Abstract
Background
Innate immunity including macrophages (Mϕ) in lupus nephritis (LN) has been gaining attention, but roles of Mϕ in LN remain uncertain.
Methods
Immunohistochemical staining was performed to determine CD68, CD163, heme oxygenase (HO)-1 (a stress-inducible heme-degrading enzyme with anti-inflammatory property), pSTAT1, and CMAF-expressing Mϕ in the glomeruli of patients with LN. Effects of type I interferons on the expression levels of CD163, HO-1, BTB and CNC homology 1 (Bach1; a transcriptional HO-1 repressor), interleukin (IL)-6, and IL-10 by human M2-like Mϕ, which were differentiated in vitro from peripheral monocytes with macrophage colony-stimulating factor, were assessed by RT-PCR and immunocytostaining. Clinical manifestations, anti-double-stranded DNA (anti-dsDNA), and local HO-1 expression were compared in Bach1-deficient and wild-type MRL/lpr mice.
Results
The number of glomerular M2-like Mϕ correlated with the amounts of proteinuria in patients with LN. Unlike monocyte-derived M2-like Mϕ, HO-1 expression was defective in the majority of glomerular M2-like Mϕ of patients with LN. Stimulation of human M2-like Mϕ with type I interferons led to reduced HO-1 expression and increased Bach1 and IL-6 expression. Bach1-deficient MRL/lpr mice exhibited increased HO-1 expression in kidneys, prolonged survival, reduced urine proteins, and serum blood urea nitrogen levels, but serum anti-dsDNA antibody levels were comparable. Increased expression of CD163 and HO-1 was found in peritoneal Mϕ from Bach1-deficient MRL/lpr mice.
Conclusions
Our data suggest that dysregulated M2-like Mϕ play a proinflammatory role in LN. Bach1 is a potential therapeutic target that could restore the anti-inflammatory property of M2 Mϕ.
Electronic supplementary material
The online version of this article (10.1186/s13075-018-1568-1) contains supplementary material, which is available to authorized users.
Keywords: Lupus nephritis, Heme oxygenase 1, Bach1, Type I interferons, Macrophage polarization
Background
Systemic lupus erythematosus (SLE) is an autoimmune disease with a broad spectrum of clinical presentations [1]. Lupus nephritis (LN) occurs in approximately 25–50% of patients with SLE and remains one of the leading causes of morbidity [2]. New immunosuppressive therapies such as mycophenolate mofetil have been improving disease outcomes in patients with SLE, but some patients are refractory to standard treatments [3, 4]. Unlike rheumatoid arthritis, development of biologics to treat LN has been challenging, partially owing to its disease heterogeneity [5]. Thus, unmet needs remain for patients with LN who are refractory to conventional remission-induction therapy.
Although abnormalities in acquired immunity, such as the presence of autoreactive T and B cells and autoantibodies, are considered a hallmark of SLE, recent studies have also highlighted the critical roles of innate immunity, including macrophages (Mϕ), in SLE. It has been shown that Mϕ are abundantly present in LN glomerulus and that the number of glomerular Mϕ positively correlates with proteinuria level [6]. Moreover, depletion of Mϕ ameliorated antibody-induced LN in a nephritis model [7]. Recently, the novel concept of Mϕ subsets emerged, comprising proinflammatory, classically activated M1 Mϕ and anti-inflammatory, alternatively activated M2 Mϕ [8]. The balance between M1 and M2 Mϕ has been implicated in the pathogenesis of nephritis [9]. In an adriamycin-induced nephritis model, IL-10/transforming growth factor-β-modified M2 Mϕ adequately protected against renal injury [9]. In renal biopsy specimens from patients with LN, M2c Mϕ (CD68+/CD163+) predominates over M1 Mϕ (CD68+/inducible nitric oxide synthase-positive) [10]. Collectively, these data suggest potential roles of M2 Mϕ in LN.
M2 Mϕ highly express CD163, a scavenger receptor of hemoglobin-derived heme that is widely accepted as one of the surface markers for M2 Mϕ [11, 12]. Also, phosphorylated signal transducer and activator of transcription 1 (pSTAT1) and CMAF have been proposed as M1 and M2 Mϕ markers [13]. Haptoglobin-bound heme captured by CD163 is engulfed into Mϕ lysosomes and degraded into biliverdin and Fe2+ by inducible enzyme heme oxygenase (HO)-1. Among the leukocytes, HO-1 is expressed mainly in monocytes/Mϕ lineage cells [14]. Expression of HO-1 is tightly controlled by the transcriptional balance between activator nuclear factor erythroid 2-related factor 2 (Nrf2) and repressor BTB and CNC homology 1 (Bach1) [15, 16]. We and others have previously shown that HO-1 has anti-inflammatory effects and that its induction is beneficial for the treatment of various inflammatory animal models [17]. We previously reported that peritoneal injection of hemin, a chemical inducer of HO-1, into lupus-prone MRL/lpr mice suppressed proteinuria and kidney injury [18]. In line with our findings, Nrf2-deficient mice developed lupus-like autoimmune nephritis [19], whereas treatment with Nrf2 activator dimethyl fumarate ameliorated pristane-induced LN [20]. These results reinforce the notion that induction of HO-1 could be beneficial for the treatment of LN. However, it is still unclear whether M2 Mϕ play a pathological role in human LN or whether induction of HO-1 is useful for the treatment of patients with LN.
In the present study, we demonstrate that M2-like Mϕ lacking HO-1 expression are found in LN kidneys. Supplementation of HO-1 by targeting Bach1 genes ameliorated LN in mice, suggesting that dysregulated HO-1low M2 Mϕ contribute to augmenting the inflammation of LN.
Methods
Patients
All of the patients fulfilled the revised 1997 American College of Rheumatology criteria for the classification of systemic lupus erythematosus [21]. Patients enrolled in the study signed a written informed consent form that was approved by ethics committee of Yokohama City University Hospital (B130905030).
Mice
MRL/MpJ JmsSlc-lpr/lpr (MRL/lpr) mice were obtained from Japan SLC (Hamamatsu, Japan). The Bach1−/− mice (on the C57BL/6J background) used in this study have been described previously [22]. We obtained congenic mice by backcrossing with Bach1−/− C57/BL6J for 12 generations. Male Bach1+/− and female Bach1+/+ MRL/lpr mice were interbred. Mice were genotyped by PCR using primers previously described [23]. Bach1−/− MRL/lpr female mice and Bach1+/+ MRL/lpr female mice were used in this study. Animals were maintained under specific pathogen-free conditions within the animal facility at Yokohama City University. Animal treatment protocols were approved by the Yokohama City University animal protocol ethics committee.
Urine was collected for 6 h from individual 24-week-old mice in metabolic cages (Shinano Manufacturing Co., Tokyo, Japan). Urine protein and creatinine concentrations were determined by using DC Protein Assay Reagent (Bio-Rad Laboratories, Hercules, CA, USA) and the Parameter Creatinine assay kit (R&D Systems, Minneapolis, MN, USA). Sera were collected from the tails of 20-week-old mice. Serum anti-double-stranded DNA (anti-dsDNA) antibody (immunoglobulin G [IgG]) and blood urea nitrogen (BUN) were measured using an enzyme-linked immunosorbent assay (Shibayagi, Shibukawa, Japan) [18] and a BUN colorimetric detection kit (Arbor Assays, Ann Arbor, MI, USA).
Mϕ from MRL/lpr mice were collected by peritoneal lavage with ice-cold PBS. These cells underwent positive selection by using a CD11b+ MACS antibody (Miltenyi Biotec, Bergisch Gladbach, Germany), followed by incubation at 37 °C for 40 minutes to remove floating cells [24].
In vitro polarization of human M1- and M2-like Mϕ
Human peripheral blood mononuclear cells were obtained from heparinized peripheral blood by gradient density centrifugation using Ficoll-Paque medium. Monocytes were purified by nonmonocyte depletion with antibody-conjugated magnetic-activated cell sorting microbeads (MACS II Monocyte Isolation Kit; Miltenyi Biotec). Cells were cultured in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% FBS (2917354; MP Biomedicals, Santa Ana, CA, USA), 50 mg/ml streptomycin, and 50 U/ml penicillin. To differentiate cells into M2- or M1-like Mϕ, 50 ng/ml macrophage colony-stimulating factor (M-CSF) (216-MC; R&D Systems) or 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (215-GM; R&D Systems) was added, respectively [25]. Cells were incubated for 10 consecutive days, and medium change was performed at days 2, 5, and 8. For some experiments, M1- and M2-like Mϕ were stimulated with lipopolysaccharide (LPS) (1 μg/ml, serotype 0111:B4; InvivoGen, San Diego, CA, USA), interferon (IFN)-α2b (1 U/μl; PBL Assay Science, Piscataway, NJ, USA), or IFN-β (1 U/μl; PBL Assay Science).
Immunohistochemical analysis
Paraffinized renal biopsy specimens were preserved at Yokohama City University Hospital. Renal pathological findings were evaluated by the pathologists in accordance with the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification of LN [26]. Renal biopsy specimens were sectioned at 3-μm thickness, deparaffinized in xylene, hydrated in ethanol, and pretreated with citrate buffer (10 mM sodium citrate, pH 6.0). Slides were autoclaved for 20 minutes, followed by a 30-minute incubation at room temperature. Endogenous peroxidase activity was disrupted with 0.3% H2O2 in methanol for 30 minutes. Immunohistochemistry was performed on consecutive sections using the following antibodies (Abs): CD68 (PGM1) (1:100 dilution, M0876; Dako/Agilent Technologies, Santa Clara, CA, USA), CD163 (1:200 dilution, UKNDL-L-CD163; Novocastra Laboratories, Newcastle, UK), HO-1 (1:500 dilution, ADI-OSA-110; Enzo Biochem, Farmingdale, NY, USA), and pSTAT1 (1:400 dilution, D3B7; Cell Signaling Technology, Danvers, MA, USA), and CMAF (1:50 dilution, M-153, Santa Cruz Biotechnology, Dallas, TX, USA). Abs were applied for 60 minutes at 25 °C. Slides were developed using the Dako EnVision kit (Agilent Technologies). After hematoxylin staining, total numbers of CD68+, CD163+, pSTAT1+, CMAF+, and HO-1+ cells within the glomeruli and representative ex-glomerular lesions were counted. The number of M1-like Mϕ and M2-like Mϕ were calculated using the following formula: < CD163 × pSTAT1/(pSTAT1 + CMAF) > and < CD163 × CMAF/(pSTAT1 + CMAF) >, where “CD163 × pSTAT1” refers to cells that are double expressors of CD163 and pSTAT1 and “CD163 × CMAF” refers to cells that are double expressors of CD163 and CMAF. For immunocytochemistry, cells incubated on chamber slides were fixed with 4% paraformaldehyde for 10 minutes, followed by application of the Abs and protocols used in the aforementioned immunohistochemical analysis. In immunofluorescence staining, blocking was performed for 1 h by 1% Tris-buffered saline (TBS)-4% goat serum-TBS. Staining was achieved using primary Ab (CD163, pSTAT1, and CMAF) for 45 minutes at room temperature as described above. We used Alexa Fluor 555 antimouse IgG (A21425, dilution 1:500; Thermo Fisher Scientific, Waltham, MA, USA) and Alexa Fluor 488 antirabbit IgG (A11034, dilution 1:500; Thermo Fisher Scientific) as a second antibody.
RT-PCR
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and complementary DNA was prepared with Invitrogen SuperScript II enzyme (Life Technologies, Carlsbad, CA, USA), according to the manufacturers’ protocols. Primers and probes for human and mouse Hmox1 (Hs00157965_m1, Mm00516004_m1), CD163 (Hs00174705_m1, Mm00474091_m1), Bach1 (Hs00230917_m1), IL-6 (Hs00174131_m1), IL-10 (Hs00961622_m1), and Gapdh (4326317E, Mm99999915_g1) for RT-PCR were purchased from Applied Biosystems (Foster City, CA, USA). Primers for Ifnα and hypoxanthine phosphoribosyltransferase (Hprt) are listed in Additional file 1: Table S1. RT-PCR was performed using TaqMan Fast Advanced Master Mix (Applied Biosystems) or SYBR Green (Fast SYBR Green Master Mix; Applied Biosystems), and the data were analyzed with the StepOnePlus Real-Time PCR System (Applied Biosystems). The data were standardized to the expression of Gapdh or Hprt. The comparative cycle threshold method was used to semiquantify messenger RNA (mRNA) levels.
Western blot analysis
Kidney and spleen samples were lysed in lysis buffer (150 mM NaCl, 50 mM 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid, 1 mM ethylenediaminetetraacetic acid, 1% Triton X-100) in the presence of a protease inhibitor (Roche, Mannheim, Germany). After 10-minute incubation, cell membranes were disrupted by ultrasonication (Emerson Electric, St. Louis, MO, USA). Supernatants were collected after centrifugation at 15,000 rpm for 30 minutes and adjusted to an appropriate concentration with LDS sample buffer (Life Technologies) and 2-mercaptoethanol (Sigma-Aldrich). Each lysate was resolved by NuPAGE 4–12% Bis-Tris gel electrophoresis (Life Technologies) and transferred onto polyvinylidene fluoride (PVDF) membrane (Merck Millipore, Darmstadt, Germany). After blocking with 5% skim milk PBS, the PVDF membrane was probed with antimouse HO-1 monoclonal antibody (1:500 dilution) or antimouse Bach1 monoclonal antibody (1:100 dilution, F-9; Santa Cruz Biotechnology) overnight at 4 °C, followed by incubation with ECL antimouse IgG horseradish peroxidase-linked whole antibody (1:5000 dilution; GE Healthcare Life Sciences, Little Chalfont, UK) for 60 minutes at room temperature. Blots were developed using ECL Prime Western Blotting Detection Reagent (GE Healthcare Life Sciences) and exposed to the LAS-3000 Mini Imaging System (FUJIFILM, Tokyo, Japan) for 1–5 minutes.
Statistical analysis
Statistical analysis was performed by using Prism software (GraphPad Software, La Jolla, CA, USA). Data are presented as mean and SEM. p < 0.05 was considered statistically significant.
Results
Characteristics of M2-like Mϕ in glomeruli of patients with LN
In this study, we enrolled 19 patients with LN, including 13 patients with LN newly diagnosed via renal biopsy (Table 1). The histopathological diagnosis of LN was made by light microscopy and a routine set of immunofluorescence studies. According to ISN/RPS classification, 18 of the 19 patients were categorized into class III or class IV, and pure class V was found in the remaining patient (Table 1). Five patients with LN classes I and II were also evaluated.
Table 1.
Clinical features of patients with lupus nephritis at renal biopsy
| Characteristics of all patients with SLE (n = 19) | Data |
|---|---|
| Female sex, n (%) | 15 (78.9) |
| Age, years | 31.0 ± 10.9 |
| Duration of SLE, years | 6.1 ± 7.6 |
| Duration of LN, years | 2.8 ± 5.8 |
| Initial diagnosis of LN at renal biopsy, n (%) | 13 (68.4) |
| ISN/RPS classification, n | |
| III (A) | 4 |
| (A/C) | 6 (3)a |
| IV-S (A) | 0 |
| (A/C) | 3 (1)a |
| -G (A) | 2 |
| (A/C) | 3 |
| V | 1 |
| SLEDAI score | 16.0 ± 8.9 |
| PSL, n (%) | 12 (63.2) |
| Dosage of PSL, mg/day | 15.0 ± 12.4 |
| Concomitant immunosuppressantsb, n (%) | 4 (21.1) |
| Urine protein, g/24 h | 1.88 ± 1.93 |
| Serum C3, mg/dl | 60.1 ± 22.7 (normal range 70–129) |
| Serum C4, mg/dl | 10.6 ± 6.9 (normal range 12–36) |
| Serum creatinine, mg/dl | 0.65 ± 0.18 (normal range 0.48–0.82) |
| Anti-DNA- or anti-dsDNA antibody-positivec, n (%) | 17 (89.4) |
Abbreviations: dsDNA Double-stranded DNA, ISN/RPS International Society of Nephrology/Renal Pathology Society, LN Lupus nephritis, SLE Systemic lupus erythematosus, SLEDAI SLE Disease Activity Index, PSL Prednisolone, C3 Complement component C3, C4 Complement component C4
Data are shown as number (%) or mean ± SD
aNumbers in parentheses show number of patients with class V component
bConcomitant immunosuppressants included mycophenolate mofetil, cyclophosphamide, azathioprine, and tacrolimus
cAnti-DNA and anti-dsDNA antibody were determined by radioimmunoassay and enzyme-linked immunosorbent assay, respectively
Expression of CD68, CD163, HO-1, pSTAT1, CMAF, and HO-1 was determined in the renal biopsy specimens using immunohistochemistry. Representative immunohistochemical data from a patient with LN (ISN/RPS class IV-G [A/C]) are shown in Fig. 1a–e. Average cell numbers of CD68, CD163, and HO-1 per glomerulus in the renal tissues from the patients with LN were 9.52, 7.95, and 1.78, respectively (Fig. 1f). We found that numbers of CD163+ cells were comparable to numbers of CD68+ cells in the glomerulus of LN. We also found that the number of CMAF+ M2-like cells was greater than that of pSTAT1+ M1-like cells in the glomerulus of LN (Fig. 1g). Average cell numbers of M1-like Mϕ, M2-like Mϕ, and HO-1 per glomerulus of patients with LN were 1.62, 6.33, and 1.78, respectively (Fig. 1h). Double-immunofluorescence staining in a representative case indicated that CD163+CMAF+ M2-like Mϕ are dominant compared with CD163+pSTAT1+ cells in the glomeruli of LN (Fig. 1i, j). Numbers of M2-like Mϕ per glomeruli positively correlated with urine protein levels (Fig. 1k), consistent with a previous report [6]. The number of Mϕ in glomerulus were few in LN classes I and II (Additional file 1: Figure S1). In extraglomerular lesions, of LN classes III, IV, and V, the number of M2-like Mϕ was greater than M1-like Mϕ. Also, HO-1+ cells were significantly fewer than numbers of M2-like Mϕ (Additional file 1: Figure S2).
Fig. 1.
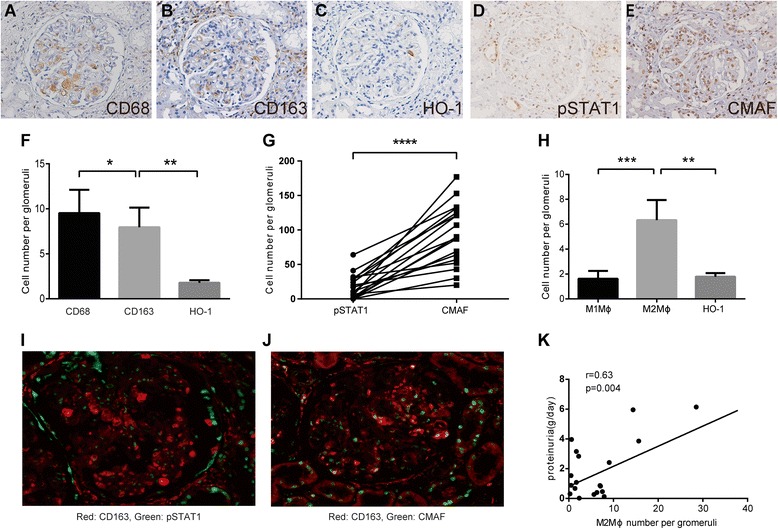
Numbers of CD68+, CD163+, and heme oxygenase (HO)-1+ cells in the glomerulus of patients with lupus nephritis. Representative immunohistochemical images of the renal biopsy specimen obtained from a patient with lupus nephritis (International Society of Nephrology/Renal Pathology Society [ISN/RPS] class IV-G [A/C]) are shown. Serial sections of a glomerulus stained with antibodies against (a) CD68, (b) CD163, (c) HO-1, (d) phosphorylated signal transducer and activator of transcription 1 (pSTAT1), and (e) CMAF (original magnification × 400). f Numbers of CD68+, CD163+, and HO-1+ cells in glomerulus of renal tissue from patients with systemic lupus erythematosus (SLE) (n = 19) were counted. Data shown are mean ± SEM. g Numbers of pSTAT1+ and CMAF+ cells in a glomerulus of renal tissue from patients with SLE (n = 19) were counted. h Numbers of estimated M1 macrophage (Mϕ), M2 Mϕ, and HO-1+ cells in glomerulus in the renal tissues from patients with SLE (n = 19). Data shown are mean ± SEM. i and j Representative immunofluorescently stained images of the renal biopsy specimen from a patient with lupus nephritis (ISN/RPS class IV-G [A/C]). Red shows CD163; green shows pSTAT1 (i) or CMAF (j). Double-positive cells indicate M1-like Mϕ and M2-like Mϕ in (i) and (j), respectively. k Correlation between proteinuria levels (g/day) and numbers of M2-like Mϕ within the glomeruli (n = 19). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by Student’s t test
Previous reports have shown that HO-1 mediates the anti-inflammatory property of M2 Mϕ [27–29]. Nevertheless, the numbers of HO-1+ cells were fewer than those of M2-like Mϕ. These data suggest that HO-1 expression is downregulated in glomerular Mϕ of human LN in spite of the M2-like property.
HO-1 expression is high but is repressed by type I interferons in human M2-like Mϕ generated in vitro
HO-1 is an inducible protein, and the expression is positively and negatively modulated by external environments [27]. We hypothesized that local inflammatory conditions of LN are responsible for the discrepancy between CD163/CMAF positivity and HO-1 expression in glomerular Mϕ. In the present study, we focused on effects of IFN-α on mRNA expression of both molecules in human monocyte-derived M2-like Mϕ generated in vitro, because type I IFN and the related genes are upregulated in patients with LN, including the renal tissues as a so-called IFN signature [1, 3, 30].
Concordant with previous reports [11, 12, 28], our results confirmed that both CD163 and HO-1 mRNA and protein expression was high in M2-like Mϕ compared with M1-like Mϕ in our in vitro culture systems (Fig. 2a, b, g–l). The in vitro M2-like polarization was associated with increased mRNA of IL-10, another hallmark of M2 Mϕ, whereas that of IL-6 was comparable between M1- and M2-like Mϕ (Fig. 2c, d). The established M2-like Mϕ were further treated with type I IFNs for 4 h. The results showed that HO-1 expression was suppressed by IFN-α in M2-like Mϕ (Fig. 2b, k, and l) [14, 31], whereas mRNA of Bach1, an HO-1 transcriptional repressor, was upregulated (Fig. 2e). Simultaneously, IL-6 mRNA levels, but not IL-10 levels, were upregulated by IFN-α. The findings were also found in M2-like Mϕ treated with either IFN-β or LPS (Additional file 1: Figure S3A). Moreover, the same results were reproduced in M2-like Mϕ derived from patients with SLE (Fig. 2f). The results suggest that the type I IFN-enriched condition in the glomerular lesions of LN suppresses HO-1 expression through upregulated Bach1 and modulates anti-inflammatory properties in M2-like Mϕ.
Fig. 2.
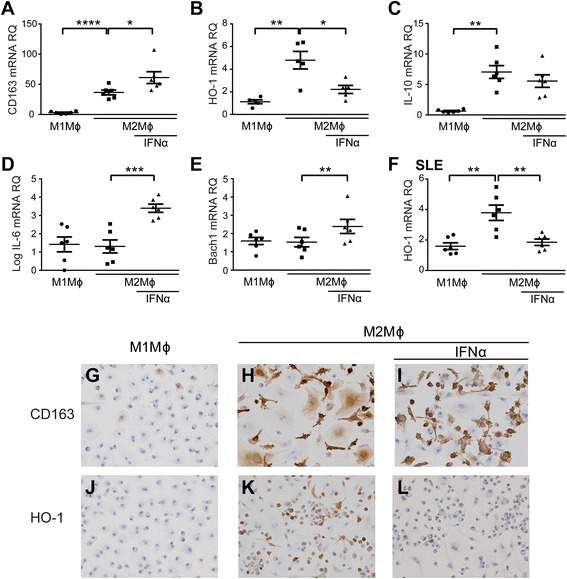
Expression of CD163, heme oxygenase (HO-1), and BTB and CNC homology 1 (Bach1) in human monocyte-derived M1- and M2-like macrophages (Mϕ) from healthy donors (HD). Purified peripheral monocytes were cultured in the presence of granulocyte-macrophage colony-stimulating factor or macrophage colony-stimulating factor for 10 days to induce differentiation of M1- or M2-like Mϕ, respectively. Messenger RNA (mRNA) expression was semiquantitatively determined from M1- and M2-like Mϕ generated from HD in vitro (n = 6) by RT-PCR of (a) CD163, (b) HO-1, (c) interleukin (IL)-10, (d) IL-6, and (e) Bach1. f HO-1 mRNA expression of M1- and M2-like Mϕ from patients with systemic lupus erythematosus (SLE) stimulated with interferon (IFN)-α (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by Student’s t test. Data shown are mean ± SEM. Representative immunocytochemical staining shows results for M1- and M2-like Mϕ stimulated with or without human recombinant IFN-α. CD163 (g–i) and HO-1 (j–l) (original magnification × 400). RQ Relative quantification
HO-1 upregulation by genetic ablation of Bach1 ameliorates LN in mice
We and others have reported that induction of HO-1 expression is beneficial in the treatment of LN [18, 19]. Our in vitro experiments suggest the involvement of Bach1 in IFN-α-dependent reduction of HO-1 expression in LN. To determine the role of Bach1 in the development of LN, we generated LN-prone MRL/lpr mice lacking Bach1 and asked whether recovery of HO-1 expression in M2 Mϕ by Bach1 deficiency leads to amelioration of LN.
Microsatellite analysis (ICLAS Monitoring Center, Kawasaki, Japan) confirmed the genetic purity of Bach1−/− MRL/MpJ JmsSlc-lpr/lpr was > 99.4% compared with wild type (Additional file 1: Figure S4). PCR analysis confirmed Bach1 deficiency in these congenic mice (Additional file 1: Figure S5). Using female mice, we observed their phenotypic changes, including renal function, over the time course. As shown in Fig. 3a, the survival rate of Bach1−/− MRL/lpr mice was better than that of Bach1+/+ MRL/lpr mice. Urine total protein/creatinine ratio at 24 weeks and serum BUN at 20 weeks were decreased in Bach1−/− mice compared with Bach1+/+ mice (Fig. 3b, c), though there was no difference in anti-dsDNA Ab(IgG) between the mice (Fig. 3d). The discrepancy between proteinuria/serum BUN and the serological findings suggest that Bach1 gene targeting modulates the innate immune process after immune complex (IC) deposition, resulting in favorable clinical outcomes of Bach1−/− MRL/lpr mice. Therefore, we further focused on the analysis of the renal lesions (Fig. 4a–j).
Fig. 3.
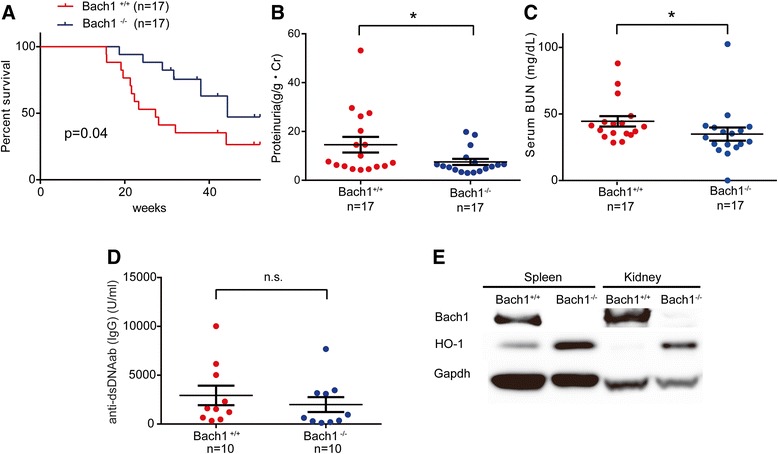
Phenotypic analysis of BTB and CNC homology 1 (Bach1)-deficient MRL/lpr mice. Clinical and renal findings were compared between Bach1+/+ and Bach1−/− MRL/lpr mice as follows. a Kaplan-Meier survival analysis. b Proteinuria (g/g*Cr). c Serum blood urea nitrogen (BUN) (mg/dl) at 24 weeks. d Anti-double-stranded DNA (anti-dsDNA) antibody (Ab) immunoglobulin G (IgG) (U/ml). *p < 0.05 by Student’s t test. n.s. Not significant. Data shown are mean ± SEM. e Immunoblot analysis of Bach1, heme oxygenase (HO)-1, and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) in whole spleens and kidneys
Fig. 4.
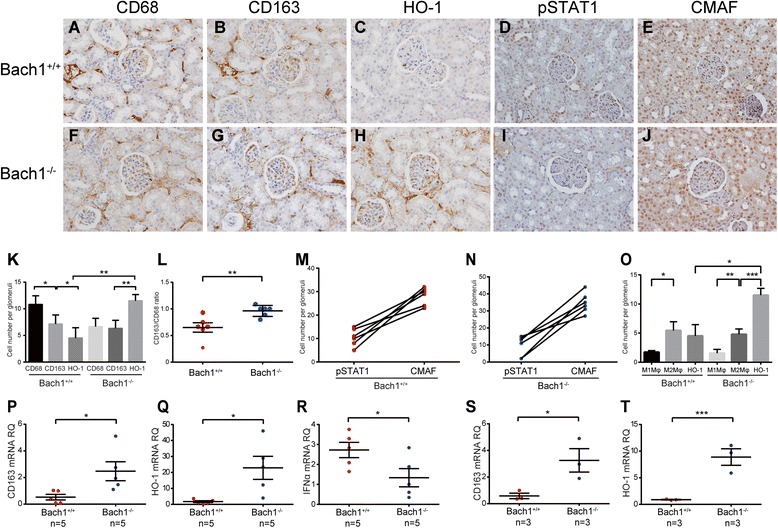
Characteristics of cells within the glomeruli of BTB and CNC homology 1 (Bach1)-deficient MRL/lpr mice. Immunohistochemical staining of the renal tissues for CD68 (a, f), CD163 (b, g), heme oxygenase (HO)-1 (c, h), phosphorylated signal transducer and activator of transcription 1 (pSTAT1) (d, i), and CMAF (e, j) (original magnification × 400). k Numbers of CD68+, CD163+, and HO-1+ cells in glomeruli of mice. l Ratio of CD163+ and CD68+ cells within the glomerulus of Bach1+/+ and Bach1−/− MRL/lpr mice. m and n Numbers of pSTAT1+ and CMAF+ cells in glomeruli of Bach1+/+ and Bach1−/− MRL/lpr mice (n = 2 from each group). o Numbers of estimated M1 macrophage (Mϕ), M2 Mϕ, and HO-1+ cells in glomeruli in the renal tissues from Bach1+/+ and Bach1−/− MRL/lpr mice. Data shown are mean ± SEM. Three glomeruli from each mouse were evaluated. (p) CD163, (q) HO-1, and (r) interferon (IFN)-α messenger RNA (mRNA) from the whole kidneys. mRNA expression of (s) CD163 and (t) HO-1 of CD11b+ peritoneal Mϕ. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test. Data shown are mean ± SEM
Immunoblot analysis confirmed the deficiency of Bach1 and increased expression of the HO-1 protein in the kidneys and spleens of Bach1−/− compared with Bach1+/+ MRL/lpr mice (Fig. 3e). Concordantly, immunohistochemical analysis revealed more HO-1-expressing cells in glomeruli from Bach1−/− mice than in those from Bach1+/+ MRL/lpr mice, whereas no differences were found in CD68- or CD163-expressing cells (Fig. 4c, h, and k). However, M2-like polarization was more prominent in glomerular Mϕ of Bach1−/− MRL/lpr mice than in the wild-type mice, as shown by the increased ratio of CD163+ cells per CD68+ cells (Fig. 4l). CMAF+ cells were much more numerous than pSTAT1+ cells in both Bach1+/+ and Bach1−/− mice (Fig. 4m, n). The estimated number of M2-like Mϕ was greater than M1 Mϕ in both Bach1+/+ and Bach1−/− mice. However, numbers of HO-1+ cells in glomeruli were greater in Bach1−/− mice (Fig. 4o). PCR analysis also revealed higher CD163 and HO-1 mRNA expression in the kidneys of Bach1−/− mice than in wild-type mice, whereas the IFN-α expression level was rather decreased in Bach1−/− MRL/lpr mice (Fig. 4p–r).
To further compare the characteristics of Mϕ in these mice, Mϕ obtained from peritoneal lavage were examined (Fig. 4s, t). We found that Mϕ from Bach1−/− MRL/lpr mice expressed higher levels of CD163 and HO-1 mRNA than wild-type mice did, suggesting that M2-like polarization is more evident in Bach1 deficiency, consistent with the results of a previous paper [24]. Collectively, these data suggest that Bach1 negatively regulates HO-1 expression and M2 Mϕ polarization, leading to the inflammation of LN.
Discussion
In the present study, we showed high numbers of M2-like Mϕ than M1-like Mϕ within the glomerulus of patients with LN, suggesting an M2 shift in LN. However, HO-1 expression was reduced in these abundantly present M2-like Mϕ (Fig. 1). Moreover, human monocyte-derived M2-like Mϕ treated with type I IFNs showed reduced HO-1 and increased Bach1 expression. Mϕ from Bach1-deficient mice showed an M2 shift along with high HO-1 expression, consistent with a previous report [24]. These data suggest that HO-1-expressing M2 Mϕ are necessary to regulate LN.
Over the past few decades, authors of hundreds of papers have reported the anti-inflammatory properties of HO-1 in various inflammation settings. Indeed, HO-1 deficiency in humans and mice exhibits marked inflammation caused by oxidative stress [32, 33]. Moreover, we previously reported that HO-1 knockdown by small interfering RNA resulted in an enhanced inflammatory response in human monocytes [14]. Therefore, it is likely that these “HO-1-deficient M2-like Mϕ” in LN are proinflammatory rather than anti-inflammatory as in ordinary M2 Mϕ. Indeed, the results of the present study show low HO-1 and high IL-6 production in human M2 Mϕ stimulated with IFN-α (Fig. 2b and d). Besides, a previous study demonstrated that chemical induction of HO-1 was beneficial in the LN murine model [18]. Along the same line, the present study provides hope that the cell population could be reversed into anti-inflammatory M2 Mϕ through induction of HO-1 via inhibition of Bach1.
LN is considered a representative IC disease caused by anti-dsDNA Ab IC deposition in the glomerulus. Subsequently, the deposited IC triggers activation of the complement system and preferentially promotes M2 Mϕ polarization [34]. Our data show that anti-inflammatory properties of glomerular M2 Mϕ are abrogated by a systemic and local IFN signature that suppresses HO-1 through upregulation of Bach1. Moreover, costimulation with Toll-like receptor ligands, which reduced HO-1 expression in this study, and IgG promotes inflammatory responses of M2 Mϕ instead of suppressing inflammation via Fcγ receptor IIa [35]. Our in vitro analysis suggests that the functional changes of M2-like Mϕ are associated with an altered cytokine profile characterized by increased IL-6 synthesis while maintaining IL-10 production capacity (Fig. 2). Both cytokines are implicated in the pathogenesis of SLE, because recent genetic studies have identified the IL10 risk allele in SLE [36]. Our hypothesis proposed on the basis of this study is summarized in Fig. 5. Collectively, M2 Mϕ dysfunction may facilitate the inflammation in LN. Bach1-mediated HO-1 supplementation is one of the promising strategies to “tame” these aberrantly activated M2 Mϕ in LN.
Fig. 5.
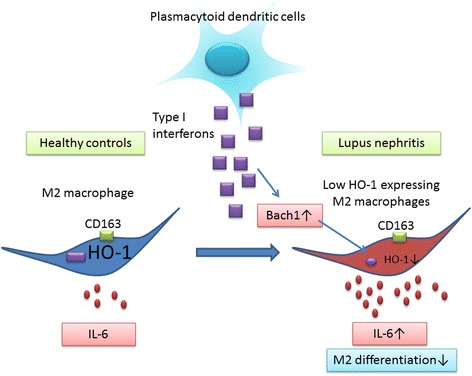
Schema describing our hypothesis of BTB and CNC homology 1 (Bach1)-mediated M2 dysregulation in lupus nephritis. Type I interferons are highly produced by plasmacytoid dendritic cells in systemic lupus erythematosus [1]. The secreted interferons suppress heme oxygenase (HO)-1 expression in CD163+ M2-like macrophages via induction of HO-1 transcriptional repressor Bach1. These aberrantly functional HO-1 low M2-like macrophages produce inflammatory cytokines such as interleukin (IL)-6, which is facilitated by reduced HO-1 expression. Bach1 also has the property of modulating M2-like macrophage differentiation, as suggested in our Bach1−/− mouse experiments
In the present study, we demonstrate that Bach1 is induced by type I IFNs in human M2-like Mϕ (Fig. 2e). Elucidating the mechanisms of how type I IFNs regulate Bach1 expression is essential. Bach1 was initially identified as a Maf-binding protein, and it now is well established that Bach1 forms a heterodimer with small Maf [37]. A recent study showed that MafB is involved in the regulation of IRF3-dependent type I IFN-inducible genes [38]. Interestingly, genome-wide association studies (GWAS) identified Bach2, which belongs to the same family as Bach1, as being associated with SLE [39]. These findings and our data suggest that Bach1 is critically involved in type I IFN-mediated inflammation, including SLE.
Bach2 regulates antibody class switch in B cells [40], thus contributing to acquired immunity because it is identified as a susceptible gene in GWAS of various autoimmune diseases [39]. In contrast, a primary source of Bach1 is monocytes (BioGPS GeneAtlas U133A, gcrma), and Bach1 was identified as one of the core Mϕ-associated genes in mice [41], suggesting that Bach1 plays a strong role in innate immunity. In this context, Bach1-deficient mice showed a milder LN phenotype than wild-type mice without affecting anti-dsDNA antibody production (Fig. 3c). This fact suggests that innate immune responses after IC deposition on glomerulus are also significant in this model. Although the mainstay of current treatment strategies for LN is to suppress acquired immunity, including pathogenic autoantibody production, our data support an alternative treatment strategy directed against innate immunity via Bach1. The approach is expected to have an advantage over conventional therapies regarding safety issues, especially for complications with infection because systemic immunosuppressive effects are marginal.
There are several possible strategies to treat LN by targeting the Bach1, HO-1, and M2 Mϕ. First, inhibition of Bach1 transcription is promising. Recently, the novel Bach1 inhibitor HPP971, which increases HO-1 expression, was developed [42]. Nrf2, a competitor to Bach1, is an alternative because Nrf2-deficient mice showed a phenotype resembling LN [43]. Treatment with novel Nrf2 inducer TFM-735 ameliorated experimental autoimmune encephalomyelitis [44]. Finally, supplementation or generation of normal HO-1-expressing M2 Mϕ could also be beneficial for LN. It has been shown that adoptive transplant of M2 Mϕ, but not of M1 Mϕ, reduced SLE severity in clodronate- and activated lymphocyte-derived DNA-treated mice [45].
Conclusions
Our data suggest that functional alteration of M2 Mϕ plays an important role in LN and that Bach1 is a therapeutic target for LN.
Additional file
Table S1. Primer sequences used for qRT-PCR. Figure S1. Numbers of CD68, CD163, HO-1 positive cells in the glomerulus of lupus nephritis patients (ClassI or II). Figure S2. Numbers of CD68, CD163, HO-1 positive cells in the extra-glomerulus of lupus nephritis patients. Figure S3. HO-1 mRNA expression in M1 and M2 Mϕ stimulated with various regents. Figure S4. Genomic background of congenic mice. Figure S5. Genotyping of Bach1 knockout mice. (DOCX 953 kb)
Acknowledgements
We express our gratitude to Yukihiro Toyota, Kento Ichikawa, Takayuki Akagi, and Masato Kawashima for their assistance. We thank Tom Kiper for his critical review in preparing the manuscript.
Funding
YKi is supported by grants from the Japanese Society for the Promotion of Science Grants-in-Aid for Scientific Research (grants 26713036, 15 K15374), the Yokohama Foundation for Advancement of Medical Science, the Naito Foundation, the Uehara Memorial Foundation, the Japan Intractable Diseases Research Foundation, and the Japan Rheumatism Foundation. RY is supported by grants from the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (grant 26461468), Takeda Science Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and Yokohama Foundation for Advancement of Medical Science. MTak is supported by grants from the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (grant 26461469). MTam is supported by grants from the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (grant 26860756). KN is supported by grants from the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (grant 16 K08698). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- Ab
Antibody
- Bach1
BTB and CNC homology 1
- BUN
Blood urea nitrogen
- C3
Complement component C3
- C4
Complement component C4
- dsDNA
Double-stranded DNA
- Gapdh
Glyceraldehyde 3-phosphate dehydrogenase
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GWAS
Genome-wide association studies
- HD
Healthy donors
- HO-1
Heme oxygenase 1
- Hprt
Hypoxanthine phosphoribosyltransferase
- IC
Immune complex
- IFN
Interferon
- IgG
Immunoglobulin G
- IL
Interleukin
- ISN/RPS
International Society of Nephrology/Renal Pathology Society
- LN
Lupus nephritis
- LPS
Lipopolysaccharide
- M-CSF
Macrophage colony-stimulating factor
- mRNA
Messenger RNA
- Mϕ
Macrophage
- Nrf2
Nuclear factor erythroid 2-related factor 2
- PSL
Prednisolone
- pSTAT1
Phosphorylated signal transducer and activator of transcription 1
- PVDF
Polyvinylidene fluoride
- RQ
Relative quantification
- SLE
Systemic lupus erythematosus
- SLEDAI
Systemic Lupus Erythematosus Disease Activity Index
- TBS
Tris-buffered saline
Authors’ contributions
DK performed experiments, analyzed data, and wrote the manuscript. MTam, KTM, YKu, H.Nakano, and IK performed some experiments. MTam, MTak, KN, RY, KI, IA, and H.Nakajima coordinated research work and critically revised the manuscript. YKi conceived of the experimental design, analyzed data, and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Each patients enrolled in this study signed a written informed consent form, which was approved by the ethics committee of Yokohama City University Hospital (B130905030). Animals were maintained under specific pathogen-free conditions within the animal facility at Yokohama City University. Animal treatment protocols were approved by the Yokohama City University Animal Protocol Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13075-018-1568-1) contains supplementary material, which is available to authorized users.
Contributor Information
Daiga Kishimoto, Email: daiga@yokohama-cu.ac.jp.
Yohei Kirino, Phone: +81-45-787-2630, Email: kirino@yokohama-cu.ac.jp.
Maasa Tamura, Email: mtamura@nagaoka.jrc.or.jp.
Mitsuhiro Takeno, Email: m-takeno@nms.ac.jp.
Yosuke Kunishita, Email: a050014@yokohama-cu.ac.jp.
Kaoru Takase-Minegishi, Email: kaoru_t@yokohama-cu.ac.jp.
Hiroto Nakano, Email: hnakano@yokohama-cu.ac.jp.
Ikuma Kato, Email: triebjp@yahoo.co.jp.
Kiyotaka Nagahama, Email: ki-nagahama@ks.kyorin-u.ac.jp.
Ryusuke Yoshimi, Email: yoshiryu@med.yokohama-cu.ac.jp.
Kazuhiko Igarashi, Email: igarashi@med.tohoku.ac.jp.
Ichiro Aoki, Email: 1202aoki@gmail.com.
Hideaki Nakajima, Email: hnakajim@yokohama-cu.ac.jp.
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Cameron JS. Lupus nephritis. J Am Soc Nephrol. 1999;10(2):413–424. doi: 10.1681/ASN.V102413. [DOI] [PubMed] [Google Scholar]
- 3.Mok CC. Towards new avenues in the management of lupus glomerulonephritis. Nat Rev Rheumatol. 2016;12(4):221–234. doi: 10.1038/nrrheum.2015.174. [DOI] [PubMed] [Google Scholar]
- 4.Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353(21):2219–2228. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 5.Furie R, Nicholls K, Cheng TT, Houssiau F, Burgos-Vargas R, Chen SL, Hillson JL, Meadows-Shropshire S, Kinaszczuk M, Merrill JT. Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol. 2014;66(2):379–389. doi: 10.1002/art.38260. [DOI] [PubMed] [Google Scholar]
- 6.Hill GS, Delahousse M, Nochy D, Remy P, Mignon F, Mery JP, Bariety J. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 2001;59(1):304–316. doi: 10.1046/j.1523-1755.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers SA, Chitu V, Herlitz LC, Sahu R, Stanley ER, Putterman C. Macrophage depletion ameliorates nephritis induced by pathogenic antibodies. J Autoimmun. 2015;57:42–52. doi: 10.1016/j.jaut.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Q, Wang Y, Zheng D, Sun Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, Harris DC. IL-10/TGF-β-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21(6):933–942. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olmes G, Büttner-Herold M, Ferrazzi F, Distel L, Amann K, Daniel C. CD163+ M2c-like macrophages predominate in renal biopsies from patients with lupus nephritis. Arthritis Res Ther. 2016;18:90. doi: 10.1186/s13075-016-0989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409(6817):198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 12.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13(9):621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 13.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8(11):e80908. doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirino Y, Takeno M, Murakami S, Kobayashi M, Kobayashi H, Miura K, Ideguchi H, Ohno S, Ueda A, Ishigatsubo Y. Tumor necrosis factor α acceleration of inflammatory responses by down-regulating heme oxygenase 1 in human peripheral monocytes. Arthritis Rheum. 2007;56(2):464–475. doi: 10.1002/art.22370. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki T, Kirino Y, Takeno M, Samukawa S, Hama M, Tanaka M, Yamaji S, Ueda A, Tomita N, Fujita H, et al. Expression of heme oxygenase-1 in human leukemic cells and its regulation by transcriptional repressor Bach1. Cancer Sci. 2010;101(6):1409–1416. doi: 10.1111/j.1349-7006.2010.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi K, Watanabe-Matsui M. Wearing red for signaling: the heme-Bach axis in heme metabolism, oxidative stress response and iron immunology. Tohoku J Exp Med. 2014;232(4):229–253. doi: 10.1620/tjem.232.229. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Adhikari R, Yamada KM, Dhawan S. Hemin activation of innate cellular response blocks human immunodeficiency virus type-1-induced osteoclastogenesis. Biochem Biophys Res Commun. 2015;464(1):7–12. doi: 10.1016/j.bbrc.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda Y, Takeno M, Iwasaki M, Kobayashi H, Kirino Y, Ueda A, Nagahama K, Aoki I, Ishigatsubo Y. Chemical induction of HO-1 suppresses lupus nephritis by reducing local iNOS expression and synthesis of anti-dsDNA antibody. Clin Exp Immunol. 2004;138(2):237–244. doi: 10.1111/j.1365-2249.2004.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, Morito N, Koyama A, Yamamoto M, Takahashi S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60(4):1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 20.Ebihara S, Tajima H, Ono M. Nuclear factor erythroid 2-related factor 2 is a critical target for the treatment of glucocorticoid-resistant lupus nephritis. Arthritis Res Ther. 2016;18(1):139. doi: 10.1186/s13075-016-1039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 22.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21(19):5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hama M, Kirino Y, Takeno M, Takase K, Miyazaki T, Yoshimi R, Ueda A, Itoh-Nakadai A, Muto A, Igarashi K, et al. Bach1 regulates osteoclastogenesis in a mouse model via both heme oxygenase 1-dependent and heme oxygenase 1-independent pathways. Arthritis Rheum. 2012;64(5):1518–1528. doi: 10.1002/art.33497. [DOI] [PubMed] [Google Scholar]
- 24.Harusato A, Naito Y, Takagi T, Uchiyama K, Mizushima K, Hirai Y, Higashimura Y, Katada K, Handa O, Ishikawa T, et al. BTB and CNC homolog 1 (Bach1) deficiency ameliorates TNBS colitis in mice: role of M2 macrophages and heme oxygenase-1. Inflamm Bowel Dis. 2013;19(4):740–753. doi: 10.1097/MIB.0b013e3182802968. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, Stone DL, Chae JJ, Rosenzweig SD, Bishop K, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370(10):911–920. doi: 10.1056/NEJMoa1307361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15(2):241–250. doi: 10.1097/01.ASN.0000108969.21691.5D. [DOI] [PubMed] [Google Scholar]
- 27.Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys. 2014;564:83–88. doi: 10.1016/j.abb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Sierra-Filardi E, Vega MA, Sanchez-Mateos P, Corbi AL, Puig-Kroger A. Heme oxygenase-1 expression in M-CSF-polarized M2 macrophages contributes to LPS-induced IL-10 release. Immunobiology. 2010;215(9–10):788–795. doi: 10.1016/j.imbio.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 30.Parikh SV, Malvar A, Song H, Alberton V, Lococo B, Vance J, Zhang J, Yu L, Birmingham D, Rovin BH. Molecular imaging of the kidney in lupus nephritis to characterize response to treatment. Transl Res. 2017;182:1–13. doi: 10.1016/j.trsl.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirino Y, Takeno M, Watanabe R, Murakami S, Kobayashi M, Ideguchi H, Ihata A, Ohno S, Ueda A, Mizuki N, et al. Association of reduced heme oxygenase-1 with excessive Toll-like receptor 4 expression in peripheral blood mononuclear cells in Behcet’s disease. Arthritis Res Ther. 2008;10(1):R16. doi: 10.1186/ar2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103(1):129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94(20):10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spivia W, Magno PS, Le P, Fraser DA. Complement protein C1q promotes macrophage anti-inflammatory M2-like polarization during the clearance of atherogenic lipoproteins. Inflamm Res. 2014;63(10):885–893. doi: 10.1007/s00011-014-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogelpoel LT, Hansen IS, Rispens T, Muller FJ, van Capel TM, Turina MC, Vos JB, Baeten DL, Kapsenberg ML, de Jong EC, et al. Fcγ receptor-TLR cross-talk elicits pro-inflammatory cytokine production by human M2 macrophages. Nat Commun. 2014;5:5444. doi: 10.1038/ncomms6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakurai D, Zhao J, Deng Y, Kelly JA, Brown EE, Harley JB, Bae SC, Alarcón-Riquelme ME, Edberg JC, Kimberly RP, et al. Preferential binding to Elk-1 by SLE-associated IL10 risk allele upregulates IL10 expression. PLoS Genet. 2013;9(10):e1003870. doi: 10.1371/journal.pgen.1003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol. 1996;16(11):6083–6095. doi: 10.1128/MCB.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Seed B. The transcription factor MafB antagonizes antiviral responses by blocking recruitment of coactivators to the transcription factor IRF3. Nat Immunol. 2010;11(8):743–750. doi: 10.1038/ni.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris DL, Sheng Y, Zhang Y, Wang YF, Zhu Z, Tombleson P, Chen L, Cunninghame Graham DS, Bentham J, Roberts AL, et al. Genome-wide association meta-analysis in Chinese and European individuals identifies ten new loci associated with systemic lupus erythematosus. Nat Genet. 2016;48(8):940–946. doi: 10.1038/ng.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muto A, Tashiro S, Nakajima O, Hoshino H, Takahashi S, Sakoda E, Ikebe D, Yamamoto M, Igarashi K. The transcriptional programme of antibody class switching involves the repressor Bach2. Nature. 2004;429(6991):566–571. doi: 10.1038/nature02596. [DOI] [PubMed] [Google Scholar]
- 41.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11):1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attucks OC, Jasmer KJ, Hannink M, Kassis J, Zhong Z, Gupta S, Victory SF, Guzel M, Polisetti DR, Andrews R, et al. Induction of heme oxygenase I (HMOX1) by HPP-4382: a novel modulator of Bach1 activity. PLoS One. 2014;9(7):e101044. doi: 10.1371/journal.pone.0101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Stein TD, Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics. 2004;18(3):261–272. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- 44.Higashi C, Kawaji A, Tsuda N, Hayashi M, Saito R, Yagishita Y, Suzuki T, Uruno A, Nakamura M, Nakao K, et al. The novel Nrf2 inducer TFM-735 ameliorates experimental autoimmune encephalomyelitis in mice. Eur J Pharmacol. 2017;802:76–84. doi: 10.1016/j.ejphar.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Yang Y, Zhu X, Huang L, Xu J. Macrophage polarization modulates development of systemic lupus erythematosus. Cell Physiol Biochem. 2015;37(4):1279–1288. doi: 10.1159/000430251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer sequences used for qRT-PCR. Figure S1. Numbers of CD68, CD163, HO-1 positive cells in the glomerulus of lupus nephritis patients (ClassI or II). Figure S2. Numbers of CD68, CD163, HO-1 positive cells in the extra-glomerulus of lupus nephritis patients. Figure S3. HO-1 mRNA expression in M1 and M2 Mϕ stimulated with various regents. Figure S4. Genomic background of congenic mice. Figure S5. Genotyping of Bach1 knockout mice. (DOCX 953 kb)
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.


