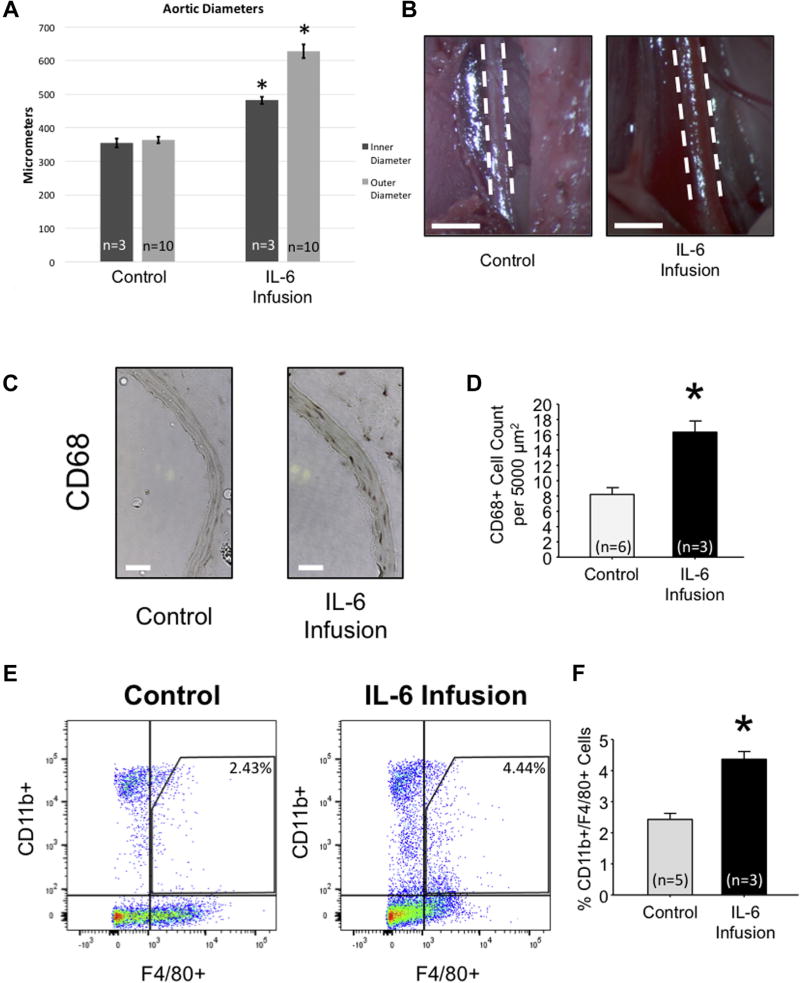
An IL-6 infusion model (4.36 µg/kg/day) was created utilizing an osmotic pump in normotensive mice. No mortality was noted in this model, and there was no change in murine blood pressure (data not shown). Following 4-week IL-6 infusion, digital microscopy revealed a 72.5% increase in AA outer diameter compared to nontreated control mice (627.32 ± 20.47 µm vs. 363.62 ± 12.29 µm; P < 0.05; Fig. 1A, B). Furthermore, the inner (lumen) diameter was calculated from histologic sections, and an increase of 35.97% was noted (354.18 ± 13.22 µm vs. 481.57 ± 10.13 µm; P < 0.05; Fig. 1A), thereby suggesting aortic wall thickening as well as outward remodeling under conditions of elevated circulating IL-6.
Fig. 1.
Interleukin-6 infusion induces AA dilation and infiltration of macrophages into the aortic media. (A) Inner abdominal aortic diameter as calculated from histology (n = 3) and outer abdominal aortic diameter (n = 10) as determined by calibrated video microscopy (µm) compared among wild-type normotensive mice (control) and wild-type mice infused with IL-6 for 28 days. (B) Representative images of the abdominal aorta from control and IL-6—treated mice. (C) Representative CD68 immunohistochemistry on control and IL-6 infused abdominal aortic tissue (DAB stains CD68+ cells brown). (D) Quantitation of CD68 + cells per 5,000 µm2 abdominal aortic tissue section of control (n = 6) and IL-6 infused aortas (n = 3). (E) Representative dot plot and (F) flow cytometric quantitation of % cells stained positive for CD11b and F4/80 in control (n = 5) and IL-6 (n = 3) abdominal aortic tissue. *P < 0.05 versus control.
Immunohistochemical analysis of perfusionfixed, paraffin-embedded AA tissue segments revealed increased CD68 + staining in mice infused with IL-6 (16.33 ± 1.47 cells per 5,000 µm2) when compared to nontreated controls (8.20 ± 0.886 cells per 5,000 µm2; P < 0.05; Fig. 1C, D). Flow cytometric analysis was performed on AA tissue utilizing a dual-marker approach (CD11 b and F4/80), allowing for the identification of mature macrophages isolated from fresh AA digested tissue. Flow cytometric analysis demonstrated an increase in CD11b+/F4/80 + cells in mice infused with IL-6 for 4 weeks as compared to controls (4.37 ± 0.25% vs. 2.43 ± 0.20%, P < 0.05; Fig. 1E, F). Together, these results suggest an approximate 2-fold increase in macrophage accumulation in the AA of mice infused with IL-6.
Interleukin-6 Stimulates Phosphorylation of STAT3 in Vascular Smooth Muscle Cells
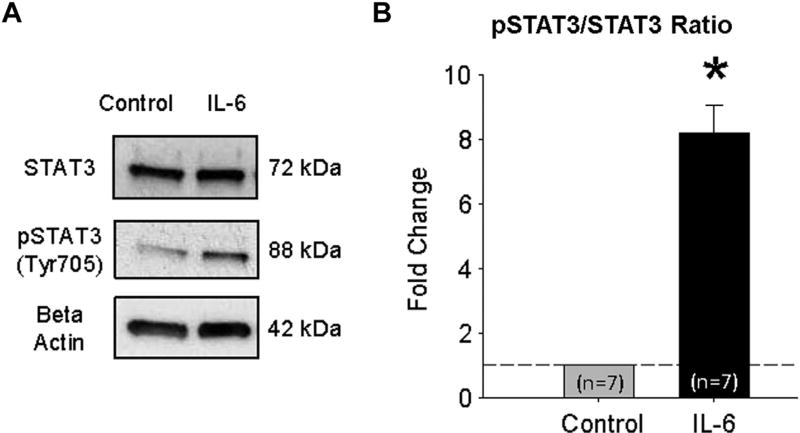
To examine the effects of IL-6 on phosphorylation of STAT3, VSMCs were exposed to 10 ng/mL IL-6 for 3 hr. VSMCs were then collected and homogenized for protein analysis by immunoblot. IL-6 stimulation resulted in an 8.19 ± 0.88 fold increase (P < 0.05) in pSTAT3 (Tyr703)/STAT3 ratio (Fig. 2).
Fig. 2.
Interleukin-6 stimulates phosphorylation of STAT3 in vascular smooth muscle cells. (A) Representative STAT3, phospho-STAT3 (pSTAT3) and beta-actin (loading control), immunoblot of VSMCs, serum starved for 24 hr, then treated without (control) or with 10 mg/mL IL-6 for 3 hr. (B) Densitometric quantification of the fold change in the ratio of pSTAT3/STAT3 abundance in VSMCs treated without (control), or with 10 mg/mL IL-6 for 3 hr (n = 7). *P < 0.05 versus control.
Mechanical Tension Increases IL-6 and MCP-1 mRNA Expression through Phosphorylation of STAT3
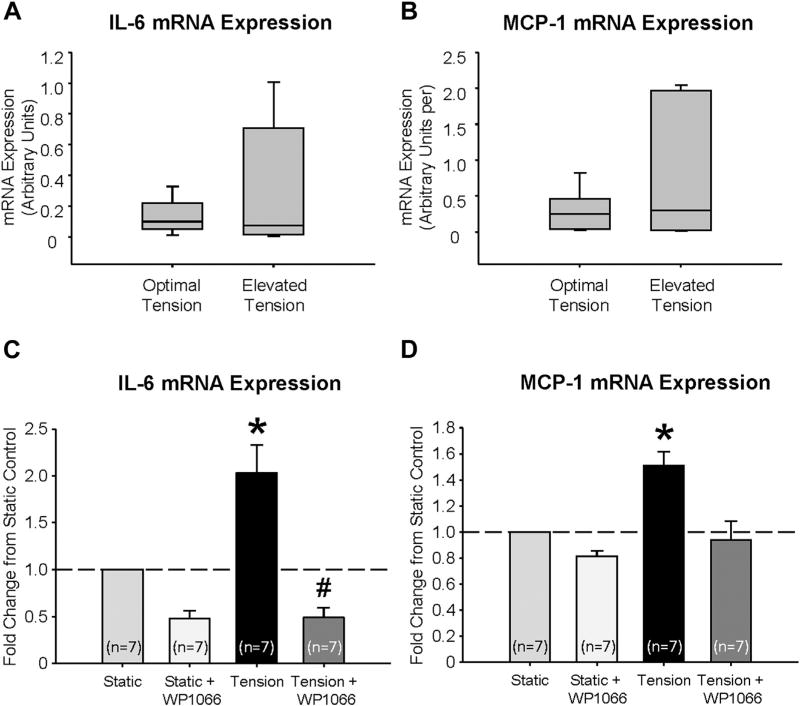
Utilizing an ex vivo tissue myograph, mRNA expression of IL-6 (Fig. 3A) and MCP-1 (Fig. 3B) were examined in AA segments exposed to 3 hr of ET, and no significant stimulation was noted. Addition of AngII treatment was also insufficient to elicit cytokine production. Isolated aortic VSMCs were then subjected to 3 hr of 12% biaxial cyclic stretch alone, and a 2.03 ± 0.3 fold increase (P < 0.05) in IL-6 and a 1.51 ± 0.11 fold increase (P < 0.05) in MCP-1 mRNA expression were acquired. Since STAT3 can be latent in the cytoplasm and activation may be accompanied by tyrosine phosphorylation at Tyr705 to induce dimerization, nuclear translocation, and binding to DNA,16 the effect of a 24-hr pretreatment with 2.5 µM of WP1066 was also completed. This specific STAT3 inhibitor prevented tension-induced expression of IL-6 and MCP-1 (P < 0.05; Fig. 3C, D).
Fig. 3.
Mechanical tension increases IL-6 and MCP-1 mRNA expression through phosphorylation of STAT3. (A) IL-6 and (B) MCP-1 mRNA expression in aortic tissue held at optimal (1.2 g, n = 7) or elevated tension (1.7 g, n = 7) on an ex vivo tissue myograph. (C) IL-6 and (D) MCP-1 mRNA expression in VSMCs held at static or tension, defined by 12% biaxial cyclic stretch, for 3 hr in complete media with or without 2.5 µM WP1066 (n = 7). *P < 0.05 versus control (static); #P < 0.05 versus tension.
AngII Stimulation Has No Effect on IL-6 and MCP-1 mRNA Expression
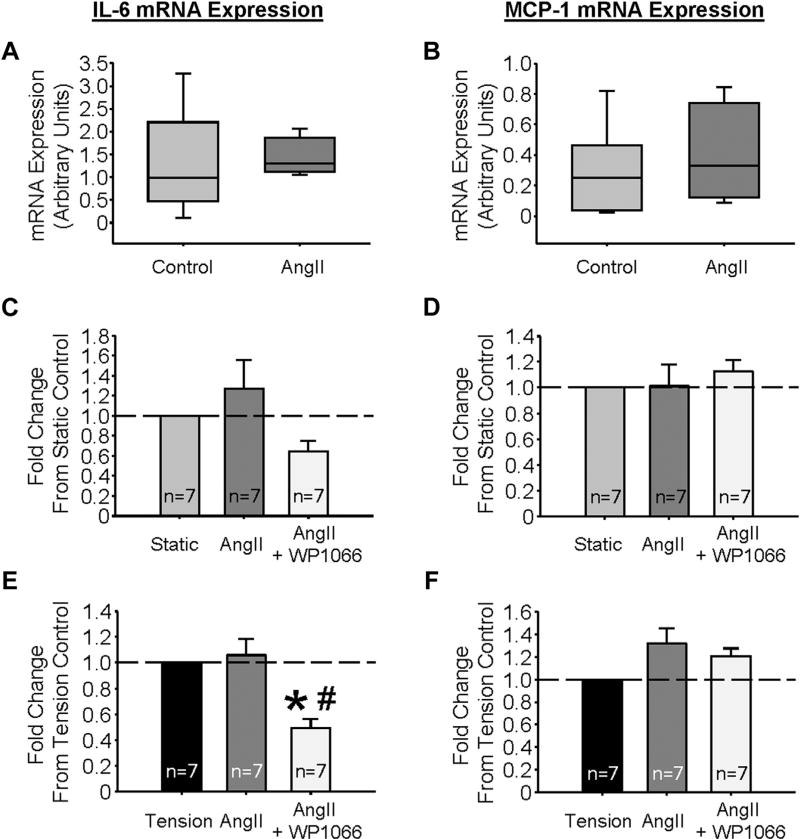
In AA segments held at OT in the presence of 100 nM AngII for 3 hr, no change in IL-6 or MCP-1 mRNA expression was detected (Fig. 4A, B, respectively). IL-6 and MCP-1 expressions were also assessed in VSMCs with or without 3 hr of 12% biaxial cyclic tension. Under static conditions, treatment with AngII did not augment expression of either cytokine (Fig. 4C, D), and interestingly, AngII failed to amplify IL-6 or MCP-1 mRNA abundance induced by tension alone (Fig. 4E, F, respectively), but pretreatment with WP1066 did significantly inhibit IL-6 transcription (Fig. 4E), supporting STAT3 as an operative transcription factor in this response.
Fig. 4.
Angiotensin II stimulation has no effect on IL-6 and MCP-1 mRNA expression. (A) IL-6 and (B) MCP-1 mRNA expression, in aortic tissue held at optimal (1.2 g, n = 7) tension in a solution void or containing 100 nM angiotensin II (AngII, n = 4). (C) IL-6 and (D) MCP-1 mRNA expression in VSMCs held under static conditions in media void or containing 100 nM AngII or AngII and 2.5 µM WP1066. (E) IL-6 and (F) MCP-1 mRNA expression in VSMCs held under tension (12% biaxial cyclic stretch) conditions in media void or containing 100 nM AngII or AngII and 2.5 µM WP1066. *P < 0.05 versus static; #P < 0.05 versus tension.
DISCUSSION
HTN contributes to cardiovascular morbidity and mortality 17 and is an independent risk factor for AAA.18 Investigations into the pathophysiology of hypertensive cardiovascular disease have identified a strong association with the proinflammatory cytokine IL-6, 19,20 and this peptide is likewise increased in plasma of patients with AAA.11 The mechanism of IL-6 production in vascular inflammation has previously been linked to AngII, but this investigation has identified that tension alone can initiate VSMC production of IL-6. VSMCs are also sensitive to IL-6 and signaling through phosphorylation of the transcription factor STAT3 may lead to downstream amplification of MCP-1. Moreover, a continuous infusion of IL-6 led to macrophage accumulation in the aortic media and propagated infrarenal aortic lumen dilation as well as aortic wall thickening, suggesting that this IL-6 infusion model may effectively emulate the early stages of aortic remodeling, as hypothesized in Figure 5. Further studies determining the specific role of the IL-6-STAT3 pathway in promoting macrophage accumulation and potential activation of matrix degrading protease production will enable engineering of therapeutic agents to attenuate this cascade and inhibit AAA growth.
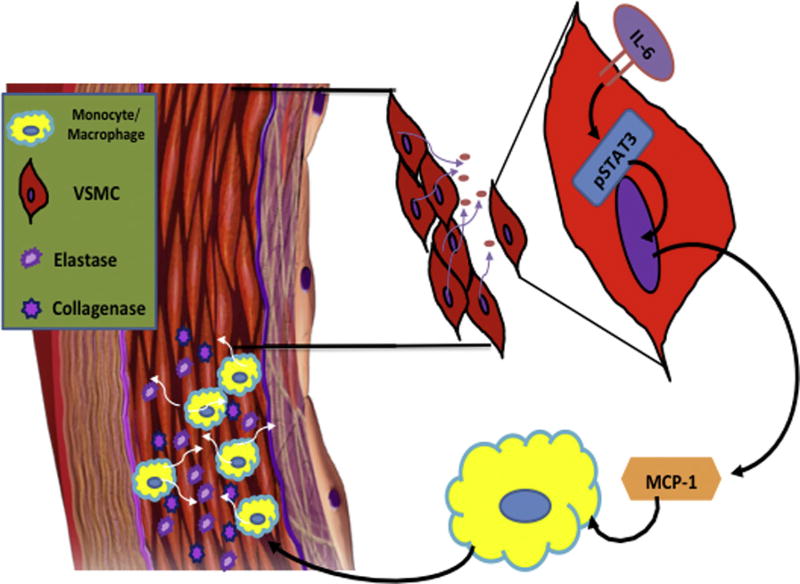
Fig. 5.
Hypothesized IL-6 pathway leading to early aortic remodeling. This model demonstrates a putative pathway whereby circulating IL-6 may bind to vascular smooth muscle cell (VSMC) IL-6 receptors, resulting in the phosphorylation of STAT3, which, once phosphorylated, will translocate into the nucleus and act as a transcription factor driving the production and subsequent secretion of the monocyte chemoattractant protein-1 (MCP-1). Secretion of MCP-1 may then stimulate migration and infiltration of monocytes/macrophages into the aortic wall, which secrete elastase and collagenase, contributing to vascular remodeling.
The clinical relationship between HTN and atherosclerotic plaque deposition has been attributed to incremental increases in IL-6 plasma levels correlating to climbing blood pressure measurements, even in otherwise healthy subjects.19,21 As a potent vasoactive peptide, AngII contributes to vasoconstriction to alter vascular wall tension, but more importantly IL-6 production in VSMCs can be augmented by AngII receptor-mediated signaling through the angiotensin type I receptor (AT1R).8,22,23 Accordingly, pharmacologic modulation of AngII activity has decreased IL-6 production in vitro,8 but widespread clinical utilization of these medications has had minimal impact on the incidence of vascular disease, suggesting additional contributing forces. Since the AT1R demonstrated mechanosensitivity in other cardiovascular systems,24,25 this project has hypothesized that tension alone may stimulate signaling through this receptor in aortic VSMCs and initiate IL-6 production. Utilizing an ex vivo tissue myograph, 3 hr of consistent elevated tension was unable to initiate IL-6 production. To overcome the potential confounding effects of the medial extracellular matrix, drug penetration, and the heterogeneous cell population of the murine AA, isolated VSMCs were then exposed to biaxial cyclic stretch and an increase in IL-6 and MCP-1 mRNA levels was observed, supporting the premise that tension can drive production of inflammatory mediators. Furthermore, pretreatment with the STAT3 inhibitor WP1066 prevented tension-induced expression of both IL-6 and MCP-1 in isolated aortic VSMCs, identifying STAT3 as a potential operative pathway.
With the close association between AngII and HTN, the receptor-mediated effects of AngII on IL-6 and MCP-1 expression were likewise examined in VSMCs with or without concurrent tension application. Interestingly, treatment with AngII had no effect on IL-6 or MCP-1 transcription under static conditions in isolated aortic VSMCs, and AngII did not augment the IL-6 and MCP-1 expression induced by tension alone. Pretreatment with WP1066 did prevent IL-6 expression under conditions of dual stimulation, however, further supporting STAT3 as a key transcription factor in this response.
Many clinical investigations have documented elevated plasma IL-6 levels in patients with AAA,4,11 and the correlation to aneurysm size has suggested that this cytokine is a contributor rather than consequence of degenerative aortic remodeling. 26 For instance, epidemiologic analysis of patients with a common IL-6 receptor polymorphism has demonstrated a lower rate of AAA formation with dysfunctional signaling.27,28 Alternatively, treatment with tocilizumab, an IL-6 receptor blocker, has been demonstrated to reverse aortic stiffening in patients with rheumatoid arthritis,5 indicating that IL-6 directly contributes to aortic cellular function and maintenance of the extracellular matrix. To examine this interaction more thoroughly, this investigation employed an IL-6 infusion model extrapolated from a murine model of colitis, 10 and this cytokine treatment led to significantly greater aortic inner and outer diameters at 4 weeks compared to untreated controls, suggesting that IL-6 directly impacts wall thickness as well as outward remodeling of the abdominal aorta. Additional experimentation to establish a correlation between murine plasma IL-6 levels and aortic diameter ought to be pursued. Moreover, this model may represent a new mechanism of initiating aneurysmal degeneration in mice, but further examination of protease and structural alterations in the abdominal aorta are needed for validation. Harvest techniques in this project included stripping of periadventitial adipose tissue, a process which also demonstrated histologic evidence of adventitial loss, compromising our ability to fully assess the effect of IL-6 on vascularity, leukocyte density, and thickness of the outermost layer of the aortic wall. If elevated IL-6 plasma levels can be related to HTN, atherosclerosis, and AAA, then modulating this cytokine production or receptor activity may significantly alter integrity of all aortic layers, further emphasizing the value of transmural investigation to refine therapeutic targeting.
Aortic production and response to IL-6 may also reflect regional heterogeneity. For instance, previous experimentation with plasma sampling from patients with AAA in different locations (ascending aorta versus descending thoracic aorta versus aneurysmal infrarenal aorta versus iliac artery) has suggested that the aneurysmal region itself has higher local IL-6 levels and therefore must produce IL-6. 29 The tension-dependent VSMC production of IL-6 documented in this study may directly relate to regional variance in plasma IL-6 values since the Law of Laplace dictates that the wall of the AAA experiences more tension compared to the nearby healthy region. Alternatively, this in vivo model of systemically elevated IL-6 levels in normotensive mice appeared to preferentially affect aortic morphology in the infrarenal location because preliminary investigations showed no change in descending thoracic aortic diameters (data not shown). When IL-6 was inhibited during murine aneurysm induction by the elastase method, however, AAA progression was unabated while TAA growth was significantly abrogated.30 This discrepancy in regional aortic response to IL-6 implies the complexity of this pathophysiology and emphasizes that further investigation into this pathway is imperative to adequately understanding degenerative aortic remodeling. Future directives of this project will explore AAA growth in mice undergoing IL-6 infusion as well as in IL-6 inhibition utilizing the periadventitial application of CaCl2. This model has the advantage of slow aneurysm growth to separate early versus late impact of IL-6, such as monocyte/macrophage infiltration versus macrophage maturation versus stimulation of protease production. Concurrent induction of additional clinical correlates such as HTN, diabetes mellitus, or obesity, may also be superimposed on this model to maximize translational efficacy to meaningfully impact patient care. In addition, defining time-and region-dependent impact of IL-6 on aneurysm growth will strengthen the argument for pharmaceutical development of IL-6 blockade to treat small AAA.
One potential mechanism by which IL-6 alters abdominal aortic integrity is by stimulating transcription of potent chemokines such as MCP-1 to accumulate leukocytes in the media. More specifically, monocyte/macrophages have been extensively studied in the initiation and progression of AAA,31,32 and knockout of the MCP-1 receptor, CCR-2, can abrogate murine aneurysm growth.33 Having demonstrated in vitro that IL-6 induces MCP-1 production from VSMCs and that the system is STAT3 dependent, this project went on to localize and quantify aortic macrophages by 2 different methods. Immunohistochemistry with a mature macrophage marker CD68 demonstrated localization to the media layer and confirmed a nearly 2-fold increase in macrophage abundance. Additional flow cytometric analysis of cells expressing the myeloid lineage marker CD11b + along with the murine mature macrophage marker F4/80 detected increased aortic macrophage accumulation under conditions of continuous IL-6 infusion, presumably due to local MCP-1 production and secretion. Since these cells are the documented source of several matrix metalloproteinases (MMPs) and cathepsins (Cts) known to be instrumental in AAA development,34,35 and more specifically, that MMP-9 and Cts contain a STAT3 binding site in their promoter regions,36,37 the medial accumulation of macrophages supports the pivotal role of the IL-6-STAT3 pathway in pathophysiology of aortic remodeling. Furthermore, accumulation of macrophages with an anti-inflammatory phenotype, frequently referred to as M2 or nonclassical macrophages, may be directed by the IL-6-STAT3 pathway with potential for subsequent profibrotic contribution to account for not only increased wall thickness but also elevated lumen diameter.38–40 Localizing these mechanisms to the media versus adventitia of the infrarenal aorta will be a major objective of future experimentation focused on the role of IL-6 in AAA.
Limitations
While this project has defined increased mechanical tension as an activator of the IL-6-STAT3 pathway and demonstrated that IL-6 infusion can accumulate monocyte/macrophages in the aortic media to promote aortic wall thickening as well as lumen dilation, several limitations have been identified. For instance, plasma levels associated with the IL-6 infusion model have not yet been quantified to demonstrate consistent penetration and potentially establish a correlation to aortic diameter. Similarly, the structural changes elicited by the IL-6 infusion need to be examined to determine whether the matrix degradation and protease production in the murine AA are consistent with those of clinical aneurysm samples to support this intervention as a new model of murine AAA. Moreover, the vascular layer contributing to the increase in wall thickness (media versus adventitia) ought to be further explored to determine how these fibrotic changes may be supported by IL-6, if production of a vasa vasorum has been stimulated, and whether the response may be abrogated. To address these inquiries, alterations in the manner of aortic harvest will need to be incorporated into further investigations. Considering the possibility that the nature of the accumulated macrophages may effect wall thickening, the methodology used to quantitate monocyte/macrophage accumulation after IL-6 infusion in this study cannot distinguish between monocyte/macrophage infiltration versus resident macrophage proliferation. Additional studies exploring cellular origin will be necessary to make that determination. With this in mind, the present project proposed that macrophage accumulation may be inhibited by decreasing MCP-1 production through treatment with the STAT3 inhibitor WP1066, but alterations in abundance and activity of the IL-6 receptor may also prove therapeutic. Finally, the specific mechanosensitive receptor responsible for IL-6 production has not been identified but the potential affiliation with AT1R will be further explored with the use of commercially available pharmaceutical receptor blockers.
CONCLUSION
An IL-6 infusion model can initiate macrophage accumulation as well as aortic dilation, and under conditions of elevated tension, this proinflammatory cytokine can be produced by aortic VSMCs. Through IL-6 activation of STAT3, MCP-1 is also expressed by VSMCs and may increase macrophage abundance in the aortic media to create an environment susceptible to dilation. This biomechanical association between HTN and aortic dilation may allow for the identification of novel therapeutic strategies to attenuate aneurysm growth.
Acknowledgments
This research project was supported by internal funding from the Medical University of South Carolina, Department of Surgery.
References
- 1.Middleton RK, Lloyd GM, Bown MJ, et al. The proinflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. J Vase Surg. 2007;45:574–80. doi: 10.1016/j.jvs.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Qin Y, Cao X, Yang Y, et al. Cysteine protease cathepsins and matrix metalloproteinases in the development of abdominal aortic aneurysms. Future Cardiol. 2013;9:89–103. doi: 10.2217/fca.12.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forsdahl SH, Singh K, Solberg S, et al. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994-2001. Circulation. 2009;119:2202–8. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 4.Juvonen J, Surcel HM, Satta J, et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vase Biol. 1997;17:2843–7. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 5.Kume K, Amano K, Yamada S, et al. Tocilizumab monotherapy reduces arterial stiffness as effectively as etanercept or adalimumab monotherapy in rheumatoid arthritis: an open-label randomized controlled trial. J Rheumatol. 2011;38:2169–71. doi: 10.3899/jrheum.110340. [DOI] [PubMed] [Google Scholar]
- 6.Protogerou AD, Zampeli B, Fragiadaki K, et al. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis. 2011;219:734–6. doi: 10.1016/j.atherosclerosis.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Frister A, Wang S, et al. Interaction of vascular smooth muscle cells and monocytes by soluble factors syner-gistically enhances IL-6 and MCP-1 production. Am J Physiol Heart Circ Physiol. 2009;296:H987–996. doi: 10.1152/ajpheart.01158.2008. [DOI] [PubMed] [Google Scholar]
- 8.Kranzhofer R, Schmidt J, Pfeiffer CA, et al. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vase Biol. 1999;19:1623–9. doi: 10.1161/01.atv.19.7.1623. [DOI] [PubMed] [Google Scholar]
- 9.Ruddy JM, Jones JA, Stroud RE, et al. Differential effects of mechanical and biological stimuli on matrix metalloprotei-nase promoter activation in the thoracic aorta. Circulation. 2009;120(11 Suppl):S262–268. doi: 10.1161/CIRCULATIONAHA.108.843581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan SL, Russell J, Granger DN. Platelet activation and platelet-leukocyte aggregation elicited in experimental colitis are mediated by interleukin-6. Inflamm Bowel Dis. 2014;20:353–62. doi: 10.1097/01.MIB.0000440614.83703.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takagi H, Watanabe T, Mizuno Y, et al. All-literature investigation of cardiovascular evidence G. Circulating interleukin-6 levels are associated with abdominal aortic aneurysm presence: a meta-analysis and meta-regression of case-control studies. Ann Vase Surg. 2014;28:1913–22. doi: 10.1016/j.avsg.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 12.Ruddy JM, Akerman AW, Kimbrough D, et al. Differential hypertensive protease expression in the thoracic versus abdominal aorta. J Vase Surg. 2017;66:1543–52. doi: 10.1016/j.jvs.2016.07.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikonomidis JS, Gibson WC, Gardner J, et al. A murine model of thoracic aortic aneurysms. J Surg Res. 2003;115:157–63. doi: 10.1016/s0022-4804(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 14.Ray JL, Leach R, Herbert JM, et al. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci. 2001;23:185–8. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 15.Ruddy JM, Jones JA, Stroud RE, et al. Differential effect of wall tension on matrix metalloproteinase promoter activation in the thoracic aorta. J Surg Res. 2010;160:333–9. doi: 10.1016/j.jss.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Ann Rev Biochem. 1995;64:621–51. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121:1768–77. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 18.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae CU, Lee RT, Rifai N, et al. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 20.Manhiani MM, Seth DM, Banes-Berceli AK, et al. The role of IL-6 in the physiologic versus hypertensive blood pressure actions of angiotensin U. Physiol Rep. 2015;3 doi: 10.14814/phy2.12595. pii: e12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamarthi B, Williams GH, Ricchiuti V, et al. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens. 2011;24:1143–8. doi: 10.1038/ajh.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–8. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funakoshi Y, Ichiki T, Ito K, et al. Induction of interleukin-6 expression by angiotensin U in rat vascular smooth muscle cells. Hypertension. 1999;34:118–25. doi: 10.1161/01.hyp.34.1.118. [DOI] [PubMed] [Google Scholar]
- 24.Barauna VG, Magalhaes FC, Campos LC, et al. Shear stress-induced Ang U ATI receptor activation: G-protein dependent and independent mechanisms. Biochem Biophys Res Commun. 2013;434:647–52. doi: 10.1016/j.bbrc.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Barauna VG, Mantuan PR, Magalhaes FC, et al. ATI receptor blocker potentiates shear-stress induced nitric oxide production via modulation of eNOS phosphorylation of residues Thr(495) and Ser(1177.) Biochem Biophys Res Commun. 2013;441:713–9. doi: 10.1016/j.bbrc.2013.10.108. [DOI] [PubMed] [Google Scholar]
- 26.Rohde LE, Arroyo LH, Rifai N, et al. Plasma concentrations of interleukin-6 and abdominal aortic diameter among subjects without aortic dilatation. Arterioscler Thromb Vase Biol. 1999;19:1695–9. doi: 10.1161/01.atv.19.7.1695. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira RC, Freitag DF, Cutler AJ, et al. Functional IL6R 358Ala allele impairs classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9:e1003444. doi: 10.1371/journal.pgen.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison SC, Smith AJ, Jones GT, et al. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J. 2013;34:3707–16. doi: 10.1093/eurheartj/ehs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson J, Cockerill GW, Choke E, et al. Aortic aneurysms secrete interleukin-6 into the circulation. J Vase Surg. 2007;45:350–6. doi: 10.1016/j.jvs.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 30.Pope NH, Salmon M, Johnston WF, et al. Interleukin-6 receptor inhibition prevents descending thoracic aortic aneurysm formation. Ann Thorac Surg. 2015;100:1620–6. doi: 10.1016/j.athoracsur.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch AE, Kunkel SL, Pearce WH, et al. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am J Pathol. 1993;142:1423–31. [PMC free article] [PubMed] [Google Scholar]
- 32.Middleton RK, Bown MJ, Lloyd GM, et al. Characterisation of Interleukin-8 and monocyte chemoattractant protein-1 expression within the abdominal aortic aneurysm and their association with mural inflammation. Eur J Vase Endovasc Surg. 2009;37:46–55. doi: 10.1016/j.ejvs.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 33.MacTaggart JN, Xiong W, Knispel R, et al. Deletion of CCR2 but not CCR5 or CXCR3 inhibits aortic aneurysm formation. Surgery. 2007;142:284–8. doi: 10.1016/j.surg.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Longo GM, Xiong W, Greiner TC, et al. Matrix metallopro-teinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–32. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong W, Knispel R, MacTaggart J, et al. Membrane-type 1 matrix metalloproteinase regulates macrophage-dependent elastolytic activity and aneurysm formation in vivo. J Biol Chem. 2009;284:1765–71. doi: 10.1074/jbc.M806239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Araki Y, Wada TT, Aizaki Y, et al. Histone methylation and STAT3 differentially regulate IL-6-induced MMP gene activation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol. 2015;68:1111–23. doi: 10.1002/art.39563. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura H, Kamon H, Sawa S, et al. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Fernando MR, Reyes JL, Iannuzzi J, et al. The proinflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One. 2014;9:e94188. doi: 10.1371/journal.pone.0094188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ju X, Ijaz T, Sun H, et al. IL-6 regulates extracellular matrix remodeling associated with aortic dilation in a fibrillin-1 hypomorphic mgR/mgR mouse model of severe Marfan syndrome. J Am Heart Assoc. 2014;3:e000476. doi: 10.1161/JAHA.113.000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Recinos A3rd, LeJeune WS, Sun H, et al. Angiotensin II induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis. 2007;194:125–33. doi: 10.1016/j.atherosclerosis.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]