Abstract
Background
Several cases of food-borne acute Chagas disease (ACD) have been reported in the Brazilian Amazon so far. Up to 2004, the occurrence of ACD by oral transmission, associated with food consumption, was rare. Recent cases of ACD in Brazil have been attributed to the consumption of juice from the açai palm containing reservoir animals or insect vectors waste, infected with Trypanosoma cruzi. This study aimed to determine the T. cruzi contamination rate and to genotype the parasite in food samples prepared from açai, which are commercialized in Rio de Janeiro and the Pará States in Brazil.
Methods
The amplificability of DNA extracted from açai samples, and T. cruzi and Triatominae detection were performed by conventional PCR. Molecular characterization was done by multilocus PCR analysis, to determine the parasite discrete type units (DTUs) based on the size of PCR products in agarose gels, using the intergenic region of the spliced leader (SL), 24 Sα rDNA and nuclear fragment A10 as targets.
Results
From the 140 samples of açai-based products analyzed, T. cruzi DNA was detected in 14 samples (10%); triatomine DNA was detected in one of these 14 samples. The parasite genotyping demonstrated that food samples containing açai showed a mixture of T. cruzi DTUs with TcIII, TcV and TcI prevailing.
Conclusions
In this study, the molecular detection and identification of T. cruzi from açai-based manufactured food samples, was performed for the first time. Although parasite DNA is a marker of possible contamination during food manufacturing, our findings do not indicate that açai is a source of Chagas disease via oral transmission per se, as live parasites were not investigated. Nevertheless, a molecular approach could be a powerful tool in the epidemiological investigation of outbreaks, supporting previous evidence that açai-based food can be contaminated with T. cruzi. Furthermore, both food quality control and assessment of good manufacturing practices involving açai-based products can be improved, assuring the safety of açai products.
Keywords: Chagas disease, Trypanosoma cruzi, Açai, Oral transmission, T. cruzi genotyping, Health surveillance
Background
Chagas disease (ChD) is an important neglected tropical illness caused by the flagellate protozoan Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae). The disease is established through a complex biological cycle, including insects belonging to the subfamily Triatominae (Hemiptera: Reduviidae) and mammalian reservoir that may belong to several classes, such as marsupials and rodents [1]. Currently, more than five million people are infected and approximately 70 million living at risk in the world [2]. In the chronic phase, the disease shows a diversity in clinical manifestations, from indeterminate to cardiac and/or digestive forms [2, 3], which can be associated with complex interactions between the genetic diversity of the parasite and the host, and environmental and epidemiologic factors [4].
The reemergence of ChD from 2005 challenged Brazilian authorities. This disease used to be characteristic of rural areas, especially the most deprived populations without access to adequate sanitary conditions; however, in recent years, it has also been disseminated in urban areas, with transmission via ingestion also reported. In this case, the disease is more aggressive and difficult to control, with lethality as high as 5% in the Amazon [5]. In Brazil, several cases of acute Chagas disease (ACD) have been reported as outbreaks, characterized by groups of individuals gathered in the same place, who ingested the same food and became sick almost simultaneously, with fever and general manifestations of a systemic infection [6]. Despite the increasing number of acute cases, reports of this form of the disease are rare in the literature.
The açai palm is most commonly associated with ACD cases in the northern Amazon and involves either contamination of the fruits or the pulp itself, via reservoir animal waste or infected insect vectors in endemic areas [7, 8]. Consequently, the ability to produce sanitary and high-quality food stuffs from the açai palm concerned the authorities and created the need for public health strategies to combat Chagas’ disease and its spread via this novel route. In cases of ACD outbreaks, elucidation of oral transmission is usually determined via clinical and/or epidemiological investigations. However, there were no official methods established for this kind of investigation in Brazil.
Molecular methods based on PCR can be used to test food samples for pathogens, or residual pathogen DNA. This is usually the case following outbreaks, due to the delay between the event and analysis. Thus, the availability of methods for the detection of T. cruzi in food samples would be a powerful tool in the epidemiological investigation of Chagas disease.
Methods
Samples
One hundred and forty samples of açai-based products were analyzed in this study, in two different periods. In the first period (2010), 47 samples were analyzed, including 17 samples gathered randomly from food markets in Rio de Janeiro, 9 samples of açai juice, 8 frozen or chilled samples of açai with guaraná or fruits, and 30 samples gathered from street markets in Pará. Those included 2 samples of açai pulps, 9 açai juices, 1 açai candy, 1 chocolate bonbon with açai, 1 açai ice cream, 1 açai popsicle, 2 açai with rice porridge, 2 açai seeds, and 11 açai fruits. During the second period (2011–2015), 93 samples were gathered from Rio de Janeiro and Pará food markets by local regulatory health authorities. In Rio de Janeiro, 48 samples consisting of 27 açai juices, 1 açai pulp, and 20 açai with guaraná or fruits were collected during 2011 and 2012. In Pará, from 2012 to 2015, 20 açai pulps, 4 açai juices and 20 açai fruits were collected, as part of a monitoring program to evaluate the sanitary conditions of street markets. In 2013, a sample of açai pulp was personally collected and sent to the laboratory.
For the purposes of sensitivity and specificity assays we obtained samples of Trypanosoma, Leishmania, bacteria, yeast, and fungi. Specifically, Trypanosoma cruzi (strains or clones Y, Dm28c, CL Brener, INPA 222, INPA 4167, COLTRYP 016, COLTRYP 043, COLTRYP 370), T. rangeli (COLPROT 273), T. cervi, T. lewisi and T. mega were supplied by the Coleção de Protozoários (COLPROT) and by the Coleção de Trypanosoma de mamíferos silvestres, doméstico e vetores (COLTRYP), which belong to the Oswaldo Cruz Institute (Rio de Janeiro, Brazil). We obtained Leishmania amazonensis (IOC-L575), L. braziliensis (IOC-L560), L. guyanensis (IOC-L565), L. lainsoni (IOC-L1023), L. naiffi (1365) and L. shawi (IOC-L1545) from the Coleção de Leishmania do Instituto Oswaldo Cruz (CLIOC). Finally, the bacteria Bacillus cereus (INCQS-00435), Cronobacter sakazakii (INCQS-00578), Escherichia coli (INCQS-00033), Staphylococcus aureus (INCQS-00015), and Salmonella sp. (INCQS-00150), the yeasts Saccharomyces cerevisiae (INCQS-40001) and Ogataea polymorpha (INCQS-400116), and the fungi Alternaria alternate (INCQS-40291) and Botrytis cinerea (UFPE2802) were provided by the Reference Microorganisms Collection in Sanitary Surveillance (CMRVS) of the National Institute of Health Quality Control (INCQS/ FIOCRUZ). Trypanosoma rangeli and Salmonella sp. were used as negative controls in the PCR assays.
Sample preparation and DNA extraction
Açai juice and pulp, açai with guaraná or fruits, açai ice cream, açai popsicles, and açai porridge samples were thawed at 4 °C, homogenized in a Seward Stomacher® 400 Laboratory Blender (Seward, West Sussex, UK), and 2 ml were transferred to a glass vial and frozen at -18 °C for 24 h. Three aliquots were then lyophilized for c.18 h. The açai candy sample was manually homogenized, and three portions of 100 mg were transferred to 1.5 ml sterile vials. Before the homogenization of the açai bonbon sample, the chocolate layer was withdrawn, and three 100 mg portions were weighted and moved to 1.5 ml sterile vials. The açai fruits and seeds were placed in plastic bag with 20 ml of sterile water, and the sample was homogenized by inversion 50 times. The water was transferred to a glass vial (100 ml), frozen at -18 °C for 24 h and then lyophilized for c.18 h. DNA was extracted in triplicate from homogenized samples using the cetyltrimethylammonium bromide (CTAB) method, as proposed by the Joint Research Center [9], with some modifications [10]. Triplicate sample were analyzed in parallel. The DNA concentrations and quality were estimated by measuring the absorbance at 260 nm (A260), 280 nm (A280) and 230 nm (A230) using the GeneQuant pro spectrophotometer (Amersham Biosciences, Buckinghamshire, UK). The average concentration of DNA extracted from açai samples was 70.6 ± 9.8 ng/μl.
Evaluation of DNA amplificability
The amplificability of DNA (i.e. monitoring for PCR inhibitors) was verified using a plant-specific primer pair VPRBCP1/VPRBCP2 targeting the ribulose 1,5-diphosphate carboxylase/ oxygenase gene (rbcL) of the plant chloroplast, as described by Mbongolo Mbella et al. [11]. The reaction was set up in a volume of 25 μl with 1× PCR buffer (pH 8.3), 200 μM of dNTP mix, 1.5 mM MgCl2, 0.24 μM of each primer, 1.5 U of Platinum® Taq DNA polymerase (Thermo Scientific, Waltham, USA) and 2 μl DNA sample, in a GeneAmp PCR System 2400 thermocycler (Applied Biosystems, Foster City, USA), as follows: 95 °C for 3 min, 40 cycles at 94 °C for 1 min, 60 °C for 1 min, and 72 °C for 1 min with an additional extension at 72 °C for 7 min. Primers were synthesized and purified by Invitrogen (Thermo Scientific). The PCR products were loaded in a 2% (w/v) agarose gel and stained with GelRed 1× (Biotium, Fremont, USA).
Detection of T. cruzi DNA by conventional PCR
To detect DNA from T. cruzi we PCR-amplified the telomeric region of the gp85/sialidase superfamily using the primer pair (Tc189F and Tc189R), as described by Chiurillo et al. [12]. The reaction was set up in a volume of 25 μl with 1× PCR buffer (pH 8.4), 0.1% Triton X-100, 160 μM of each dNTP, 1.5 mM MgCl2, 0.4 μM of each primer, 1 U of Platinum® Taq DNA polymerase, and 2 μl DNA solution, in a GeneAmp PCR System 2400 thermocycler, as follows: 94 °C for 4 min; 33 cycles at 94 °C for 1 min, 60 °C for 30 s; and 72 °C for 40 s with an additional extension at 72 °C for 3 min. The PCR products were loaded in a 2% (w/v) agarose gel and stained with GelRed 1×.
Detection of triatomine DNA by conventional PCR
To detect DNA from triatomines we PCR-amplified a portion of the 12S rRNA gene [13]. Conventional PCR was carried out in a final volume of 50 μl, containing: 5 μl DNA solution, 1× PCR buffer, 0.2 mM dNTPs, 4.5 mM MgCl2, 1.25 U Platinum® Taq DNA polymerase, and 0.1 μM of each primer. Reactions were performed in the GeneAmp PCR System 9700 thermocycler (Applied Biosystems) as follows: 94 °C for 12 min; 36 cycles at 94 °C for 30 s, 55 °C for 30 s; and 72 °C for 30 s with an additional extension at 72 °C for 10 min. The PCR products were loaded in a 2% (w/v) agarose gel and stained with GelRed 1×.
Trypanosoma cruzi genotyping
The molecular characterization of T. cruzi DNA extracted from food samples was performed based on multilocus PCR analysis [14] to discriminate between the discrete typing units (DTUs) of the parasite. The identification of genotypes was based on the PCR profile revealed for each target, using the biomarkers spliced leader (SL), 24 Sα rDNA, and nuclear fragment A10 (Fig. 1).
Fig. 1.
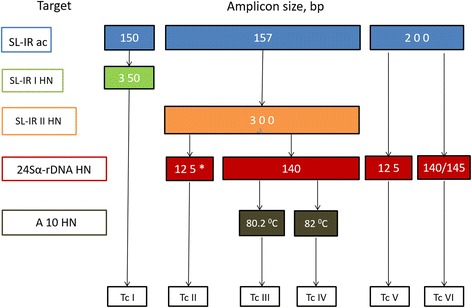
T. cruzi genotyping approach by multilocus PCR for DTU assignment. The coloured boxes indicate the expected PCR product sizes (or melting temperatures) for each target, indicated on the left. DTUs (TcI to TcVI) are indicated in white boxes
For SL, the intergenic region ac (SL-IR ac) was amplified with primers TCac and UTCC to differentiate T. cruzi DTUs TcI (150 bp), TcII, TcV, TcVI (157 bp), and TcIII, TcIV (200 bp). Reactions were performed as follows: 94 °C for 10 min, 3 cycles at 94 °C for 30 s, 70 °C for 30 s and 72 °C for 30 s, 3 cycles at 94 °C for 30 s, 68 °C for 30 s and 72 °C for 30 s, 4 cycles at 94 °C for 30 s, 66 °C for 30 s and 72 °C for 30 s, 4 cycles at 94 °C for 30 s, 64 °C for 30 s and 72 °C for 30 s, 36 cycles at 94 °C for 30 s, 62 °C for 30 s and 72 °C for 30 s, and a final step of 72 °C for 10 min. Another intergenic region of SL gene (SL-IR I and SL-IR II HN) was amplified with primers TCC, TC1 and TC2 to distinguish between TcI (350 bp) and TcII, TcV, TcVI (300 bp), and TcIII, TcIV (not amplified). Cycling conditions included 94 °C for 10 min, 5 cycles at 94 °C for 1 min, 67 °C for 1 min and 72 °C for 1 min, 5 cycles at 94 °C for 1 min, 65 °C for 1 min and 72 °C for 1 min, 5 cycles at 94 °C for 1 min, 63 °C for 1 min and 72 °C for 1 min, 30 cycles at 94 °C for 1 min, 61 °C for 1 min and 72 °C for 1 min, and a final step of 72 °C for 10 min. For D7 variable domain of 24Sα ribosomal subunit (24Sα rDNA HN), semi nested-PCR was used with primers D75 and D76 in the first round and D71 and D76 in the second round to differentiate between TcII, TcVI (140 bp), TcIII (125 bp), TcIV (140/145 bp) and TcV (125 or 125 + 140 bp). Cycling conditions consist in a first step at 94 °C for 10 min, 3 cycles at 94 °C for 30 s, 64 °C for 45 s and 72 °C for 1 min, 3 cycles at 94 °C for 30 s, 62 °C for 45 s and 72 °C for 1 min, 3 cycles at 94 °C for 30 s, 60 °C for 45 s and 72 °C for 1 min, 35 cycles at 94 °C for 30 s, 58 °C for 45 s and 72 °C for 1 min, and a final step of 72 °C for 10 min. The A10 fragment region (A10 HN) was amplified by semi-nested PCR with primers Pr1 and P6 in the first round. Cycling conditions were: the first step at 94 °C for 10 min, 35 cycles at 94 °C for 1 min and 65 °C for 1 min and 72 °C for 1 min, and a final step of 72 °C for 10 min.
Quantitative real-time PCR (second round) was performed using primers Pr3 and Pr1 to differentiate between TcII (Tm 80.2 °C) and TcVI (Tm 82 °C) (Fig. 1). Cycling conditions were the first step at 94 °C for 10 min, 35 cycles at 94 °C for 1 min and 60 °C for 1 min and 72 °C for 1 min, and a final step of 72 °C for 10 min.
The conventional PCR was set up in a volume of 30 μl containing 1× PCR buffer (pH 8.3), 250 μM dNTPs, 3 mM MgCl2, 5 U Platinum® Taq DNA polymerase, 1.67 μM of each primer, and 5 μl DNA solution. qPCR reactions were carried out in a final volume of 10 μl using 2× SYBR Green master mix buffer (Applied Biosystems), 0.5 μM of each primer, and 2 μl of DNA sample.
Results
DNA amplificability
The amplificability of DNA (i.e. monitoring the presence/absence of PCR inhibitors) extracted from all the samples was confirmed through the visualization of 95 bp amplicons resulting from the amplication of rbcl gene (Fig. 2). No PCR inhibition was observed following our methodology.
Fig. 2.
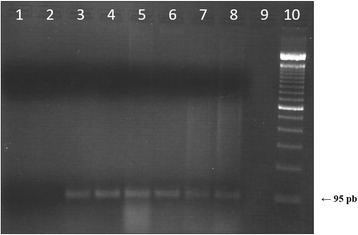
Amplification of the rbcl gene with primers VPRBCP1/VPRBCP2. Lane 1: sterile water (negative control), Lane 2: Salmonella sp. (negative control); Lane 3: rice; Lane 4: bean; Lane 5: soy; Lane 6: maize; Lane 7: acai juice; Lane 8: açai pulp; Lane 9: empty; Lane 10: 100 bp DNA ladder. The arrow indicates the PCR product size, 95 bp
Detection of T. cruzi DNA
Prior to the analysis of commercial açai-based samples, the limit of detection (LOD) and analytical sensitivity and specificity of PCR assays using primers Tc189F and Tc189R were evaluated. In Figs. 3, 2.4 ng of DNA extracted from non-contaminated açai was mixed with T. cruzi DNA, to create dilutions from 75 pg to 2.4 pg of parasite DNA. The 100 bp band, corresponding to the amplification of the telomeric junction of T. cruzi was observed from as little as 3 pg of T. cruzi DNA, which was considered LOD of the assay. In Fig. 4, the analytical sensitivity and specificity of PCR was evaluated using 2 ng of DNA from trypanosomatids, yeasts, fungi and bacteria. This assay successfully amplified DNA from four of the six T. cruzi DTUs (TcI, TcII, TcIII and TcVI) using primers Tc189F/Tc189R; DNA from all strains tested was amplified successfully. These primers did not amplify genomic DNA from other trypanosomatids (T. rangeli, T. cervi, T. lewisi, T. mega, L. amazonensis, L. braziliensis, L. guyanensis, L. lainsoni, L. naiffi and L. shawi), yeasts (O. polymorpha and S. cerevisae), fungi (A. alternate and B. cinerea), or bacteria (B. cereus, C. sakasaki, E. coli, Salmonella sp. and S. aureus).
Fig. 3.
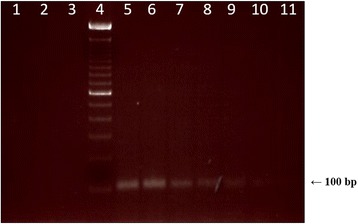
Limit of detection of T. cruzi in açai samples with primers Tc189F/Tc189R. T. cruzi DNA (from 75 pg to 2.4 pg) was mixed with 240 ng of açai DNA before the PCR assay using Tc189F/189R primers. Lane 1: sterile water (negative control); Lane 2: T. rangeli DNA (5 ng); Lane 3: Açai without T. cruzi (negative control); Lane 4: 100 bp DNA ladder. T. cruzi DNA: Lane 5: 75 pg; Lane 6: 45 pg; Lane 7: 24 pg; Lane 8: 15 pg; Lane 9: 9 pg; Lane 10: 3 pg; Lane 11: 2.4 pg
Fig. 4.
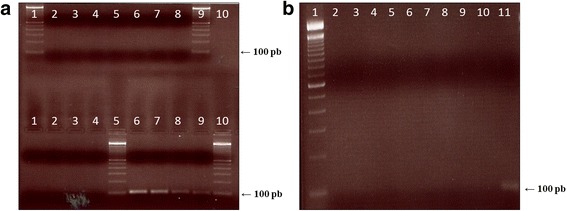
Analytical sensitivity and specificity of Tc189F/Tc189R primers to trypanosomatids and other microorganisms. PCR assays using primers Tc189F/Tc189R were performed with two ng DNA of trypanosomatids, yeast, fungi and bacteria. a Specificity assay (Non-detectable PCR). Upper lanes - Lane 1: 100 bp DNA ladder; Lane 2: sterile water (negative control); Lane 3: T. rangeli; Lane 4: T. cervi; Lane 5: T. lewisi; Lane 6: T. mega; Lane 7: L. amazonensis; Lane 8: L. braziliensis; Lane 9: 100 bp DNA ladder; Lane 10: no template. Bottom lanes - Lane 1: L. guyanensis; Lane 2: L. lainsoni; Lane 3: L. naiffi; Lane 4: L. shawi; Sensitivity assay (detectable PCR). Lane 5: 100 bp DNA ladder; Lane 6: T. cruzi Dm28c clone (TcI); Lane 7: T. cruzi Y strain (TcII); Lane 8: T. cruzi INPA 222 strain (TcIII); Lane 9: T. cruzi CL Brener clone (TcVI); Lane 10: 100 bp DNA ladder. b Specificity assay (continuation). Lane 1: 100 bp DNA ladder; Lane 2: A. alternate; Lane 3: B. cinerea; Lane 4: O. polymorpha; Lane 5: S. cerevisae; Lane 6: B. cereus; Lane 7: C. sakasaki; Lane 8: E. coli; Lane 9: Salmonella sp.; Lane 10: S. aureus; Lane 11: T. cruzi CL Brener clone (TcVI)
Eleven of the 47 (23.4%) samples collected in 2010 were positive for T. cruzi DNA, as indicated by the 100 bp amplicon visualized via agarose gel electrophoresis (Fig. 5). From the samples collected in Rio de Janeiro, 8 (47%) of 17 samples were PCR-positive for T. cruzi DNA: açai juice (n = 2), açai with guaraná (n = 1), açai with guaraná and strawberry (n = 2), açai with guaraná and banana (n = 2) and açai with guaraná, strawberry and acerola (n = 1). Among the samples collected in Pará, 3 of 30 (10%) samples were PCR-positive: açai juice (n = 1) and açai fruit (n = 2), all collected in the “Feira do açai”, at the Belém city (Table 1). Comparative analysis of PCR positivity showed no statistically significant difference (difference between independent proportions test, P = 0.5722) between the two states.
Fig. 5.
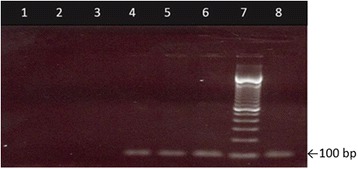
Amplification of T. cruzi DNA in açai samples with primers Tc189F/Tc189r. Lane 1: no template; Lane 2: sterile water; Lane 3: T. rangeli (negative control); Lanes 4–6: açai samples; Lane 7: 100 bp DNA ladder; Lane 8: T. cruzi (positive control)
Table 1.
Detection of T. cruzi DNA in samples collected in Rio de Janeiro and Pará States
| Sample type | Collection place | Collection year | No. of positive samples/total no. of samples |
|---|---|---|---|
| Açai juice | RJ | 2010 | 2/9 |
| PA | 2010 | 1/9 | |
| PA | 2015 | 0/4 | |
| Açai pulp | PA | 2010 | 0/2 |
| PA | 2012 | 1/15 | |
| PA | 2013 | 1/6 | |
| Açai with guaraná or fruits | RJ | 2010 | 6/8 |
| Açai candy | PA | 2010 | 0/1 |
| Chocolate bonbon with açai | PA | 2010 | 0/1 |
| Açai ice cream | PA | 2010 | 0/1 |
| Açai popsicle | PA | 2010 | 0/1 |
| Açai with rice porridges | PA | 2010 | 0/2 |
| Açai seed | PA | 2010 | 0/2 |
| Açai fruit | PA | 2010 | 2/11 |
| PA | 2012 | 1/15 | |
| PA | 2013 | 0/5 |
Abbreviations: PA Pará, RJ Rio de Janeiro
From the 93 samples collected in 2011, 2012 and 2015 in Pará and Rio de Janeiro states, only three samples were positive. From the 44 samples collected in Pará, two samples collected in 2012 were positive: one açai pulp and one açai fruit. In the açai pulp sample, T. cruzi DNA was also detected. None of the 48 samples collected in Rio de Janeiro between 2011 and 2012 were positive for parasite DNA by PCR (Table 1).
Detection of triatomine DNA
The samples positive for T. cruzi DNA were analyzed for triatomine DNA by conventional PCR, to verify the simultaneous presence of parasite and invertebrate vector DNA in the same sample. From 13 samples analyzed, only 1 sample of açai juice showed an amplicon of 163 bp (Fig. 6), indicating the presence of triatomine DNA. This sample was collected in the Pará state, in 2010.
Fig. 6.
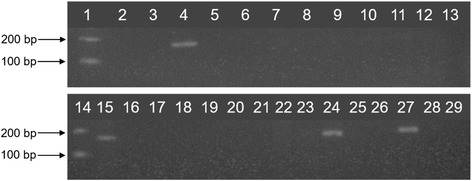
Amplification of triatomine DNA in açai samples with primers P2B/P6r. Lanes 1 and 14: 100 bp DNA ladder; Lanes 2 and 3: no template; Lane 4: uninfected triatomine (positive control); Lanes 5–7 and 9–10: açai with fruit; Lane 8: açai with guaraná; Lanes 11–13: açai; Lanes 15 and 16: açai fruit; Lanes 17 and 18: açai; Lane 19: T. cruzi; Lane 20: T. rangeli; Lane 21: açai pulp; Lane 22: açai fruit; Lane 23: T. cruzi; Lane 24: Triatomine spiked with T. cruzi (positive control); Lanes 25 and 26: T. cruzi; Lane 27: uninfected triatomine (positive control); Lane 28: T. rangeli, Lane 29: T. cruzi
Trypanosoma cruzi genotyping
From the samples positive for T. cruzi DNA, 13 were submitted for genotyping using SL intergenic region (SL-IR ac and SL-IR I and II), D7 variable domain of 24Sα ribosomal subunit, and A10 fragment region as targets. Figure 7 shows the amplified fragments generated by SL-IRac (a), SL-IR-I and II (b), and 24Sα rDNA (c). To differentiate TcII from TcVI, the melting curves generated by qPCR, corresponding to amplification of nuclear fragment A10 are also shown (Fig. 7d).
Fig. 7.
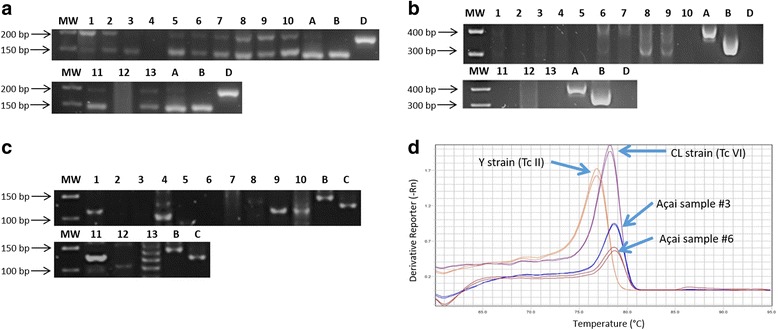
Amplified fragments generated by SL-IRac (a), SL-IR-I and II (b), and 24S rDNA (c) targets and melting curves generated by the amplification of nuclear fragment A10 in positive controls (d). a-c Lane MW: 50 and 100-bp DNA ladder; Lanes 1–13: açai samples; Lane A: Dm28c strain (Reference TCI); Lane B: Y strain (Reference TcII); Lane C: INPA 222 strain (Reference TcIII); Lane D: D INPA 4167 strain (Reference TcIV). d Melting curves for different amplification products indicated by arrows on the graph: Y strain (Reference TcII), CL Brener strain (Reference TcVI), Açai 3 sample, Açai 6 sample
As shown in Table 2, food products containing açai showed a mixture of T. cruzi DTUs, with the prevalence of TcIII, TcV, and TcI, indicating different genotypes circulate in the same region, but with the predominance of DTUs associated with the sylvatic cycle of the parasite. All the analyzed samples represented mixed infections, but the identification of at least one DTU was possible in 10 samples (76.9%). From these, 5 (38.5%) were infected with TcIII, 5 (38.5%) with TcV, 4 (31%) with TcI, 2 (15.4%) with TcIV and one sample presented TcVI genotype. In four samples (30.8%), inconclusive results were obtained: two samples were also infected with TcII or TcVI (samples 8 and 11) and another two contained TcIII or TcIV genotypes (samples 6 and 7). Three samples, consisting of açai with guaraná, açai with guaraná and banana and açai with guaraná, strawberry and acerola, could not be genotyped (Table 2).
Table 2.
Genotyping profile (DTU) of T. cruzi DNA isolated from açai samples
| Sample | Sample code | Collection place | SL-IRac (bp) | SL-IR I e II (bp) | 24S-α (bp) | A10 (°C) | DTU |
|---|---|---|---|---|---|---|---|
| 1 | AGF (S) | RJ | 150 + 157 + 200 | 300 + 350 | 125 | – | I + III + V |
| 2 | AGF (B) | RJ | 157 + 200 | 300 | 125 | – | III + V |
| 3 | AGF (B) | RJ | 200 | na | – | 78.2 | ni |
| 4 | AG | RJ | na | na | na | – | ni |
| 5 | AGF (S and A) | RJ | 150 + 157 + 200 | na | na | – | ni |
| 6 | AGF (S) | RJ | 150 + 157 + 200 | 300 + 350 | na | 78.2 | I + VI+ (III or IV) |
| 7 | A | RJ | 150 + 200 | 350 | na | – | I+ (III or IV) |
| 8 | A | RJ | 157 + 200 | 300 | 140 | na | IV+ (II or VI) |
| 9 | A | RJ | 150 + 157 + 200 | 300 + 350 | 125 | – | I + III + V |
| 10 | F | PA | 157 + 200 | na | 125 | – | III + V |
| 11 | F | PA | 157 + 200 | na | 140 | na | IV+ (II or VI) |
| 12 | P | PA | na | 300 | na | – | ni |
| 13 | F | PA | 157 + 200 | na | 125 | – | III + V |
Abbreviations: A açai, P pulp, F fruit, AG açai with guaraná, AGF açai with guaraná and fruit, S strawberry, B banana, S and A strawberry and acerola, RJ collected in Rio de Janeiro, PA collected in Pará, na no amplification, ni not identified, not performed, DTU discrete typing unit
Discussion
Trypanosoma cruzi and T. rangeli are protozoan species of sympatric occurrence between South and Central America. They share the same invertebrate hosts as well as wild and domestic mammalian hosts including humans [15]. Considering that the contamination of açai fruits occurs by faeces deposition or triatomine maceration during the açai fruit processing, the PCR-based method used here can differentiate the two species and is highly recommended to avoid false-positive results. The conventional PCR with primers described by Chiurillo et al. [12] resulted in a robust method to detect T. cruzi without non-specific amplification of T. rangeli DNA in the açai-based matrix. In fact, the analytical specificity assay indicates 100% specificity to T.cruzi detection. The selectivity of this set of primers could be explained by the high interspecific variability of the trypanosomatids sub-telomeric sequences used as targets. Thus, the primers Tc189F and Tc189R were used instead of primers that anneal to kinetoplast minicircle DNA (kDNA) and nuclear satellite DNA (satDNA) as those sets of primers may amplify DNA from T. rangeli [16, 17].
Besides the considerable but not statistically significant in T. cruzi DNA detection between samples from Pará and Rio de Janeiro states, the differences in positivity among the type of food were quite remarkable. In the Rio de Janeiro state, the consumption of açai pulp with guaraná syrup is common, because açai is not part of local diet. Curiously, the majority of samples positive for T. cruzi DNA consisted of guaraná syrup and fruits such as strawberry, banana and acerola. These samples were produced by food industries that should be applying strict manufacturing practices, including raw material selection, to assure food safety for consumption.
Previous studies have shown that heating above 45 °C and pasteurization can inactivate T. cruzi in açai pulp [18, 19]. In contrast, there is no consensus about the efficacy of freezing to kill the parasite in pulp. Neves et al. [20] demonstrated that T. cruzi is inactivated after 2 h at -20 °C. However, Barbosa-Labello et al. [21] showed that T. cruzi maintained its virulence after staying in contact with frozen pulp for up to 26 h. In addition, carbohydrates in guaraná syrup could act as cryopreservant to maintain parasite viability. Although some rules have been established for açai manufacture and processing in Brazil [22, 23], the methods still require validation for quality control of commercialized products. Thus, there is a possibility of açai-based foods enhancing the risk of oral Chagas disease transmission. However, more studies using animal models are needed to properly evaluate the infection rate of viable T. cruzi from açai-based products.
Triatomines are numerous, diversified, and colonize temperate, tropical, and subtropical ecotypes on the American continent. Rhodnius spp. and Panstrongylus megistus are highly associated with palm trees and bromeliads [24, 25]. Curiously, many kinds of tropical fruits such as palm tree fruits show a soft pericarp similar to açai fruit, which is associated with Chagas disease transmission in Brazil [26]. Diaz-Albiter et al. [26] described the first report of phytophagy and sugar feeding of Rhodnius prolixus, suggesting that plants may have an important nutritional role in triatomine maintenance and could contribute to increasing its life-time. These authors also verified that all five Rhodnius instars, always considered strict hematophagous, can feed themselves with 10% glycose solution and can get nutrients through the perforation of vegetal tissues such as tomato. Therefore, the acquisition of nutrients from flowers and fruits can be an additional explanation for the association of triatomine with tropical plants.
It is known that when triatomines are starved for a long period there is a population decrease of T. cruzi in the rectum and an increase of infecting metacyclic trypomastigote forms [27, 28]. Passos et al. [29] analyzed mice inoculated with T. cruzi after contact with açai and showed that a longer period of contact between parasite and açai pulp enhances the virulence and anticipates the onset of parasitemia and death. Xavier et al. [30] demonstrated that the urban cases of ACD were related to wild infected triatomines that were accidentally transported in boats from the islands to açai planting areas. In our work, we analyzed a sample of açai gathered from an urban area “Feira de Açai” that showed a PCR positive reaction for the presence of triatomine DNA. This demonstrated a tenuous link between triatomines and açai and may suggest these insects are the source of food contamination with T. cruzi. This finding highlights the need for research on the phytophagia of triatomines, the effect of açai in development, reproduction, and survival of these insects, and the behaviour of T. cruzi population in triatomines when the diet is based on phytophagia and especially açai. This anomalous finding highlights the need for an eco-epidemiological study to assess the frequency of contamination inside palm trees.
Fregonesi et al. [31] analyzed 30 frozen açai samples (pulp and juice) gathered from markets and snacks bars in Ribeirão Preto, SP and verified that 63% did not meet the standards set by local authorities, i.e. total solids vs moisture content. In addition to moisture content, this study found 50% of edible products were composed of insect fragments, mites, sand crystals, and human hair, which demonstrated quality control failures in food production practices. Another sample presented a rodent pelage, being considered unfit for human consumption according to Brazilian legislation [32]. Freitas et al. [33] also found samples presenting insect fragments. Fragments of insects found in some foods are not commonly identified because of their small size. Even with the initial data reported here, we can raise the possibility that the presence of tiny fragments in açai samples can be related to contamination with insect vectors that were infected with T. cruzi making the product a vehicle of ACD by oral transmission.
The lack of uniformity in the production of açai pulp and açai-based products combined with inadequate sanitary quality may lead to the devaluation of the food product. Given the variety of new açai-based products being launched in Brazil, the development of analytical tools becomes a challenge for laboratories testing complex food matrices. Samples collected from Pará in 2012 were part of a monitoring program led by local regulatory health authorities to establish sanitary procedures for manipulation and marketing of açai juice throughout the production chain. After that, 30 samples (15 açai fruits and 15 açai pulps) were gathered from 15 different commercial establishments. Only one açai pulp sample was positive for T. cruzi DNA demonstrating that there were still gaps in the implementation of good manufactures practices despite these efforts. In 2015, this scenario changed with no sample positive for T. cruzi DNA. Taken together, our results emphasize the presence of parasite DNA as a marker of the absence of good manufacturing practices in acai-based commercial food. It is important to point it out that the detection of T. cruzi DNA cannot be directly associated with the oral transmission of Chagas disease by the consumption of açai as DNA can also be detected from recently inactivated parasites. Nevertheless, previous studies showed that, at high humidity, T. cruzi in triatomine faeces preserves their mobility and infectivity up to 30 min at 33 °C [34]. Furthermore, T. cruzi can remain infective inside dead triatomines stored at 10 °C for six days and between 26 and 30 °C for at least two months [35]. In açai fruit, T. cruzi can be viable, at room temperature, for up to nine hours after contamination [20] and in açai pulp for up to 28 h after contamination [36]. Therefore, a robust study using a molecular marker specific to T. cruzi viability in açai-based food, such as the parasite RNA, remains necessary to investigate the potential of infected açai as a source of contamination. The T. cruzi genotyping analysis here, demonstrated that açai-based products contaminated with T. cruzi DNA showed a mixture of DTUs, including TcI, TcIII and TcIV. These genotypes are in agreement with other studies in the literature [4, 5], which showed a correlation between triatomines and Didelphis in food outbreaks.
The genotype TcI seems to be prevalent in the Amazon Basin [37] and is associated with ACD outbreaks by oral transmission [38]. The sylvatic vectors usually include species of Rhodnius, Panstrongylus, Triatoma and Eratyrus. The sylvatic hosts are usually arboreal and semi-arboreal animals, especially Didelphis, arboreal rodents, primates, Tamandua, and terrestrial rodents. The main ecotypes are palm, tree holes, rocky landscapes, and terrestrial Amazonian environments.
The genotype TcIII shows ecological terrestrial and fossorial niches, and armadillos, especially species of Dasypus, Chaetophractus, Euphractus and Didelphis and Monodelphis are the sylvatic hosts [4]. Panstrongylus geniculatus are the sylvatic vectors, widespread in South America. This genotype is rare in humans with few cases described in Amazon and Brazilian south-east region [39]. It is also rare in domestic dogs with a few reports in Mato Grosso do Sul [40]. The acute cases attributed to this DTU occurred in the Brazilian Amazon, showing subclinical symptoms.
The TcV genotype is found in the Southern Cone (Brazil, Paraguay, Uruguay, Argentina and Chile), in the extreme South of Brazil, and great Gran Chaco region (Bolivia, Argentina, Paraguay and Brazil). Patients from the Southern cone show cardiac forms and present megasyndromes as symptoms of Chagas disease [4]. Although the description of TcV in ecological niches, sylvatic vectors, and hosts (the most usual are armadillos, especially Dasypus spp. and Euphractus spp., and rodents Octodon spp.) is rare, Araujo et al. [41] have made the first report of TcV infecting a wild host, the caviomorph rodent species Thrichomys laurentius in Brazil. In addition, Lima et al. [42], showed, for the first time, the presence of hybrid DTUs (Tc V or Tc VI) from the triatomine Rhodinus pictipes and a dog in the state of Pará in the Brazilian Amazon. Here, five (38.5%) of the açai samples presented the genotype TcV, mixed with other DTUs. Even more unusual, the prevalence of TcV is a consequence of the epidemiological changes occurring in the Amazon Basin area.
Valente et al. [43] studied an outbreak of ACD by açai consumption in Mazagão (Amapá) with 96 affected subjects. They identified 68 triatomines belonging to Rhodnius pictipes (n = 66) and Panstrongylus geniculatus (n = 2) captured in the local area. Of these, 45 (43 Rhodnius pictipes and 2 Panstrongylus geniculatus) were infected with T. cruzi. Thirteen isolates (eight from human and five from Rhodnius pictipes) showed Z3 and TcI genotypes, using the T. cruzi mini-exon as a target for genotyping. Also, a mixed infection of Z3 and T. rangeli in two isolates of Rhodnius pictipes was observed. This method does not distinguish between TcIIa from TcIIc and classifies both genotypes as Z3. The authors assumed that these isolates belong to genotype TcIIa that has been associated with human disease. Therefore, following the classification of Zingales et al. [44], these isolates correspond to DTU TcI and TcIV (former TcIIa).
Steindel et al. [45] genotyped 13 T. cruzi isolates from humans, opossums (Didelphis aurita and Didelphis albiventris), and the vector (Triatoma tibiamaculata) involved in an outbreak of ACD in Santa Catarina in 2005, by ingestion of sugar cane juice. The isolates were characterized by multilocus enzyme electrophoresis (MLEE) and analysis of the spliced-leader and 24Sα rDNA genes. The molecular genotyping demonstrated that all isolates from humans belong to TcII, while the isolates from opossum belong to TcI and the isolates from triatomines showed a mix contamination with TcI and TcII, according to the classification (in Anonymous [46]).
Brazil is still in the early stages of controlling Chagas disease transmission by açai-based products, despite important recent prevention strategies [6]. Recently, quantitative real-time PCR to detect and quantify T. cruzi from açai-based samples with high sensitivity have been developed by Mattos et al. [47] and Souza Godoi et al. [48], reaching LOD of 0.1 parasite equivalent (0.35 fg/μl) and 0.44 parasite equivalents (4–10 ng/μl). Nevertheless, it is still necessary to develop more scientific researcher related to the viability of the parasite in different types of food, the mechanisms of food infection, food preservation technologies, methodologies of T. cruzi detection in food, and other strategies to upgrade the understanding of oral transmission and advance its epidemiological features, prevention, and control [49]. It also remains necessary to develop more strategies to assure the safety of açai products while keeping the nutritional and sensorial properties. Thus, the implementation of good hygienic practices, good manufacturing practices, and joint work between scientists and açai producers are essential to improve product quality.
Conclusions
This work showed, to our knowledge for the first time, a sanitary assessment of manufactured açai-based products through the detection of T. cruzi DNA. The samples were produced in all Brazilian regions and commercialized in Rio de Janeiro and Pará states. The PCR used in this study could detect small quantities of T. cruzi DNA in an açai-based matrix. Also, the genotyping analysis demonstrated that açai-based products contaminated with T. cruzi DNA showed a mixed contamination with the predominace of DTUs TcI, TcIII, and TcIV.
Acknowledgements
The authors acknowledge INCQS/FIOCRUZ, IOC/FIOCRUZ and FAPERJ for funding support. We also thank the staff of Central Laboratory of Public Health of Pará (LACEN /PA), the State Secretary of Health of Pará (SESPA) and the State Secretary of Health of Rio de Janeiro (SES/RJ) for sending us samples of acai from several localities of Pará state and Rio de Janeiro state.
Funding
Constança Britto and Otacilio Moreira are research fellows of the CNPq and FAPERJ (CNE and JCNE).
Availability of data and materials
The data sets supporting the conclusions of this article are included in the article and its additional file. Nucleotide sequences reported in this paper are available in the GenBank database under accession numbers: AF394519.1 (Triatomine 12S subunit ribosomal rDNA) and AF100651 (Trypanosoma cruzi clone VATc17 telomere-associated sequence).
Abbreviations
- ACD
acute Chagas disease
- ChD
Chagas disease
- CMRVS
Coleção de Microorganismos de Referência of the National Quality Control Institute (Rio de Janeiro, Brazil)
- COLPROT
Coleção de Protozoários da Fundação Oswaldo Cruz, Rio de Janeiro, Brazil
- DTU
discrete typing unit
- Fiocruz
Oswaldo Cruz Foundation
- HN
heminested
- INCQS
National Institute of Quality Control in Health, Oswaldo Cruz Foundation
- IOC
Oswaldo Cruz Institute, Oswaldo Cruz Foundation
- IR
intergenic region
- LACEN /PA
Central laboratory of Public Health of Pará
- MLEE
multilocus enzyme electrophoresis
- SES
State Secretary of Health of Rio de Janeiro
- SESPA
State Secretary of Health of Pará
- SL
spliced leader
- TcI, TcII, TcIII, TcIV, TcV and TcVI
Trypanosoma cruzi discrete typing units I, II, III, IV, V and VI
- Tm
melting temperature
Authors’ contributions
Conceived and designed the experiments: RTBF, PCL and OCM. Performed the experiments: MLC, RTBF, PFA and RSM. Analyzed the data: RTBF, OCM, CB, SAS, MRB and PCL. Wrote the paper: RTBF, MRB, PCL, CB and OCM. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Renata Trotta Barroso Ferreira, Email: renata.trotta@incqs.fiocruz.br.
Maria Luiza Cabral, Email: mluizacabrals@gmail.com.
Ronald Sodré Martins, Email: ron.martins21@gmail.com.
Paula Finamore Araujo, Email: paulafinamore@gmail.com.
Sérgio Alves da Silva, Email: sergio.silva@incqs.fiocruz.br.
Constança Britto, Email: cbritto@ioc.fiocruz.br.
Maria Regina Branquinho, Email: regina.branquinho@incqs.fiocruz.br.
Paola Cardarelli-Leite, Email: paola.cardarelli@incqs.fiocruz.br.
Otacilio C. Moreira, Email: otacilio@ioc.fiocruz.br
References
- 1.Deane MP, Lenzi HL, Jansen AM. Trypanosoma cruzi: vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem Inst Oswaldo Cruz. 1984;79(4):513–515. doi: 10.1590/S0074-02761984000400021. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Chagas disease (American trypanosomiasis) Wkly Epidemiol Rec. 2015;90:33–44. [Google Scholar]
- 3.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 4.Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM, et al. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. [DOI] [PubMed]
- 5.Valente VC. Estudo genotípico de Trypanosoma cruzi: epidemiologia e caracterização molecular de isolados do homem, triatomíneos e mamíferos silvestres do Pará, Amapá e Maranhão (Doctoral thesis) 2011. [Google Scholar]
- 6.Ferreira RTB, Branquinho MR, Cardarelli-Leite P. Transmissão oral da doença de Chagas pelo consumo de açaí: um desafio para a vigilância sanitária. Vigil Sanit Deb. 2014;2(4):4–11. [Google Scholar]
- 7.Passos LAC, Guaraldo AMA, Alves DP. Análise da interferência da polpa de açaí na transmissão oral de Trypanosoma cruzi, contribuindo para o surgimento de surtos de Doença de Chagas Aguda (DCA) na região Norte do Brasil: relatório final, convênio 667/ 2008 com Ministério da Saúde. Campinas: Universidade Estadual de Campinas; 2010. [Google Scholar]
- 8.Pereira KS, Schmidt FL, Guaraldo AM, Franco RM, Dias VL, Passos LA. Chagas disease as a foodborne illness. J Food Prot. 2009;72(2):441–446. doi: 10.4315/0362-028X-72.2.441. [DOI] [PubMed] [Google Scholar]
- 9.Join Research Centre (JRC). Event-specific method for the quantification of maize line NK603 using real-time PCR protocol. 2005.http://gmo-crl.jrc.ec.europa.eu/StatusOfDossiers.aspx. Accessed 17 Jun 2016.
- 10.Ferreira RTB, Melandre AM, Cabral ML, Branquinho MR, Cardarelli-Leite P. Extraction of Trypanosoma cruzi DNA from food: a contribution to the elucidation of acute Chagas disease outbreaks. Rev Soc Bras Med Trop. 2016;49(2):1–6. doi: 10.1590/0037-8682-0414-2015. [DOI] [PubMed] [Google Scholar]
- 11.Mbongolo Mbella EG, Lievens A, Barbau-Piednoir SM, Leunda-Casi A, Roosens N, Van den Bulcke M. SYBR green qPCR methods for detection of endogenous reference genes in commodity crops: a step ahead in combinatory screening of genetically modified crops in food and feed products. Eur Food Res Technol. 2011;232:485–496. doi: 10.1007/s00217-010-1408-2. [DOI] [Google Scholar]
- 12.Chiurillo MA, Crisante G, Agustina R, Peralta A, Dias M, Guevara P, et al. Detection of Trypanosoma cruzi and Trypanosoma rangeli infection by duplex PCR assay based on telomeric sequences. Clin Diag Lab Immunol. 2003;10(5):775–779. doi: 10.1128/CDLI.10.5.775-779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uehara LA, Moreira OC, Oliveira AC, Azambuja P, Lima AP, Britto C, et al. Cruzipain promotes Trypanosoma cruzi adhesion to Rhodnius prolixus midgut. PLoS Negl Trop Dis. 2012;6(12):1958. doi: 10.1371/journal.pntd.0001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgos JM, Altcheh J, Bisio M, Duffy T, Helder MSV, Seidenstein ME, et al. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int J Parasitol. 2007;37:1319–1327. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Nascimento APM. (2015). Estudo da dinâmica da co-infecção por Trypanosoma cruzi e Trypanosoma rangeli no hospedeiro invertebrado e no hospedeiro mamífero. (Master’s dissertation). https://repositorio.ufsc.br/handle/123456789/160799. Accessed 8 Oct 2016.
- 16.Ramírez JC, Cura CI, Moreira C, Lages-silva E, Juiz N, Velázquez E, et al. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. J Mol Diagnostics. 2015;17(5):605–615. doi: 10.1016/j.jmoldx.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiringer P, Pritsch M, Flores-Chaves M, Marchisio E, Helfrich K, Mengele C, et al. Comparison of four PCR methods for efficient detection of Trypanosoma cruzi in routine diagnostics. Diagn Microbiol and Infect Dis. 2017;88(3):225–232. doi: 10.1016/j.diagmicrobio.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 18.JCP DIAS. Notas sobre o Trypanosoma cruzi e suas características bio-ecológicas, como agente de enfermidades transmitidas por alimentos. Rev Soc Bras Med Trop. 2006;39(4):370–375. doi: 10.1590/S0037-86822006000400010. [DOI] [PubMed] [Google Scholar]
- 19.Barbosa RL, Pereira KS, Dias VL, Schmidt FL, Alves DP, Guaraldo AMA, Passos LAC. Virulence of Trypanosoma cruzi in açai (Euterpe oleraceae martius) pulp following mild heat treatment. J Food Prot. 2016;79(10):1807–1812. doi: 10.4315/0362-028X.JFP-15-595. [DOI] [PubMed] [Google Scholar]
- 20.Neves AL, Gomes FS, Freitas AM, Almeida RN, Valente VC, Valente AS. Estudo experimental da viabilidade do Trypanosoma cruzi no açaí e infecção em camundongos. Abstract published in 59 Encontro Anual da Sociedade Brasileira para o progresso da ciência (SBPC); 2007.
- 21.Barbosa-Labello R. (2010). Transmissão oral do Trypanosoma cruzi pela polpa de açaí em camundongos. PhD thesis, Universidade Estadual de Campinas, Campinas, Brasil http://www.bibliotecadigital.unicamp.br/. Accessed 10 Apr 2016.
- 22.BRASIL. Regulamento Técnico de Procedimento higiênico sanitários para manipulação de alimentos e bebidas preparados com vegetais (RDC 218, 29 Jul 2005). Agência Nacional de Vigilância Sanitária (ANVISA). http://portalanvisagovbr/documents/33916/388704/RDC_218pdf Acessed 22 Jan 2018.
- 23.BRASIL. Gerenciamento do Risco Sanitário na Transmissão de Doença de Chagas Aguda por Alimentos (Informe Técnico 35, 19 Jun 2008). Agência Nacional de Vigilância Sanitária (ANVISA). http://portal.anvisa.gov.br/. Accessed 11 Nov 2009.
- 24.Gaunt M, Miles M. The ecotopes and evolution of triatomine bugs (Triatominae) and their associated trypanosomes. Mem Inst Oswaldo Cruz. 2000;95:557–565. doi: 10.1590/S0074-02762000000400019. [DOI] [PubMed] [Google Scholar]
- 25.Sarquis O, Carvalho-Costa FA, Oliveira LS, Duarte R, D’Andrea PS, de Oliveira TG, Lima MM. Ecology of Triatoma brasiliensis in northeastern Brazil: seasonal distribution, feeding resources, and Trypanosoma cruzi infection in a sylvatic population. J Vector Ecol. 2010;35:385–394. doi: 10.1111/j.1948-7134.2010.00097.x. [DOI] [PubMed] [Google Scholar]
- 26.Díaz-Albiter HM, Ferreira TN, Costa SG, Rivas GB, Gumiel M, Cavalcante DR, et al. Everybody loves sugar: first report of plant feeding in triatomines. Parasit Vectors. 2016;9:114. doi: 10.1186/s13071-016-1401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kollien AH, Schaub GA. Development of Trypanosoma cruzi after starvation and feeding of the vector - a review. Tokai J Exp Clin Med. 1998;23:335–340. [PubMed] [Google Scholar]
- 28.Kollien AH, Schaub GA. The development of Trypanosoma cruzi in Triatominae. Parasitol Today. 2000;16(38):1–7. doi: 10.1016/s0169-4758(00)01724-5. [DOI] [PubMed] [Google Scholar]
- 29.Passos LAC, Guaraldo AMA, Barbosa RL, DIAS VL, Pereira KS, Schmidt FL, et al. Sobrevivência e infectividade do Trypanosoma cruzi na polpa de açaí: estudo in vitro e in vivo. Epidemiol Serv Saúde. 2012;21(2):223–32.
- 30.Xavier SCC, Roque ALR, Bilac D, de Araújo VAL, da Costa Neto SF, Lorosa ES, et al. Distantiae transmission of Trypanosoma cruzi: a new epidemiological feature of acute Chagas disease in Brazil. PLoS Negl Trop Dis. 2014;8:2878. doi: 10.1371/journal.pntd.0002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fregonesi BM, Yokosawa CE, Okada IA, Massafera G, Costa TMB, SPT P. Polpa de açaí congelada: características nutricionais, fisico-químicas, microscópicas e avaliação da rotulagem. Rev Inst Adolfo Lutz. 2010;69(3):387–395. [Google Scholar]
- 32.BRASIL. Regulamento Técnico de Avaliação de Matérias Macroscópicas e Microscópicas Prejudiciais à Saúde Humana em Alimentos Embalados (RDC 175, 08 Jul 2003). Agência Nacional de Vigilância Sanitária (ANVISA). http://www.anvisa.gov.br/anvisalegis/resol/2003/rdc/175_03rdc.htm. Accessed 22 Jan 2018.
- 33.Freitas B, Bento FS, Santos FQ, Figueiredo M, América P, Características MP. físico-químicas, bromatológicas, microbiológicas e microscópicas de polpa de açaí (Euterpe oleraceae) congeladas do tipo B. J Appl Pharm Sciences. 2015;2(2):2–13. [Google Scholar]
- 34.Soares VA, Marsden PD, Johnson C. Efeito da dessecação das fezes de triatomíneos na sobrevivência de formas metacíclicas de Trypanosoma cruzi. Rev Soc Bras Med Trop. 1986;19(4):233–238. doi: 10.1590/S0037-86821986000400006. [DOI] [PubMed] [Google Scholar]
- 35.Soares VA, Persistência MPD. de infectividade do T. cruzi em barbeiros mortos. Rev Inst Med Trop. 1978;20(4):241. [Google Scholar]
- 36.Dias JC, Prata A, Correia D. Problems and perspectives for Chagas disease control: in search of a realistic analysis. Rev Soc Bras Med Trop. 2008;41:193–196. doi: 10.1590/S0037-86822008000200012. [DOI] [PubMed] [Google Scholar]
- 37.Buscaglia CA, Di Noia JM. Trypanosoma cruzi clonal diversity and the epidemiology of Chagas disease. Microbes Infect. 2003;5:419–427. doi: 10.1016/S1286-4579(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 38.Coura JR, Transmissão d. infecção chagásica por via oral na história natural da doença de Chagas. Rev Soc Bras Med Trop. 2006;39(Suppl. 3):113–117. [PubMed] [Google Scholar]
- 39.Marcili A, Valente VC, Valente SA, Junqueira ACV, Silva FM, Pinto AY, et al. Trypanosoma cruzi in Brazilian Amazonia: lineages TcI and TcIIa in wild primates, Rhodnius spp. and in humans with Chagas disease associated with oral transmission. Int J Parasitol. 2009;39:615–623. doi: 10.1016/j.ijpara.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Umezawa ES, Souza AI, Pinedo-Cancino V, Marcondes M, Marcili A, Camargo LM, et al. TESA-blot for the diagnosis of Chagas disease in dogs from co-endemic regions for Trypanosoma cruzi, Trypanosoma evansi and Leishmania chagasi. Acta Trop. 2009;11(1):15–20. doi: 10.1016/j.actatropica.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Araújo CA, Waniek PJ, Xavier SC, Jansen AM. Genotype variation of Trypanosoma cruzi isolates from different Brazilian biomes. Exp Parasitol. 2011;27(1):308–312. doi: 10.1016/j.exppara.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Lima V dos S, Xavier SC, Maldonado IF, Roque AL, Vicente AC, Jansen AM. Expanding the knowledge of the geographic distribution of Trypanosoma cruzi TcII and TcV/TcVI genotypes in the Brazilian Amazon. PLoS One. 2014;9(12):e116137. doi: 10.1371/journal.pone.0116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valente SA, da Costa Valente V, Das Neves Pinto AY, De Jesus Barbosa César M, Dos Santos MP, Miranda CO, et al. Analysis of an acute Chagas disease outbreak in the Brazilian Amazon: human cases, triatomines, reservoir mammals and parasites. Trans R Soc Trop Med Hyg. 2009;103:291–297. doi: 10.1016/j.trstmh.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 44.Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Second Satellite Meeting Mem Inst Oswaldo Cruz. 2009;(7):1051–4. [DOI] [PubMed]
- 45.Steindel M, Kramer Pachec L, Scholl D, Soares M, de Moraes MH, Eger I, et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina state, Brazil. Diagn Microbiol Infect Dis. 2008;60(1):25–32. doi: 10.1016/j.diagmicrobio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Anonimous Recommendations from a satellite meeting. Mem Inst Oswaldo Cruz. 1999;94(Suppl. 1):429–432. doi: 10.1590/s0074-02761999000700085. [DOI] [PubMed] [Google Scholar]
- 47.Mattos EC, Meira-Strejevitch CDS, Marciano MAM, Faccini CC, Lourenço AM, Pereira-Chioccola VL. Molecular detection of Trypanosoma cruzi in acai pulp and sugarcane juice. Acta Trop. 2017;176:311–315. doi: 10.1016/j.actatropica.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 48.Souza Godoi PA, Piechnik CA, de Oliveira AC, Sfeir MZ, de Souza EM, Rogez H, Soccol VT. qPCR for the detection of foodborne Trypanosoma cruzi. Parasitol Int. 2017;66(5):563–566. doi: 10.1016/j.parint.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Gutierrez E, Salvatella R, Figueroa R. Consulta técnica em epidemiologia, prevenção e manejo da transmissão da doença de Chagas como doença transmitida por alimentos. Relatório Técnico. PANAFTOSA, Rio de Janeiro, 2006. Rev Soc Bras Med Trop. 2006;39(5):512–514. doi: 10.1590/S0037-86822006000500020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets supporting the conclusions of this article are included in the article and its additional file. Nucleotide sequences reported in this paper are available in the GenBank database under accession numbers: AF394519.1 (Triatomine 12S subunit ribosomal rDNA) and AF100651 (Trypanosoma cruzi clone VATc17 telomere-associated sequence).


