Summary
Background
Restricted diffusion that is found on magnetic resonance diffusion-weighted imaging (DWI) typically indicates acute ischaemic stroke. However, restricted diffusion can also occur in other diseases, like metastatic brain tumours, which we describe in this case report.
Case Report
A 57-year-old male, with a diagnosis of small-cell cancer of the right lung (microcellular anaplastic carcinoma), was admitted with focal neurological symptoms. Initial brain MRI revealed multiple, disseminated lesions that were hyperintense on T2-weighted images and did not enhance after contrast administration; notably, some lesions manifested restricted diffusion on DWI images. Based on these findings, disseminated ischaemic lesions were diagnosed. On follow-up MRI that was performed after 2 weeks, we observed enlargement of the lesions; there were multiple, disseminated, sharply outlined, contrast-enhancing, oval foci with persistent restriction of diffusion. We diagnosed the lesions as disseminated brain metastases due to lung cancer. To our knowledge, this is the first description of a patient with brain metastases that were characterised by restricted diffusion and no contrast enhancement.
Conclusions
Multiple, disseminated brain lesions, that are characterised by restricted diffusion on DWI, typically indicate acute or hyperacute ischemic infarcts; however, they can also be due to hypercellular metastases, even if no contrast enhancement is observed. This latter possibility should be considered particularly in patients with cancer.
Keywords: Blood-Brain Barrier, Diffusion Magnetic Resonance Imaging, Neoplasm Metastasis, Neuroimaging, Small Cell Lung Carcinoma
Background
Diffusion weighted imaging (DWI) is based on an analysis of diffusion of water molecules (the so-called Brownian motion) [1,2]. On DWI, areas that are characterised by normal diffusion of water are iso- or hypointense. On DWI, restriction of diffusion is observed as hyperintense signal, whereas a corresponding signal attenuation is seen in apparent diffusion coefficient (ADC) maps. Moreover, ADC maps allow for a quantitative analysis of true values of diffusion coefficients [2].
Differential diagnosis of disseminated brain lesions that are characterised by restricted diffusion includes primarily acute or hyperacute ischaemic infarcts, multiple bacterial abscesses, demyelinating lesions in multiple sclerosis patients, and various forms of primary central nervous lymphomas (PCNSL) [1–3]. In general, cerebral metastases do not display restricted diffusion or the decline in signal on ADC maps [4]; in contrast, cerebral metastases could display increased diffusion with preserved or increased signal on ADC maps, if they are cystic or necrotic [1,2,5]. However, cases of cerebral metastases that were characterised by DWI hyperintensities with corresponding ADC hypointensities have been described [5,6]. Because such radiological features suggest disseminated ischemic infarcts, they constitute an important clinical issue. Cases of cerebral metastases with restriction of diffusion described to date occurred in patients with lung cancer, breast cancer, colon cancer, testicular cancer, and renal cancer [6–8]. Therefore, cerebral metastases should be included in the differential diagnosis of disseminated cerebral lesions that display restricted diffusion on DWI, especially in patients with a history of cancer.
We describe a patient with small-cell lung cancer who had disseminated cerebral lesions that were characterised by DWI hyperintensities and lack of the contrast enhancement. Based on conventional MRI with DWI sequence, these lesions were initially diagnosed as disseminated ischaemic lesions, and later turned out to be cerebral metastases.
Case Report
A 53-year-old man, with a history of hypertension and small-cell cancer of the right lung (pathologically anaplastic microcellular carcinoma) that was diagnosed 2 months earlier and treated palliatively with etoposide and cisplatin, was admitted because of a clonic seizure in the right extremities that occurred one day before.
On admission, the following neurological signs were present: slight facial asymmetry to the right, slight weakness of the right upper extremity, and slight ataxia on finger-to-nose testing; all signs were present throughout hospitalisation. Moreover, we observed clonic seizures of the right extremities that were successfully treated with intravenous clonazepam.
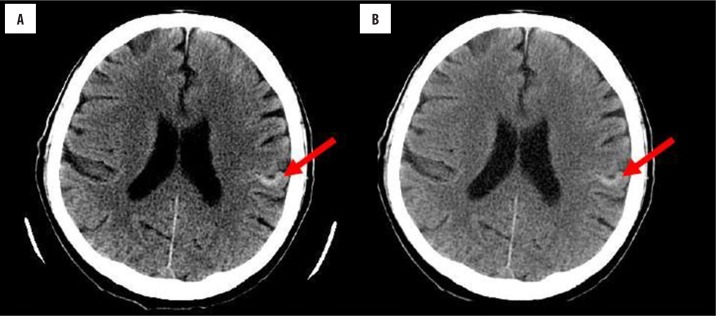
On contrast-enhanced haed computed tomography (CT) that was performed in the day of admission, we observed hyperdense foci in the frontal and parietal cortex on the left that did not enhance after contrast administration. They were interpreted as calcifications or haemorrhagic strokes (Figure 1).
Figure 1.
Brain CT performed after one day since onset of neurological symptoms: (A) before and (B) after contrast administration. A hyperdense lesion can be observed in the cortex of the left parietal gyrus.
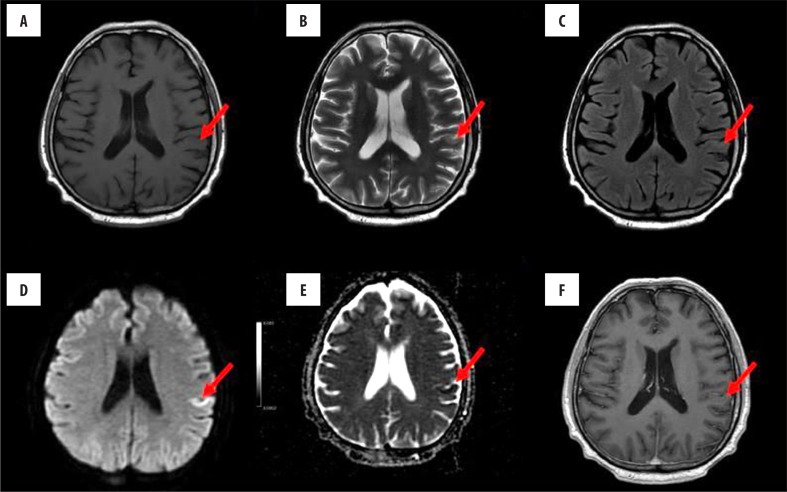
On brain MRI that was performed two days later, there were small, disseminated, T2 and FLAIR hyperintensities in the bilateral parieto-frontal areas, left temporal area, bilateral occipital areas, left thalamus, and lenticular nucleus. The lesions did not show contrast enhancement, and there was no oedema around them. Most lesions displayed restricted diffusion on DWI. Taken together, such radiological features indicated the presence of multiple acute and subacute ischaemic lesions (Figure 2).
Figure 2.
Brain MRI performed after 4 days since onset of neurological symptoms; before contrast administration: (A) T1-weighted, (B) T2-weighted, (C) FLAIR, (D) DWI-EPI, (E) ADC map images; (F) T1-weighted axial images after contrast administration. On T2-weighted and FLAIR images, a hyperintense lesion can be observed in the left parietal cortex; the lesion displays features of restricted diffusion, signal increase on DWI-EPI sequence and signal decrease on ADC map. There was no change in signal intensity after administration of contrast.
Doppler carotid ultrasound and echocardiography did not show any abnormalities that could be responsible for formation of thromboembolic material.
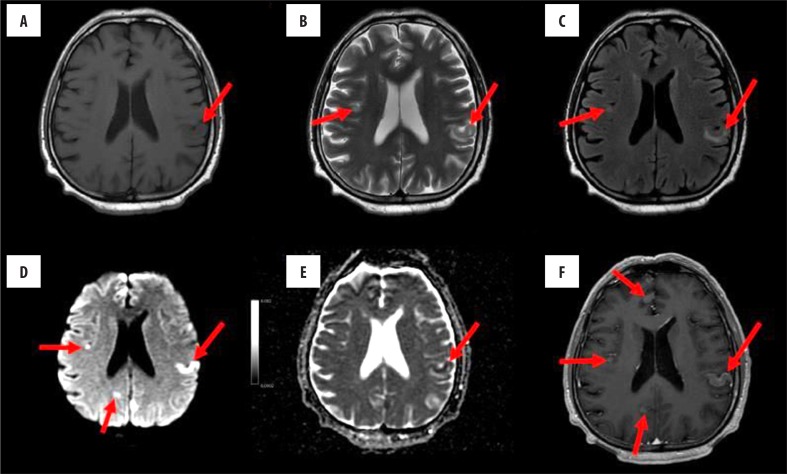
On brain MRI that was performed on the 16th day since symptom onset, there was enlargement of the lesions and persistence of restricted diffusion in the lesions in the cerebral cortex and basal ganglia. Moreover, contrast enhancement was observed in multiple, disseminated, well-delineated, oval lesions in the cerebral cortex of both brain hemispheres, pineal gland, and pons; there was no oedema around the lesions. Because a typical evolution of ischaemic lesions in DWI sequences was not observed, contrast enhancement in multiple lesions was observed, and the patient had lung cancer, we diagnosed cerebral metastases (Figure 3).
Figure 3.
Brain MRI perforemed sixteen days since onset of neurological symptoms; before contrast administration: (A) T1-weighted, (B) T2-weighted, (C) FLAIR, (D) DWI-EPI, and (E) ADC map images; (F) T1-weighted axial image after administration of contrast. On T2-weighted and FLAIR images, hyperintense foci can be observed in the left parietal cortex, together with new foci in the right frontal cortex and both parietal regions. On DWI, restricted diffusion is seen in both brain hemispheres. Signal intensity of the described lesions increased homogeneously after administration of contrast.
Discussion
Restricted diffusion on DWI is observed primarily in acute or hyperacute ischaemic infarcts, abscesses, and in certain hypercellular brain tumours. In ischaemic stroke, restriction of diffusion is due to cytotoxic oedema that leads to shrinkage of the extracellular space [1,9,10]. A relative restriction of diffusion, that is observed in abscesses, likely results from an increased density of abscess contents that include active and necrotic neutrophils, bacteria, and necrotic tissue, all of which inhibit diffusion of water molecules [1,7]. Most of demyelinating lesions in multiple sclerosis are characterised by increased ADC values, although certain acute demyelinating lesions can display restriction of diffusion with a corresponding decrease in signal in ADC maps. This finding is explained by a hypothetical presence of cytotoxic oedema in the myelin sheath, which can inhibit diffusion of water molecules in the extracellular space [11].
Primary brain tumours that display restricted diffusion include lymphomas [3], medulloblastoma [12], and highgrade gliomas (especially glioblastoma multiforme) [13]. It is suspected that restriction of diffusion that is observed in primary brain tumours is caused by hypercellular areas (i.e., areas with high cellular indices) [13–16] in which shrinkage of extracellular space impedes diffusion of water. Other potential causes of restricted diffusion in primary brain tumours include bacterial superinfection [17] and post-radiation tissue necrosis [18]. Also, one should be aware of the T2 shine-through effect that is related to T2-dependent components in DWI maps. Notably, this effect can be verified when DWI are compared with ADC maps, since only true restriction of diffusion causes decreased ADC values [2,5].
Restriction of diffusion that is observed in cerebral metastases could result from a high nuclear-cytoplasmic ratio with relative hypercellularity of cancer cells [5], liquefactive necrosis [7,18], and haemorrhage [19]. These factors could impede diffusion of water molecules in the extracellular space, thereby leading to typical findings on DWI and ADC maps [5].
A potential relationship between primary tumour cell type and presence of cerebral metastases that are characterised by restricted diffusion has been studied by some authors. Duygulu et al., in a study involving 76 patients, did not determine any significant relation between the cell type of primary tumours and presence of cerebral metastases displaying restriction of diffusion. However, those authors noted that cerebral metastases with restricted diffusion were observed primarily in patients with lung cancer (small-cell and non-small-cell carcinoma) or breast cancer. Moreover, 15 patients with colon cancer and prostate cancer also presented with cerebral metastases that displayed restricted diffusion [6].
In the study by Geijer et al., among 12 patients with cerebral metastases originating from various tumours, one patient with small-cell lung cancer had multiple lesions that suggested lacunar ischaemic infarcts on T2, T1, contrast-enhanced, DWI, and ADC sequences; in that patient, only a large number of lesions that were found throughout the brain indicated an alternative diagnosis. In the same study, half of patients had lung cancer (small-cell, non-small-cell, squamous cell carcinoma, and adenocarcinoma), and all cerebral metastases that originated from lung cancer were characterised by restricted diffusion and ring enhancement [8].
Based on the available literature [5,7,8,18], characteristic features of cerebral metastases, that are observed on CT and MRI, include distinct contrast enhancement that can be rim-like, tuberous, or homogenous [20]. These features differentiate cerebral metastases from subacute ischaemic infarcts in which contrast enhancement is seen on the borders of ischaemic foci, along the cerebral cortex (so-called luxury perfusion).
The fact that our patient had cerebral metastases that did not display contrast enhancement on initial MRI is exceptional. To the best of our knowledge, this is the first described case of cerebral metastases that were characterised by restricted diffusion and lack of contrast enhancement. Based on previous studies [21–24], we suppose that this was due to the fact that the blood-brain barrier was intact during the initial MRI examination, which prevented extravasation of contrast into the metastases. Another potential mechanism could involve a protective role of chemotherapeutics that were initially able to maintain the integrity of the blood-brain barrier [22]. Within two weeks, neoangiogenesis could contribute to disruption of the blood-brain barrier, which in turn could lead to extravasation of contrast material, as observed on the follow-up MRI examination in our patient. This hypothesis can be supported by case reports of patients who were treated with bevacizumab, an inhibitor of angiogenesis. Namely, in these patients, a decrease in or lack of contrast enhancement was observed in cerebral metastases, which was accompanied by reduction of vasogenic oedema [25]. Moreover, discontinuation of bevacizumab led to more pronounced contrast enhancement and oedema [26]. Differential diagnosis in our patient was difficult due to lack of vasogenic oedema that is usually found on T2 and FLAIR images; moreover, vasogenic oedema is characterised by facilitated diffusion on DWI [7]. To date, several cases of metastases that were not accompanied by oedema have been reported [21,27], which was explained by preservation of the blood-brain barrier at the early stage of the process of metastasizing.
An overlap between metastatic and ischaemic lesions can further hamper diagnosis. Cerebral metastases can occur in any part of the brain, but they tend to localise preferentially at sites typical for lacunar infarcts, such as the border between white and grey matter, basal ganglia, thalamus, and centrum semiovale [8,28,29]. Although most of the cerebral metastases that were observed in our patient were found in the cerebral cortex, which is atypical, similar metastases with a histopathological confirmation have been described in other reports [21,23].
It is worth to name other advanced MRI techniques that could improve the differential diagnosis of disseminated lesions that are characterised by restriction of diffusion. Magnetic resonance spectroscopy (MRS) shows an increased Cho/Cr, decreased NAA/Cr, and no change in ml/Cr ratios in the solid part of metastases that are enhancing following contrast administration [4,24,30–32]. Moreover, peaks of lactic acid and lipids can be seen in necrotic metastases [4]. As regards non-enhancing regions of metastases, MRS spectra should be similar to those for the white matter [31,32]. According to the study by Lin et al., in the centre of acute ischemic brain infarcts, one can observe decreased NAA/Cr and NAA/Cho ratios, as compared to the healthy brain hemisphere; moreover, the peak of lactic acid increases within 12 hours [33]. Due to an overlap of MRS spectra between cerebral metastases and ischaemic infarcts, MRS is not sufficient to differentiate between these diagnoses.
On perfusion-weighted imaging (PWI), cerebral metastases, having a rich vascular supply, display high relative cerebral blood volumes (rCBV), as compared to contralateral white matter [31,34–36]. Because hyperacute, acute, and subacute cerebral infracts are characterised by decreased rCBV, PWI can help differentiate between metastases and ischaemic infarcts.
Conclusions
Disseminated and non-contrast enhancing cerebral lesions with restricted diffusion on DWI typically characterise hyperacute and acute ischaemic infarcts, but they can also indicate hypercellular metastases. This latter possibility should be taken into account especially in patients with a history of cancer. If initial MRI in these patients reveals disseminated T2 and FLAIR hyperintensities with no contrast enhancement, follow-up imaging should be performed, as the blood-brain barrier can disrupt due to cancer progression, which can be visualized as contrast enhancement. This approach will help make correct diagnoses. In doubtful cases, PWI and other advanced MRI techniques can be employed.
References
- 1.Moritani T, Shrier DA, Numaguchi Y, et al. Diffusion-weighted echo-planar MR imaging: Clinical applications and pitfalls – a pictorial essay. Clin Imaging. 2000;24(4):181–92. doi: 10.1016/s0899-7071(00)00203-5. [DOI] [PubMed] [Google Scholar]
- 2.Krawczyk R, Ryterski J, Poniatowska R, et al. Diffusion-weighted magnetic resonance imaging – physical foundations and clinical application of the method. Postępy Psychiatrii i Neurologii. 2005;14(1):47–56. [Google Scholar]
- 3.Waliszewska-Prosół M, Bladowska J, Hałoń A, et al. An older woman after a first generalized seizure. Primary central nervous system lymphoma of the B cell. Eur Neurol. 2013:70(5–6): 366–68. doi: 10.1159/000353283. [DOI] [PubMed] [Google Scholar]
- 4.Al-Okaili RN, Krejza J, Wang S, et al. Advanced MR imaging techniques in the diagnosis of intraaxial brain tumors in adults. Radiographics. 2006;26(Suppl. 1):S173–89. doi: 10.1148/rg.26si065513. [DOI] [PubMed] [Google Scholar]
- 5.Mortimer A, O’Leary S, Bradley M, Renowden SA. Pitfalls in the discrimination of cerebral abscess from tumour using diffusion-weighted MRI. Clin Radiol. 2010;65(6):488–92. doi: 10.1016/j.crad.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Duygulu G, Ovali GY, Calli C, et al. Intracerebral metastasis showing restricted diffusion: correlation with histopathologic findings. Eur J Radiol. 2010;74(1):117–20. doi: 10.1016/j.ejrad.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann M, Jansen O, Heiland S, et al. Restricted diffusion within ring enhancement is not pathogenic for brain abscess. Am J Neuroradiol. 2001;22:1738–42. [PMC free article] [PubMed] [Google Scholar]
- 8.Geijer B, Holtås S. Diffusion-weighted imaging of brain metastases: Their potential to be misinterpreted as focal ischaemic lesions. Neuroradiology. 2002;44(7):568–73. doi: 10.1007/s00234-002-0792-0. [DOI] [PubMed] [Google Scholar]
- 9.Lövblad KO, Laubach HJ, Baird AE. Clinical expirience with diffusion-weighted MR in patients with acute stroke. Am J Neuroradiology. 1998;19:1061–66. [PMC free article] [PubMed] [Google Scholar]
- 10.Lövblad KO, Baird AE, Schlaug G, et al. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann Neurol. 1997;42(2):164–70. doi: 10.1002/ana.410420206. [DOI] [PubMed] [Google Scholar]
- 11.Tievsky AL, Ptak T, Farkas J. Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. Am J Neuroradiol. 1999;20(8):1491–99. [PMC free article] [PubMed] [Google Scholar]
- 12.Pillai S, Singhal A, Byrne AT, et al. Diffusion-weighted imaging and pathological correlation in pediatric medulloblastomas – “They are not always restricted!”. Childs Nerv Syst. 2011;27(9):1407–11. doi: 10.1007/s00381-011-1499-5. [DOI] [PubMed] [Google Scholar]
- 13.Ejma M, Waliszewska-Prosół M, Hofman A, et al. Rare clinical form of glioblastoma multiforme. Postepy Hig Med Dosw. 2014:68: 316–24. doi: 10.5604/17322693.1095834. [DOI] [PubMed] [Google Scholar]
- 14.Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9:53–60. doi: 10.1002/(sici)1522-2586(199901)9:1<53::aid-jmri7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita Y, Kumabe T, Higano S, et al. Minimum apparent diffusion coefficient is significantly correlated with cellularity in medulloblastomas. Neurol Res. 2009;31(9):940–46. doi: 10.1179/174313209X382520. [DOI] [PubMed] [Google Scholar]
- 16.Doskaliyev A, Yamasaki F, Ohtaki M, et al. Lymphomas and glioblastomas: differences in the apparent diffusion coefficient evaluated with high b-value diffusion-weighted magnetic resonance imaging at 3T. Eur J Radiol. 2012;81(2):339–44. doi: 10.1016/j.ejrad.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Bantra A, Tripathi RP. Atypical diffusion-weightet magnetic resonance findings in glioblastoma multiforme. Australas Radiol. 2004;48(3):388–91. doi: 10.1111/j.0004-8461.2004.01324.x. [DOI] [PubMed] [Google Scholar]
- 18.Tung GA, Evangelista P, Rogg JM, Duncan JA., 3rd Diffusion-weighted MR imaging of rim-enhancing brain masses: Is markedly decreased water diffusion specific for brain abscess? Am J Roentgenol. 2001;177(3):709–12. doi: 10.2214/ajr.177.3.1770709. [DOI] [PubMed] [Google Scholar]
- 19.Mori H, Abe O, Aoki S, et al. Hemorrhagic brain metastases with high signal intensity on diffusion-weighted images: A case report. Acta Radiol. 2002;43:563–66. doi: 10.1080/j.1600-0455.2002.430604.x. [DOI] [PubMed] [Google Scholar]
- 20.Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013;4(Suppl. 4):S209. doi: 10.4103/2152-7806.111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inomata M, Hayashi R, Kambara K, et al. Miliary brain metastasis presenting with calcification in a patient with lung cancer: A case report. J Med Case Rep. 2012:6: 279. doi: 10.1186/1752-1947-6-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemzek W, Poirier V, Salamat MS, Yu T. Carcinomatous encephalitis (miliary metastases): Lack of contrast enhancement. Am J Neuroradiol. 1993;14(3):540–42. [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa M, Kurahashi K, Ebina A, et al. Miliary brain metastasis presenting with dementia: Progression pattern of cancer metastases in the cerebral cortex. Neuropathology. 2007;27(4):390–95. doi: 10.1111/j.1440-1789.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 24.van de Pol M, van Oosterhout AG, Wilmink JT, et al. MRI in detection of brain metastases at initial staging of small-cell lung cancer. Neuroradiology. 1996;38(3):207–10. doi: 10.1007/BF00596529. [DOI] [PubMed] [Google Scholar]
- 25.Sivasundaram L, Hazany S, Wagle N, et al. Diffusion restriction in a non-enhancing metastatic brain tumor treated with bevacizumab – recurrent tumor or atypical necrosis? Clin Imaging. 2014;38(5):724–26. doi: 10.1016/j.clinimag.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Karimi S, Lis E, Gilani S, D’Ambrosio N, Holodny A. Nonenhancing brain metastases. J Neuroimaging. 2011;21(2):184–87. doi: 10.1111/j.1552-6569.2009.00389.x. [DOI] [PubMed] [Google Scholar]
- 27.Bekiesińska-Figatowska M, Kuczyńska-Zardzewiały A, Klepacka T, et al. Miliary brain metastases from papillary adenocarcinoma of the lung – unusual MRI pattern with histopathologic correlation. Pol J Radiol. 2013;78(3):57–60. doi: 10.12659/PJR.889182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang TL, Close TP, Grego JM, et al. Predilection of brain metastasis in gray and white matter junction and vascular border zones. Cancer. 1996;77(8):1551–55. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1551::AID-CNCR19>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi JA, Llena JF, Hirano A. Pathology of cerebral metastases. Neurosurg Clin North Am. 1996;7:345–67. [PubMed] [Google Scholar]
- 30.Bulakbasi N, Kocaoglu M, Ors F, et al. Combination of single-voxel proton MR spectroscopy and apparent diffusion coefficient calculation in the evaluation of commonrsingle-voxel proton MR ain tumors. Am J Neuroradiol. 2003;24(2):225–33. [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang IC, Kuo YT, Lu CY, et al. Distinction between highgrade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology. 2004;46(8):619–27. doi: 10.1007/s00234-004-1246-7. [DOI] [PubMed] [Google Scholar]
- 32.Fan G, Sun B, Wu Z, et al. In vivo single-voxel proton MR spectroscopy in the differentiation of high-grade gliomas and solitary metastases. Clin Radiol. 2004;59(1):77–85. doi: 10.1016/j.crad.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Lin AQ, Shou JX, Li XY, et al. Metabolic changes in acute cerebral infarction: Findings from proton magnetic resonance spectroscopic imaging. Exp Ther Med. 2014;7(2):451–55. doi: 10.3892/etm.2013.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bendini M, Marton E, Feletti A, et al. Primary and metastatic intraaxial brain tumors: prospective comparison of multivoxel 2D chemical-shift imaging (CSI) proton MR spectroscopy, perfusion MRI, and histopathological findings in a group of 159 patients. Acta Neurochir (Wien) 2011;153(2):403–12. doi: 10.1007/s00701-010-0833-0. [DOI] [PubMed] [Google Scholar]
- 35.Calli C, Kitis O, Yunten N, et al. Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur J Radiol. 2006;58(3):394–403. doi: 10.1016/j.ejrad.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 36.Hakyemez B, Erdogan C, Gokalp G, et al. Solitary metastases and high-grade gliomas: radiological differentiation by morphometric analysis and perfusion-weighted MRI. Clin Radiol. 2010;65(1):15–20. doi: 10.1016/j.crad.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer PW, Ozsunar Y, He J, et al. Assessing tissue viability with MR diffusion and perfusion imaging. Am J Neuroradiol. 2003;24(3):436–43. [PMC free article] [PubMed] [Google Scholar]