Abstract
Background
Obstructive sleep apnea (OSA) is highly prevalent in patients with coronary artery disease (CAD) and is associated with recurrent cardiovascular risk. However, whether treatment with continuous positive airway pressure (CPAP) reduces this risk remains unclear. We performed a systematic review and meta-analysis to assess the effect of CPAP on long-term cardiovascular outcomes in patients with concomitant CAD and OSA.
Methods
We searched the PubMed, EMBASE, and Cochrane library from their inceptions to October 7, 2017. We included observational studies and randomized controlled trials (RCTs) that described the association of CPAP treatment with cardiovascular events in patients with CAD and OSA. The primary outcome of interest was major adverse cardiovascular event (MACE), including all-cause or cardiovascular death, myocardial infarction, stroke, repeat revascularization, or hospitalization for heart failure. Outcomes data were pooled using random effects models and heterogeneity assessed with the I2 statistic.
Results
We identified 9 studies (2 RCTs and 7 observational studies) with 1430 participants. The median follow-up duration was from 36 to 86.5 months. Treatment with CPAP was associated with a significantly lower risk of MACE in 6 observational studies (RR 0.61, 95% CI: 0.39–0.94, P = 0.02), but this was not reproduced in 2 RCTs (RR 0.57, 95% CI: 0.32–1.02, P = 0.06). Similarly, CPAP significantly reduced the risk of all-cause death (4 observational studies) and cardiovascular death (3 observational studies), which were also not confirmed in RCTs.
Conclusions
The use of CPAP in patients with CAD and OSA might prevent subsequent cardiovascular events, which was only demonstrated in observational studies, but not in RCTs. The value of CPAP therapy as second prevention for CAD needs further investigation.
Electronic supplementary material
The online version of this article (10.1186/s12931-018-0761-8) contains supplementary material, which is available to authorized users.
Keywords: Continuous positive airway pressure, Coronary artery disease, Meta-analysis, Obstructive sleep apnea
Background
Obstructive sleep apnea (OSA) is highly prevalent in patients with cardiovascular diseases. Compared to the general population, OSA is more common in patients with coronary artery disease (CAD), with a reported prevalence of 38% to 65% [1]. Observational studies have shown OSA was associated with increased risk of subsequent cardiovascular events in various subsets of CAD patients [2–6]. Continuous positive airway pressure (CPAP) is recommended for symptomatic patients with OSA, but multiple observational studies [7–13] and randomized controlled trials (RCTs) [14, 15] have shown inconsistent results of CPAP therapy in reducing cardiovascular events in patients with established CAD. Moreover, there is no meta-analysis focusing on patients with concomitant CAD and OSA and evaluating the role of CPAP in preventing recurrent adverse events. Therefore, we conducted a systematic review and meta-analysis to assess whether adding CPAP therapy would improve long-term cardiovascular outcomes in patients with CAD and OSA.
Methods
Search strategies
This meta-analysis was conducted in accordance to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement [16]. The searches included the PubMed, EMBASE, and Cochrane library from their inceptions to October 7, 2017, without language restrictions. We used Medical Subject Heading terms “Continuous Positive Airway Pressure”, “Sleep Apnea Syndromes”, “Myocardial Ischemia”, and related text words including CPAP, sleep apnea, and coronary disease. We also checked the reference lists of all included studies, relevant review articles, and conference abstracts manually for potential citations. An example search strategy is presented in Additional file 1: Table S1.
Study selection and eligibility criteria
Two authors (X.W. and Y.Z., both cardiologists) assessed the eligibility of articles by initially screening the titles and abstracts. Articles that reported the impact of CPAP versus standard therapy (control group) among patients with OSA and CAD were considered for inclusion. Each full-text article was then reviewed in duplicate by these authors. Studies that were not performed in patients with CAD, studies that did not report on outcomes of interest (cardiovascular events), and studies with less than 1-year follow-up were excluded. Any disagreement was resolved by consensus through referral to a third reviewer (S.N.).
Data extraction and validity assessment
Data extraction was performed independently and in duplicate by two reviewers (X.W. and Y.Z.) using a standardized electronic form, and verified by a senior author (S.N.). Any discrepancies were resolved by consensus. We recorded the following information: study design, location, and time span, number of participants, inclusion criteria for OSA, demographic characteristics, methods of OSA assessment, duration and completeness of follow-up, cardiovascular events, and potential confounders included in adjusted analysis.
The potential risk of bias of RCTs was appraised according to the Cochrane Collaboration guidelines [17]. The quality items included random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias, which were each classified as low, unclear, or high.
The quality of observational studies was evaluated using the Newcastle-Ottawa Scale for cohort studies [18]. A quality score was calculated according to a maximum of one star for each item upon selection (4 items: representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, demonstration that outcome was not present at study start), comparability (2 items: controls for the most important factor and any additional factor), and outcomes (3 items: assessment, duration, and adequacy of follow-up) categories.
The primary outcome of interest was major adverse cardiovascular events (MACE), defined as a composite of all-cause or cardiovascular death, myocardial infarction (MI), stroke, repeat revascularization, or hospitalization for heart failure. Secondary endpoints included all-cause death, cardiovascular death, MI, stroke, and repeat revascularization. Definitions of events were in accordance to guidelines during each study period. Endpoints were assessed at the longest follow-up.
Data synthesis and analysis
In general, we collected multivariable-adjusted hazard ratio (HR) or risk ratio (RR) from original studies. In case of unreported HR or RR of the outcomes of interest, we calculated unadjusted RR using crude values. Summary RR with 95% confidence interval (CI) were estimated for primary and secondary outcomes by DerSimonian and Laird random-effects model. We used the Cochran Q test and I2 statistic to assess heterogeneity across studies with a significance level of p < 0.10. All analyses were performed with Cochrane Review Manager software (version 5.3). A 2-sided p value < 0.05 was deemed significant.
Results
Study selection and characteristics
The literature search yielded 1452 citations of which 21 were retained for full-text review (Fig. 1). We subsequently excluded 12 studies, of which 7 studies did not specify patients with CAD, and 4 studies did not report on the outcomes of interest, and 1 study had less than 1-year follow-up.
Fig. 1.
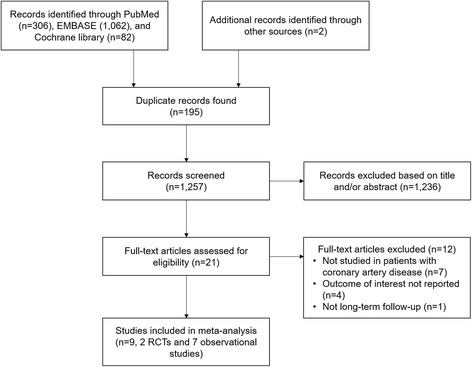
Flow chart of the study selection process for meta-analysis
Finally, a total of 9 studies [7–15] with 1430 participants were included in this meta-analysis. Study characteristics are listed in Tables 1 and 2. Seven studies were prospective cohort [7, 9–11, 13], 2 studies were retrospective cohort [8, 12], and 2 studies were RCTs [14, 15]. All studies enrolled patients with CAD and OSA. OSA was assessed primarily by overnight polysomnography in 7 studies [7–9, 11, 12, 14, 15], and by validated portable diagnostic devices in 2 studies [10, 13]. The definition of OSA was based on standardized assessment of apnea-hypopnea index (AHI) in all studies, with AHI ≥ 15 as cut-off value in most studies [7, 8, 10, 12, 14, 15]. In 2 studies, either CPAP (84% to 98%) or upper airway surgery (2% to 16%) was used, and effect measures were assessed for the entire modality [7, 8], whereas patients were exclusively treated with CPAP in others studies. Two retrospective cohort identified those who refused CPAP therapy or were not adherent to CPAP as untreated group [8, 12], whereas the others considered all patients receiving CPAP as being treated regardless of adherence. CPAP adherence data were available in 2 cohort studies [7, 9] (5.7 h [7] and 6.1 h [9] per night) and in 2 RCTs (4.5 h per night in Huang’s study [14] and 4.4 to 6.9 h per night during 6 years follow-up in Peker’s study [15]).
Table 1.
Study design and clinical characteristics of included studies
| Source | Study design, location, years | Number of participants | Main inclusion criteria | Mean age (Years) | Male (%) | Mean BMI (kg/m2) | Mean AHI (events/h) | Mean ESS (points) | OSA assessment |
|---|---|---|---|---|---|---|---|---|---|
| Milleron et al., 2004 [7] | Prospective cohort, single-center in France, 1991–1999 | 54 | AHI ≥ 15 | 57.3 | 98.1 | 28.3 | 31.2 | NR | Polysomnography |
| Cassar et al., 2007 [8] | Retrospective cohort, single-center in US, 1992–2004 | 371 | AHI ≥ 15 | 64.0 | 87.6 | 34.1 | 44.2 | NR | Polysomnography |
| Garcia-Rio et al., 2013 [9] | Prospective cohort, single-center in Spain, 2003–2005 | 123 | AHI ≥ 5 | 58.0 | 86.2 | 27.3 | 21.7 | 8.5 | Polysomnography |
| Capodanno et al., 2014 [10] | Prospective cohort, single-center in Italy, 2008 | 129 | AHI ≥ 15 | 68.3 | 80.6 | 27.3 | 22.4 | 7 | Portable diagnostic device |
| Nakashima et al., 2015 [11] | Prospective cohort, single-center in Japan, 2003–2009 | 95 | AHI ≥ 20 | 71.0a | 77.0a | NR | NR | NR | Polysomnography |
| Wu et al., 2015 [12] | Retrospective cohort, single-center in China, 2002–2012 | 295 | AHI ≥ 15 | 55.1 | 84.4 | 29.7 | 42.8 | NR | Polysomnography 72.1%, Portable diagnostic device 27.9% |
| Leão et al., 2016 [13] | Prospective cohort, single-center in Portugal, NR | 46 | AHI ≥ 5 | 63.5 | 82.6 | 27.8 | 30.6 | 8.8 | Portable diagnostic device |
| Huang et al., 2015 [14] | RCT, parallel, single-center in China, 2009–2012 | 73 | AHI ≥ 15, ESS < 15 | 62.4 | 82.2 | 27.7 | 28.5 | 8.8 | Polysomnography |
| Peker et al., 2016 [15] | RCT, parallel, single-center in Sweden, 2005–2010 | 244 | AHI ≥ 15, ESS < 10 | 66.0 | 84.1 | 28.5 | 28.8 | 5.5 | Polysomnography |
AHI apnea-hypopnea index, BMI body mass index, ESS Epworth Sleepiness Scale, NR not reported, OSA obstructive sleep apnea, RCT randomized controlled trial
aIndicate values in patients with AHI ≥ 15
Table 2.
Study groups, outcomes, results, and risk of bias
| Source | Follow-up | Loss to follow-up, % | Outcomes of interest (Primary) | Results | Confounders included in adjusted analysis |
|---|---|---|---|---|---|
| Milleron et al., 2004 [7] | 86.5 months (median) | 0 | MACE (Cardiovascular death, ACS, hospitalization for heart failure, or revascularization) | Adjusted HR, 0.24 (0.09–0.62) | Age, AHI, BMI, hypertension, and hypercholesterolaemia |
| Cassar et al., 2007 [8] | 3 year (median) | 0 | MACE (severe angina, MI, PCI, CABG, stroke, or death | Unadjusted RR, 0.93 (0.79–1.11) | NR |
| Garcia-Rio et al., 2013 [9] | 6.5 years (mean) | 2.4% | Recurrent MI | Adjusted HR, 0.16 (0.03–0.76) | Age, sex, body mass index, smoking habit, packs×year, LVEF, diabetes, hypertension, dyslipidemia, metabolic syndrome, smoking cessation and long-term pharmacological treatment |
| Capodanno et al., 2014 [10] | 3 years | 0 | MACE (all-cause death, MI, stroke, or repeat revascularization either percutaneous or surgical) | Adjusted HR, 0.18 (0.04–0.78) | BMI, smoking status, previous MI, prior stroke, and LVEF < 40% |
| Nakashima et al., 2015 [11] | 4 years (median) | 4.9 | MACE (cardiac death, ACS recurrence, and re-admission for heart failure) | Unadjusted RR, 0.46 (0.21–1.03) | NR |
| Wu et al., 2015 [12] | 4.8 years (median) | 1.5 | MACE (death, non-fatal MI, repeat revascularization, stent thrombosis, or stroke) | Adjusted HR, 0.82 (0.53–1.27) | Age, sex, BMI, clinical presentation, smoking, hypertension, type 2 diabetes, dyslipidemia, history of MI, cerebrovascular disease, peripheral arterial disease, renal failure, heart failure (LVEF ≤40%), extent of diseased or treated vessel, adjunctive medical therapy |
| Leão et al., 2016 [13] | 75 months (median) | 0 | MACE (death for any cause, MI, and myocardial revascularization | Unadjusted RR, 0.87 (0.31–2.46) | NR |
| Huang et al., 2015 [14] | 36 months (median) | 2.4 | MACE (new-onset acute MI, hospitalization for heart failure, need for repeated coronary revascularization, stroke, and death associated with cardiovascular and cerebrovascular disease) | Unadjusted RR, 0.21 (0.03–1.67) | NR |
| Peker et al., 2016 [15] | 56.9 months (median) | 0.4 | MACE (repeat revascularization, MI, stroke, and cardiovascular mortality) | Adjusted HR, 0.62 (0.34–1.13) | Age, sex, AHI, BMI, CABG vs. PCI, current smoking, hypertension, diabetes mellitus, acute MI, previous PCI or CABG, pulmonary disease, LVEF |
ACS indicates acute coronary syndrome, AHI apnea-hypopnea index, BMI body mass index, CABG coronary artery bypass graft, CPAP continuous positive airway pressure, HR hazard ratio, LVEF left ventricular ejection fraction, MACE major adverse cardiovascular events, MI myocardial infarction, NR not reported, PCI percutaneous coronary intervention, RR risk ratio
The median duration of follow-up was from 36 months to 86.5 months, and a small proportion of patients were lost to follow-up (up to 4.9%). Most of the studies reported adjusted risk estimates for the primary endpoint except 3 cohort studies [8, 11, 13] and 1 RCT [14], thus contributing to potential bias.
The 2 RCTs were open-label study and did not include blinding of participants and personnel to the intervention, but all did blinded assessment of cardiovascular outcomes [14, 15] (Additional file 1: Table S2). All the observational studies except 1 retrospective cohort [8] showed moderate-to-high quality (Newcastle-Ottawa Scale score > 6) (Additional file 1: Table S3).
Association of CPAP with MACE
Eight studies (6 observational studies and 2 RCTs) with 1307 patients reported outcome of MACE. Treatment with CPAP was associated with a significantly lower risk of MACE in 6 observational studies (RR 0.61, 95% CI: 0.39–0.94, P = 0.02). However, this result was not confirmed in 2 RCTs (RR 0.57, 95% CI: 0.32–1.02, P = 0.06) (Fig. 2). There was evidence of statistical heterogeneity for the composite endpoint in the observational studies (Q statistic P = 0.01; I2 = 66%). We further did subgroup analysis and showed that the decreased risk of MACE remained significant in 4 prospective cohort studies (RR 0.39, 95% CI: 0.21–0.74, P = 0.003; I2 = 34%), but was not significant in 2 retrospective cohort studies (RR 0.92, 95% CI: 0.78–1.07, P = 0.29; I2 = 0%), and the heterogeneity was attenuated in both subgroups (Additional file 1: Figure S1).
Fig. 2.
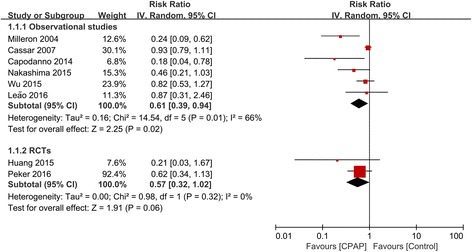
Forest plot of the risk estimates for major adverse cardiovascular events (MACE) in patients treated with continuous positive airway pressure (CPAP) compared to control
Association of CPAP with all-cause and cardiovascular death
There were 5 studies (1080 participants) that reported outcomes of all-cause death. CPAP significantly reduced the risk of all-cause death in 4 observational studies (RR 0.60, 95% CI 0.39–0.94, P = 0.03; I2 = 0%). However, the only RCT did not show significant risk reduction (RR 0.78, 95% CI 0.30–2.02, P = 0.61) (Fig. 3).
Fig. 3.
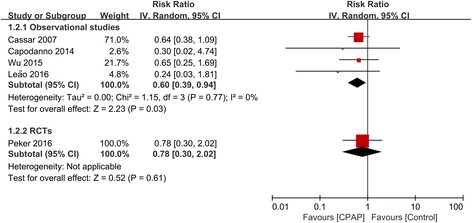
Forest plot of the risk estimates for all-cause death in patients treated with continuous positive airway pressure (CPAP) compared to control
Cardiovascular death was evaluated in 5 studies with 866 participants. Similarly, CPAP therapy significantly reduced the risk of cardiovascular death in 3 observational studies (RR 0.28, 95% CI 0.12–0.68, I2 = 0%), which were also not reproduced in 2 RCTs (RR 0.41, 95% CI 0.12–1.41, I2 = 0%) (Fig. 4).
Fig. 4.
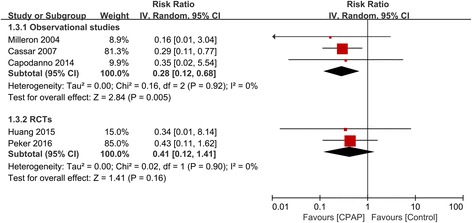
Forest plot of the risk estimates for cardiovascular death in patients treated with continuous positive airway pressure (CPAP) compared to control
Association of CPAP with individual cardiovascular events
We also evaluated the effect of CPAP on outcomes of MI in 5 studies (3 observational and 2 RCTs; 781 participants), stroke in 3 studies (1 observational and 2 RCTs; 612 participants), and repeat revascularization in 6 studies (5 observational and 1 RCTs; 886 participants). There was no association of CPAP with all individual cardiovascular events in both observational studies and RCTs (Figs. 5, 6, and 7).
Fig. 5.
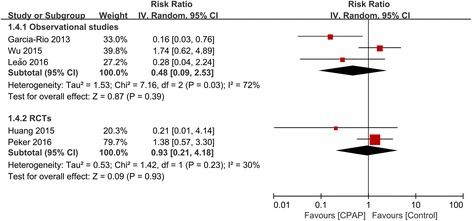
Forest plot of the risk estimates for myocardial infarction in patients treated with continuous positive airway pressure (CPAP) compared to control
Fig. 6.

Forest plot of the risk estimates for stroke in patients treated with continuous positive airway pressure (CPAP) compared to control
Fig. 7.
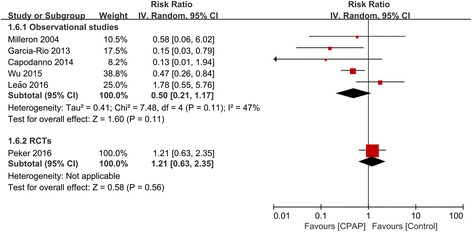
Forest plot of the risk estimates for repeat revascularization in patients treated with continuous positive airway pressure (CPAP) compared to control
Discussion
In the present meta-analysis, the associations of CPAP use with risk reduction of composite cardiovascular events, all-cause and cardiovascular death in patients with concomitant CAD and OSA were only demonstrated in observational studies, but not RCTs. There were also no significant associations between CPAP treatment with individual cardiovascular outcomes. Based on these results, there is still no clear evidence to prescribe CPAP with the purpose of preventing future cardiovascular events in patients with OSA and established CAD.
OSA was linked to a series of cardiovascular risk factors and outcomes. CPAP is effective in reversing upper airway obstruction and hypoxemia. Randomized trials have demonstrated that CPAP treatment improves cardiovascular surrogate endpoints, such as blood pressure [14, 19] and insulin resistance [20]. However, in the trials evaluating MACE, no significant beneficial effects of CPAP were shown in patients with OSA [15, 21, 22]. In the most recently SAVE (Sleep Apnea Cardiovascular Endpoints) trial that randomized 2717 participants with coronary or cerebrovascular disease and moderate-to-severe OSA, CPAP did not result in a lower rate of the composite cardiovascular events at a median follow-up of 3.7 years [21]. Furthermore, several meta-analyses of randomized trials also showed no effect of CPAP therapy on MACE for OSA with or without cardiovascular morbidities [23–25]. However, the study populations of included studies are diverse, from general population to patients with severe CAD (such as MI), thus precluding definitive conclusions.
To the best of our knowledge, the present meta-analysis is the first attempt to focus on a relatively homogenous group of patients with established CAD. Our findings suggested adding CPAP as a secondary prevention for patients with CAD and concomitant OSA might be beneficial in the long-term follow-up, but this was only shown in observational studies, and not verified in RCTs. The enrolled studies in the prospective and retrospective cohorts were usually conducted more than 10 years ago and had a wide range of follow-up, therefore they do not represent contemporary medical and interventional therapy. Also, the results could be underpowered due to a relatively small sample size and variations in study populations and definitions of events. The negative results of RCTs were mainly derived from the RICCADSA (Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea) trial, which enrolled 224 patients with OSA and revascularized CAD. There is no significant difference in the composite endpoint of repeat revascularization, MI, stroke, or cardiovascular death between CPAP and untreated OSA patients [15]. It should be noted that CPAP therapy tended to be associated with a reduced risk of MACE in the 2 RCTs (RR 0.57, 95% CI: 0.32–1.02), although there was no significant difference. Due to the small number of included RCTs, this result should be interpreted with caution. In the RICCADSA trial, adjusted on-treatment analysis exhibited better outcomes among patients who were adherent to CPAP therapy (≥4 h per night). In addition, patients in the RICCADSA trial were heterogeneous with variable risk profiles, including both percutaneous coronary intervention (PCI) and CABG, and both acute or elective PCI, thus attenuating the anticipated treatment effect. In case of second prevention of CAD patients, the treatment effects of CPAP are still needed to be evaluated in a high-risk group with homogenous CAD populations (ACS, MI, or PCI, etc.).
In the contemporary era, with the extensive use of lipid-lowering and blood pressure lowering agents, antiplatelet therapy, and drug-eluting stents, treatment of OSA with CPAP might not add more benefits for CAD patients based on current evidence. As OSA is highly prevalent and is associated with subsequent cardiovascular risk, we should still pay more attention to this condition when assessing patients with CAD in clinical practice. Whether increased compliance to CPAP or novel treatment options can lead to better cardiovascular outcomes needs further investigation.
Study limitations
First, we observed significant statistical heterogeneity in the outcome measure of MACE in the observational studies, which could be partly explained by different study design, small sample size, and study quality according to whether adjustment for confounders was performed. We did subgroup analysis based on prospective or retrospective cohorts, and the heterogeneity was attenuated in both subgroups. Second, the study population varies across studies, from general CAD patients to MI with or without revascularization (PCI or CABG). The treatment effects of CPAP need to be further investigated in more homogenous patients. Third, there are significant differences in the definitions of the use and adherence of CPAP, which could impact the treatment effects compared to the control group. Fourth, there are not enough studies (less than 10) to test for publication bias for the primary endpoint. Fifth, the risk estimates of individual cardiovascular events could be underpowered due to a small number of included studies and variations in events definition.
Conclusions
Compared to standard therapy alone, the use of CPAP in patients with OSA and concomitant CAD was associated with a reduced risk of major cardiovascular events, all-cause and cardiovascular mortality, which was only observed in observational studies, but not in RCTs. There is a need for large-scale RCTs to further explore the value of CPAP therapy as a second prevention in a high-risk and homogenous CAD population.
Additional file
Supplemental Material. (DOCX 353 kb)
Acknowledgements
Not applicable.
Funding
This study was funded by grants from International Science & Technology Cooperation Program of China (2015DFA30160), Beijing Municipal Science & Technology Commission (Z141100006014057), National Natural Science Foundation of China (81600209), Beijing Municipal Administration of Hospitals’ Youth Program (QML20160605), Beijing Municipal Administration of Hospitals Incubating Program (PX2016048), and Beijing Municipal Organization Department (2016000021469G194).
Availability of data and materials
Data are available from the authors upon request.
Abbreviations
- ACS
Acute coronary syndrome
- AHI
Apnea-hypopnea index
- AMI
Acute myocardial infarction
- BMI
Body mass index
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- CI
Confidence interval
- CPAP
Continuous positive airway pressure
- HR
Hazard ratio
- MACE
Major adverse cardiovascular event
- MI
Myocardial infarction
- NR
Not reported
- OSA
Obstructive sleep apnea
- PCI
Percutaneous coronary intervention
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analysis
- RCTs
Randomized controlled trials
- RICCADSA
Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea
- RR
Risk ratio
- SAVE
Sleep Apnea Cardiovascular Endpoints
- STEMI
ST-segment elevation myocardial infarction
Authors’ contributions
SN and YW conceived the study. XW, SN, and YW designed the study. XW, YZ, and SN conducted the literature search and data extraction. XW and YZ performed the meta-analysis. XW, YZ, ZD, and JF interpreted the data. XW and YZ wrote the draft of the manuscript, and all authors critically revised the manuscript and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12931-018-0761-8) contains supplementary material, which is available to authorized users.
Contributor Information
Shaoping Nie, Phone: 86-10-84005256, Email: spnie@ccmu.edu.cn.
Yongxiang Wei, Phone: 86-10-64456768, Email: weiyongxiang@vip.sina.com.
References
- 1.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CH, Sethi R, Li R, Ho HH, Hein T, Jim MH, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133(21):2008–2017. doi: 10.1161/CIRCULATIONAHA.115.019392. [DOI] [PubMed] [Google Scholar]
- 3.Xie J, Sert Kuniyoshi FH, Covassin N, Singh P, Gami AS, Wang S, et al. Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc. 2016;5(8):e003162. doi: 10.1161/JAHA.115.003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazaki T, Kasai T, Yokoi H, Kuramitsu S, Yamaji K, Morinaga T, et al. Impact of sleep-disordered breathing on long-term outcomes in patients with acute coronary syndrome who have undergone primary percutaneous coronary intervention. J Am Heart Assoc. 2016;5(6):e003270. doi: 10.1161/JAHA.116.003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CH, Khoo SM, Chan MY, Wong HB, Low AF, Phua QH, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7(6):616–621. doi: 10.5664/jcsm.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99(1):26–30. doi: 10.1016/j.amjcard.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 7.Milleron O, Pilliere R, Foucher A, de Roquefeuil F, Aegerter P, Jondeau G, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25(9):728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50(14):1310–1314. doi: 10.1016/j.jacc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Rio F, Alonso-Fernandez A, Armada E, Mediano O, Lores V, Rojo B, et al. CPAP effect on recurrent episodes in patients with sleep apnea and myocardial infarction. Int J Cardiol. 2013;168(2):1328–1335. doi: 10.1016/j.ijcard.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Capodanno D, Milazzo G, Cumbo M, Marchese A, Salemi A, Quartarone L, et al. Positive airway pressure in patients with coronary artery disease and obstructive sleep apnea syndrome. J Cardiovasc Med (Hagerstown) 2014;15(5):402–406. doi: 10.2459/JCM.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima H, Kurobe M, Minami K, Furudono S, Uchida Y, Amenomori K, et al. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2015;4(1):75–84. doi: 10.1177/2048872614530865. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Lv S, Yu X, Yao L, Mokhlesi B, Wei Y. Treatment of OSA reduces the risk of repeat revascularization after percutaneous coronary intervention. Chest. 2015;147(3):708–718. doi: 10.1378/chest.14-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leao S, Conde B, Fontes P, Calvo T, Afonso A, Moreira I. Effect of obstructive sleep apnea in acute coronary syndrome. Am J Cardiol. 2016;117(7):1084–1087. doi: 10.1016/j.amjcard.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Liu Z, Luo Q, Zhao Q, Zhao Z, Ma X, et al. Long-term effects of continuous positive airway pressure on blood pressure and prognosis in hypertensive patients with coronary heart disease and obstructive sleep apnea: a randomized controlled trial. Am J Hypertens. 2015;28(3):300–306. doi: 10.1093/ajh/hpu147. [DOI] [PubMed] [Google Scholar]
- 15.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London: The Cochrane Collaboration; 2011. Available from http://handbook.cochrane.org.
- 18.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 19.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, Lloberes P, Diaz de Atauri MJ, Somoza M, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Ceron E, Barquiel B, Bezos AM, Casitas R, Galera R, Garcia-Benito C, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(4):476–485. doi: 10.1164/rccm.201510-1942OC. [DOI] [PubMed] [Google Scholar]
- 21.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 22.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, Martinez-Alonso M, Carmona C, Barcelo A, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156–166. doi: 10.1001/jama.2017.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abuzaid AS, Al Ashry HS, Elbadawi A, Ld H, Saad M, Elgendy IY, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apnea. Am J Cardiol. 2017;120(4):693–699. doi: 10.1016/j.amjcard.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 25.Guo J, Sun Y, Xue LJ, Huang ZY, Wang YS, Zhang L, et al. Effect of CPAP therapy on cardiovascular events and mortality in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2016;20(3):965–974. doi: 10.1007/s11325-016-1319-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material. (DOCX 353 kb)
Data Availability Statement
Data are available from the authors upon request.


