Abstract
Abscisic acid (ABA) is required for the regulation of seed maturation in maize (Zea mays L.). Mutants blocked in ABA synthesis (such as viviparous-5) do not mature to quiescent, desiccation-tolerant seeds, but germinate on the ear midway through kernel development. Because gibberellins (GA) and ABA act antagonistically in many aspects of plant development, we hypothesized that ABA antagonizes a positive GA signal for precocious germination in maize. In these experiments, we show that a GA deficiency early in seed development, induced genetically or via biosynthesis inhibitors, suppresses vivipary in ABA-deficient developing kernels. The resulting seeds have both desiccation tolerance and storage longevity. Temporal analysis of GA accumulation in wild-type kernels revealed the accumulation of bioactive GA1 and GA3 prior to the peak in ABA content. We speculate that these GAs stimulate a developmental program leading to vivipary in the absence of normal amounts of ABA, and that a reduction of GA content re-establishes an ABA/GA ratio appropriate for suppression of germination and induction of maturation. In contrast, the induction of a GA deficiency did not suppress vivipary in viviparous-1 mutant kernels, suggesting that VP1 acts downstream of both GA and ABA in programming seed development.
Early in their formation, the embryos of flowering plants complete rudimentary organogenesis and acquire two developmental potentials: germination and maturation. Within the seed environment, the germination potential of the embryo is suppressed and maturation occurs. The maturation pathway is characterized by the accumulation of storage products, preparation for desiccation, and the first stages of normal germination (Bewley and Black, 1994). However, maturation is not necessarily an obligate process. If removed from the seed and placed in culture, many types of embryos germinate and develop into morphologically normal seedlings (for review, see Kermode, 1990), while others display a mixture of post-germinative and embryonic development (Fernandez, 1997).
The phytohormone abscisic acid (ABA) plays a central role in embryo maturation, both to suppress precocious germination and to induce the expression of maturation-associated genes for storage product accumulation and acquisition of desiccation tolerance. Mutants of Arabidopsis and tomato that are deficient in ABA synthesis have impaired seed maturation and dormancy (Koornneef and van der Veen, 1980; Koornneef et al., 1982). In maize (Zea mays L.), ABA-deficient mutants are viviparous, precociously germinating on the ear during kernel development (Robertson, 1955; Neill et al., 1986).
The ABA-insensitive mutant viviparous-1 (vp1) identifies another critical factor in regulating maize maturation. Unlike other maize viviparous mutants, vp1 kernels have normal ABA content (Neill et al., 1986), but lack seed-specific responses to the hormone. The vp1 mutant embryos germinate precociously even when cultured with exogenous ABA, and they do not accumulate many maturation products in vivo or in vitro (Kriz et al., 1990; Rivin and Grudt, 1991; White and Rivin, 1995a, 1995b). The Vp1 gene product is expressed exclusively during seed maturation. Transient expression assays of reporter genes driven by maturation-specific gene promoters indicate that VP1 functions as a transcriptional activator of ABA-inducible gene expression in developing maize kernels (McCarty et al., 1991; Hattori et al., 1992). The related ABI3 (ABA-insensitive 3) protein of Arabidopsis plays an analogous role during seed development (Parcy et al., 1994). VP1 also appears to repress the precocious expression of germination-specific genes in the aleurone independently of ABA (Hoecker et al., 1995).
The focus on the action of ABA in suppressing precocious germination has superseded assessment of other factors that may play roles in modulating the vivipary versus maturation decision. Frequently, germination has been considered to be a default developmental program that is suppressed by an appropriately timed ABA signal. While such a model is attractive in its simplicity, some data suggest that a positive factor is needed for germination of immature maize embryos. For example, fluridone-inhibition of ABA synthesis induces precocious germination only when treatments are applied during a narrow window of maize kernel development (Fong et al., 1983). Also, we have seen that the ability of excised maize embryos to germinate varies in a stage-specific manner that cannot be directly correlated with endogenous ABA content (Rivin and Grudt, 1991). Similar results have been reported in wheat (Radley, 1979).
GAs are likely candidates to have a positive role in precocious germination. Biologically active GAs are known to be present during early embryogenesis in some species, and GAs are clearly important in the germination of many types of mature seeds. In wheat and barley, GA induces the expression of various hydrolytic enzyme genes, stimulating the mobilization of endosperm reserves (for review, see Jacobsen et al., 1995). GA is also involved in the release from dormancy of various species; GA-deficient mutants of Arabidopsis and tomato are impaired in this process (Koornneef and van der Veen, 1980; Liu et al., 1994), although GA-deficient mutants of other species germinate efficiently (for review, see Reid, 1986). In many cases, ABA antagonizes these effects of GA, both at the level of gene expression (for review, see Jacobsen et al., 1995) and in modulating germination per se (Liu et al., 1994; Steber et al., 1998). In maize, a clear requirement for GA in the germination of mature seeds has not been demonstrated by the behavior of mutants, and measurements of GA in developing maize kernels have been limited to very early development (Murofushi et al., 1991).
In this report, we show that biologically active GAs are present in the developing maize kernel, and provide evidence that GA and ABA play antagonistic roles in controlling vivipary in maize. Measurements of GA and ABA content were made during maize kernel development. These revealed that the bioactive species GA1 and GA3 are present in developing maize embryos in levels that decline prior to the peak of ABA concentration. The role of GA in precocious germination was then tested by reducing the amount of endogenous GA in ABA-deficient (vp5) and vp1 mutant kernels. When the level of endogenous GAs was reduced, either genetically or with inhibitors, we observed a suppression of vivipary in vp5 kernels, leading to desiccation tolerance and storage longevity. However, in the absence of a functional Vp1 gene, vivipary occurred regardless of the GA content. We suggest that the balance of ABA to GA is a determinant in signaling maturation over germination in maize, and acts in conjunction with an absolute requirement for the Vp1 gene product.
MATERIALS AND METHODS
Plant Material
The maize (Zea mays L.) viviparous mutants vp1 and vp5 were originally obtained from D. Robertson (Iowa State University, Ames) and have been propagated in primarily W22 genetic backgrounds. The inbred line W22 was originally obtained from J. Kermicle (University of Wisconsin, Madison). The GA-deficient dwarf mutant d1 (Spray et al., 1996) was obtained from the Maize Genetics Cooperative (University of Illinois, Urbana). All stocks were propagated in Ohio State University greenhouse facilities and at the Botany and Plant Pathology Department farm (Corvallis, OR). The vp5 mutant was maintained as a heterozygote; homozygous vp5 kernels were identified on segregating ears by their lack of carotenoid pigments. The vp1 mutant stock was maintained as a homozygote by removing precociously germinating seeds for planting before the ear was dry. The experiments reported here were undertaken in summer nursery plots and repeated for two successive years.
Kernel Treatment with Hormone Biosynthesis Inhibitors
Paclobutrazol and ancymidol were obtained as commercial formulations with surfactant (Bonzi, ICI Americas, Bridgewater, NJ, and A-rest, Dow-Elanco, Indianapolis, respectively). Dilutions to 100 μm were made with water according to manufacturer's instructions. Gibberellic acid (GA3) was obtained from Sigma Chemical Co. (St. Louis) and diluted to 100 μm according to manufacturer's instructions. Fluridone was obtained from Dow-Elanco and diluted to 150 μg/mL in 1% (v/v) acetone (Fong et al., 1983). Ears were treated with hormones and inhibitors in the following manner: Following hand pollination, ears were covered and allowed to develop for various times. Kernels on one-half of each ear were then exposed by peeling back the husk and removing the silks, and treated with saturating sprays of growth regulator solutions; husks were then replaced and the ears re-covered with waterproof bags. Control ears were sprayed with water alone. Morphological staging of kernel development was according to the scheme of Abbe and Stein (1954).
Ears were harvested at 55 to 60 d after pollination (DAP) and dried. Individual kernels to be scored were removed from the center of the treatment area, taking kernels from the middle three or four rows in a strip of approximately 3 inches (30–50 kernels). In segregating vp5 ears, the small number of mutant kernels necessitated sampling from a wider zone of the treatment area. Radicle emergence, plumule extension past the scutellar border, or swelling and necrosis of the embryo were all used as criteria indicating germination; embryos having one of these characters were scored positive for germination, even if axis expansion was not extensive. Data were pooled from a minimum of three ears per treatment using plants with various pollination dates to avoid bias from environmental variables in embryo development. The values are given as mean percentage germinating ± se.
Hormone Analysis
Samples of flash-frozen kernels (5 g) were homogenized in 80% (v/v) methanol containing [17-2H2]GA1 (3 ng), [17-2H2]-GA3 (3 ng), [17-2H2]-GA19 (30 ng), [17-2H2]-GA20 (25 ng), and [20-2H1]GA44 (25 ng) (provided by Prof. L.N. Mander, Australian National University, Canberra), and [17-13C]GA8 (3 ng) and [17-13C]-GA29 (3 ng) (gifts from Prof. B.O. Phinney, University of California, Los Angeles). 3H-Labeled GA1, GA19 (Amersham International, Buckinghamshire, UK), and GA20 (from Prof. J. MacMillan, Long Ashton Research Station, University of Bristol, UK) (each 500 Bq) were also added to determine the HPLC elution profiles. The samples were extracted overnight, and the GAs were purified and analyzed by gas chromatography (GC)-mass spectrometry (MS) with selected ion monitoring (SIM), as described previously (Proebsting et al., 1992). ABA was analyzed in a similar manner. [2H3]ABA (10–50 ng) (gift from Dr. R. Horgan, University of Wales, Aberystwyth, UK) and [3H]ABA. Five-hundred becquerels (Amersham International) were added as a standard to homogenized kernels (1 g), and the purification was as described for the GAs. The ions monitored were: ABA, m/z 190 and 162 and [6-2H3]ABA, m/z 193 and 165.
RESULTS
GA and ABA Concentrations in Developing Maize Kernels
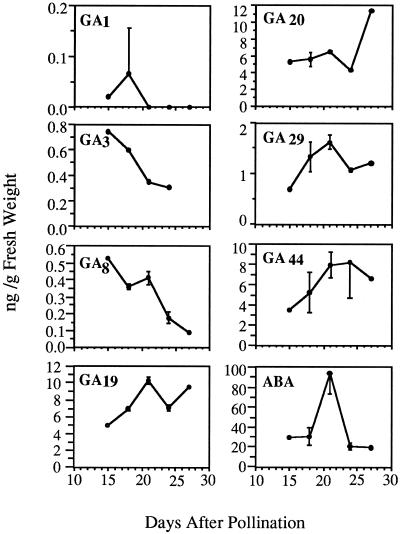
We determined the concentrations of GA1, GA3, GA8, GA19, GA20, GA29, GA44, and ABA in developing kernels of wild-type maize (inbred stock W22) between 15 and 27 DAP (Fig. 1). The concentrations of the two biologically active species, GA1 and GA3, were highest in the earliest samples (embryo stage 2, according to the scheme of Abbe and Stein [1954]) and declined markedly as embryos matured. GA8 content declined similarly. GA19, GA29, and GA44 were highest in 21 DAP kernels (embryo stage 3), when ABA levels were also at their peak, while the amount of GA20 was highest at 27 DAP.
Figure 1.
GA and ABA content of developing wild-type kernels. GAs and ABA were extracted from flash-frozen W22 kernels harvested at intervals between 15 and 27 DAP (embryo stages 2–4), separated by reversed-phase HPLC, and analyzed by GC-MS (see “Materials and Methods”). Where measureable, bars indicate sd.
Inhibition of GA Synthesis Suppresses Precocious Germination in ABA-Deficient Kernels
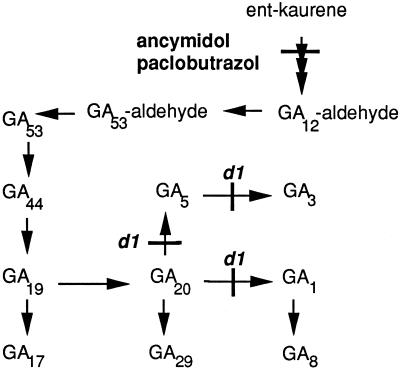
Maize kernels that are deficient in ABA synthesis germinate precociously. To determine whether precocious germination depends on a GA stimulus, we examined whether that vivipary could be suppressed by reducing the endogenous GA levels in these seeds. Hormone levels in the developing seeds were manipulated using a combination of genetic and chemical means. ABA-deficient kernels were created by self-pollinating plants heterozygous for a vp5 mutation. The homozygous kernels segregating on these ears are deficient in ABA due to a block in the carotenoid synthesis pathway (Neill et al., 1986; Hable et al., 1998). These mutant seeds fail to mature and instead precociously germinate while still on the ear. For some experiments, ABA deficiency was induced in wild-type kernels by treating with the herbicide fluridone at 11 DAP. Like the vp5 block, fluridone inhibits biosynthesis of both ABA and carotenoids (Fong et al., 1983). To reduce endogenous GA content, paclobutrazol or ancymidol was applied to developing ears at various times during embryogenesis. Both of these compounds retard oxidation of ent-kaurene to ent-kaurenoic acid (Coolbaugh et al., 1978; Hedden and Graebe, 1985), thus blocking GA synthesis at an early step in the pathway (Fig. 2). Other experiments were performed in a GA-deficient dwarf mutant background, d1/d1. The d1 mutation impairs three biosynthetic steps in the conversion of biologically inactive GA20 to the active forms GA1 and GA3 (Spray et al., 1996) (Fig. 2).
Figure 2.
Hypothetical pathway for GA biosynthesis in maize. The synthesis of GAs and the position of the d1 mutant block are shown (Spray et al., 1996). The position of the biosynthetic block imposed by ancymidol and paclobutrazol is also shown.
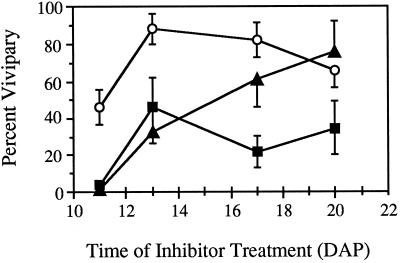
The effects of reduced GA synthesis on fluridone-treated kernels were assayed by treating half-ear sectors with saturating sprays of paclobutrazol, ancymidol, or water at the time of fluridone treatment (11 DAP) or at later times up to 20 DAP. At ear maturity, the rates of vivipary in the inhibitor-treated sectors were compared with those of fluridone-only control sectors. As expected, treatment of wild-type kernels with fluridone at 11 DAP induced a substantial amount of vivipary. When either paclobutrazol and ancymidol treatments were also applied at 11 DAP, vivipary was completely suppressed (Fig. 3). However, GA synthesis inhibitors applied at subsequent times were less effective in suppressing vivipary. The effectiveness of ancymidol gradually diminished so that treatments at 20 DAP (stage 3) had no significant effect. Paclobutrazol reduced vivipary to 30% to 40% in all treatments after 11 DAP.
Figure 3.
Suppression of vivipary in fluridone-treated kernels by treatment with GA biosynthesis inhibitors. Wild-type ears were treated at 11 DAP with fluridone. At the times indicated, these ears were also treated with a saturating spray of either 100 μm ancymidol (▴) or 100 μm paclobutrazol (▪). At ear maturity, vivipary was scored on ears receiving inhibitor treatment and on ears treated with fluridone only (○). Error bars indicate ±se.
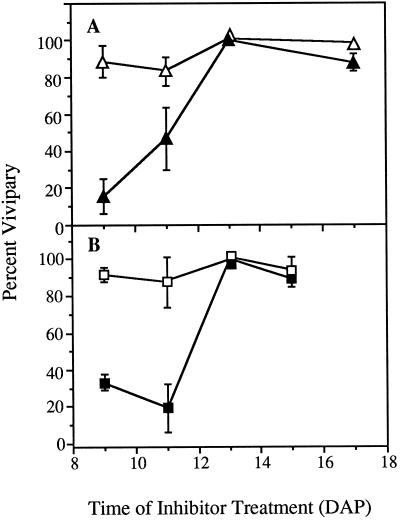
The effect of ancymidol or paclobutrazol on vivipary of vp5 kernels was assayed by treating vp5-segregating ear sectors at times between 9 and 17 DAP (Fig. 4). At ear maturity, the treated and untreated sections of the ear were compared for the fraction of precocious germination in mutant (white) kernels (see “Materials and Methods”). More than 90% of vp5 kernels were germinated on untreated ear sections. Both inhibitors significantly suppressed vivipary in the mutant kernels when ears were treated at 9 DAP. The effectiveness of ancymidol decreased quickly with increasing kernel age at time of treatment, as was seen with the fluridone-treated kernels. Neither inhibitor suppressed vivipary when treatments were applied after 13 DAP.
Figure 4.
Suppression of vivipary in vp5 mutant kernels by treatment with GA biosynthesis inhibitors. At the times indicated, one side of vp5-segregating ears was treated with either 100 μm ancymidol (A) or 100 μm paclobutrazol (B). At ear maturity, the vivipary of mutant kernels was scored from the untreated (▵, □) and treated (▴, ▪) sides of the ears. Error bars indicate ±se.
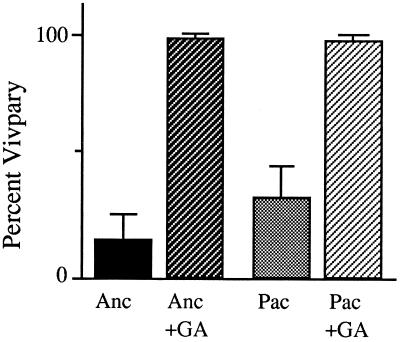
To test the presumption that both of the GA inhibitors used exert their effect by reducing kernel GA levels, vp5-segregating ears were given GA3 plus either ancymidol or paclobutrazol. As shown in Figure 5, the vivipary of vp5 embryos was restored when GA3 was supplied along with a GA synthesis inhibitor at 9 DAP, indicating that ancymidol and paclobutrazol both suppress vivipary via a reduction in kernel GA synthesis.
Figure 5.
Restoration of vivipary to inhibitor-treated vp5 kernels by exogenous GA3. vp5-segregating ears were treated at 9 DAP with either 100 μm ancymidol (Anc) or 100 μm paclobutrazol (Pac) alone or in combination with 100 μm GA3. Error bars indicate ±se.
Double Mutant vp5-d1 Kernels Have Desiccation Tolerance and Longevity
In addition to the chemical manipulations of hormone levels, a genetic lesion was used to reduce endogenous GAs in kernels. The d1 mutation blocks the formation of the active species GA1 and GA3, and homozygous d1 plants are dwarfed (Spray et al., 1996). Double mutants were constructed to test whether the d1 mutation could suppress vivipary when combined with the ABA-deficient mutant vp5.
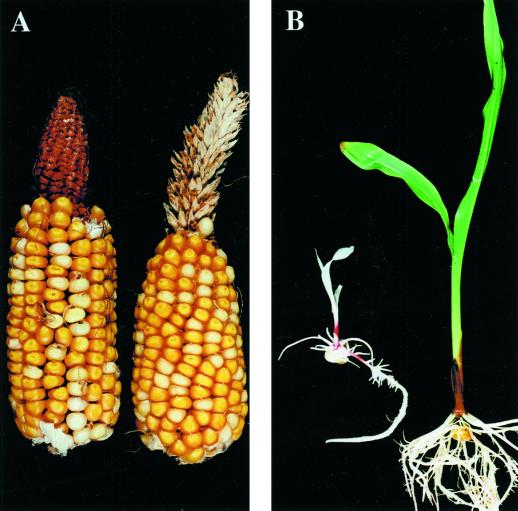
To obtain vp5 segregants in d1/d1 plants, vp5 heterozygotes were crossed with d1 homozygotes, and the resulting F1 kernels were propagated and self-pollinated. The dwarf F2 plants were self-pollinated and the vp5 segregating ears were identified by the presence of carotenoid-deficient seeds. Sibling families that carried vp5 but not d1 were also self-pollinated for controls. Figure 6A shows the difference in the vp5-segregating ears in these families. In the ears having kernels with normal GA synthesis, the vp5 kernels (carotenoid-deficient) germinated precociously. On the d1 homozygote ears, vp5 kernels did not germinate, like their sibling wild-type kernels.
Figure 6.
Phenotype of vp5-d1 double mutant kernels. A, Mature ears segregating for vp5. Mutant kernels (white) are viviparous in a normal D1 background (ear at left), but quiescent in a d1 (dwarf) background (ear at right). The ear from the dwarf plant shows partial conversion to tassel. B, Germination of vp5-d1 double mutant quiescent kernels from a segregating ear. After 10 months of dry storage, double mutant kernels germinate into white seedlings (left) that grow more slowly than their Vp5-d1 siblings (green seedling at right). vp5 seeds from D1 ears do not germinate when imbibed (not shown).
The suppression of precocious germination is one aspect of seed maturation. Another aspect is the acquisition of desiccation tolerance. The vp5-d1 homozygous dormant kernels from self-pollinated dwarf plants (d1/d1) were dried, stored for 10 months at room temperature, and then imbibed and germinated on hormone-free medium to determine if the reduction in GA during embryo development conferred desiccation tolerance and longevity. Inhibitor-treated vp5 dormant kernels were also tested in this way. Both sets of kernels germinated, albeit at delayed rates compared with their wild-type sibling kernels. These kernels produced albino seedlings, thus verifying the vp5 homozygous condition of the embryos (Fig. 6B). A small percentage of vp5 mutant kernels in normal GA backgrounds were not obviously viviparous on visual inspection of mature kernels. However, these kernels were incapable of germination before or after drying (data not shown).
Inhibition of GA Synthesis Does Not Suppress Precocious Germination in vp1 Mutants
The developmental effects of GA synthesis inhibitors were also tested on the vp1 mutant, which is viviparous in spite of having normal ABA levels (Neill et al., 1986). In contrast to the results with ABA-deficient viviparous kernels, vp1 germination was not suppressed when treated with either ancymidol or paclobutrazol at times between 6 and 26 DAP (data not shown). Vivipary occurred on all treated ears. However, plumule elongation was uniformly reduced in germinating vp1 kernels that had been sprayed with either inhibitor, demonstrating that the treatments were effective in altering GA levels in these mutant seeds.
The effect of GA on the development of vp1 seeds was also measured by constructing vp1-d1 double mutants. Homozygous vp1 plants were grown from viviparous seeds and crossed with d1 homozygotes. The resulting double heterozygotes were self-pollinated, and as expected, approximately 25% of the F2 seeds were viviparous. When the dormant F2 seeds were planted, 25% of the plants were dwarf, as predicted if the vp1 mutation had no effect on the d1 phenotype. The dwarf F2 plants were self-pollinated to look for ears segregating for vivipary. Reciprocal crosses were also made between these plants and standard vp1 homozygote stocks to confirm the presence of the vp1 allele in the event that the dwarf background suppressed the viviparous phenotype.
The double mutant phenotypes revealed no epistasis between the d1 and vp1 mutations. Of the 12 dwarf (d1/d1) F2 plants tested, seven gave fully dormant ears and these were confirmed to be Vp1/Vp1, while the five ears segregating for vivipary were confirmed to be heterozygous for vp1. Vivipary resulting from the vp1 mutation occurred in kernels of d1 homozygotes as readily as in those of plants having a normal content of active GAs. However, as with chemical inhibitors, plumule elongation was reduced on vp1 kernels developing on dwarf mother plants relative to that of kernels developing on plants of normal stature.
DISCUSSION
ABA provides an essential cue for seed maturation. ABA-deficient and ABA-insensitive mutants of tomato and Arabidopsis show no dormancy. In maize, ABA-deficient mutant kernels are viviparous, precociously germinating in spite of a large contribution of ABA from the maternal plant (Fong et al., 1983). These data have led to the supposition that a threshold level of ABA is required to block viviparous development. The timing of the ABA signal is also critical. Fong et al. (1983) showed that the ABA synthesis inhibitor fluridone produces precocious germination in maize only if given early in kernel development. These investigators speculated that embryo-derived ABA was essential in early development, and that maternal ABA or other physiological or mechanical aspects of the seed environment blocked germination at later stages (Fong et al., 1983). Another possible scenario is that maize embryos in early development respond to a positive effector of germination that is missing from mature embryos. The behavior of excised embryos is consistent with this hypothesis. Maize embryos at stages 2 and 3 (18–27 DAP) have relatively high ABA levels, but germinate efficiently when excised and grown in a hormone-free culture medium. Although endogenous ABA levels are quite low at stage 4 (28–30 DAP), these embryos do not germinate when placed in hormone-free medium, indicating that late-stage suppression of germination is embryo autonomous (Rivin and Grudt, 1991).
In contrast to ABA, a possible role for GAs in vivipary has received little attention, although biologically active GAs are known to be present during seed development in other species, including cereals (Jacobsen and Chandler, 1987). We hypothesized that GA might provide a positive stimulus for precocious germination in maize that is antagonized by the rise in ABA content at the beginning of the maturation phase. The relative amounts of ABA and GA, rather than the concentration of ABA alone, would then determine whether developing maize embryos undergo precocious germination or maturation. This hypothesis makes two predictions: (a) biologically active GAs should accumulate in developing kernels prior to or coincident with peak ABA levels, and (b) disruption of GA synthesis in vivo prior to this time should suppress vivipary in ABA-deficient viviparous kernels. The results presented here satisfy these predictions and suggest that the idea of hormone balance used to describe a variety of plant growth responses may have relevance to the regulation of seed maturation as well.
We found that two biologically active GAs (GA1 and GA3) are present in developing kernels at a time compatible with a possible function in vivipary. Both of these were most abundant in stage 2 embryos (15–17 DAP) prior to the rise in ABA level in stage 3 (21 DAP). Other measurements of GA during maize development are very limited. Murofushi et al. (1991) used GC-SIM, GC-MS, and radio-immunoassay to measure the GA content of maize kernels at 7 and 9 DAP. They detected GA1, GA8, GA19, GA20, GA29, and GA44, which were analyzed in the present work, as well as GA4, GA9, GA17, GA34, and GA53. They found greater amounts of GA1 in the 7 or 9 DAP seed than we detected at later developmental stages, although it is difficult to make direct quantitative comparisons between the sets of data because of differences in assay and genetic backgrounds.
Our data are consistent with an involvement of GAs in precocious germination in maize. Vivipary is suppressed in the d1-vp5 double mutant kernels, which are blocked in the synthesis of biologically active GAs. Chemically induced reduction of GA levels also inhibited germination of ABA-deficient kernels, while exogenous GA3 treatment restored vivipary. However, the inhibitors were only effective when applied prior to 13 DAP, implying that GA levels sufficient for a germination response are synthesized early in embryogenesis. Of the GAs we assayed in maize kernels, only GA1, GA3, and, to a lesser extent, GA 44 possess significant activity in biological assays (Reeve and Crozier, 1973). The temporal accumulation profiles for these GAs, having relatively high amounts early in embryogenesis, are consistent with the timing of GA-inhibitor effectiveness. Similar hormone accumulation patterns have been seen in developing wheat and barley seeds, where GA content peaks prior to that of ABA (Jacobsen and Chandler, 1987). These data suggest that a GA signal early in embryogenesis establishes a competence for precocious germination, and that the change in the balance of ABA to GA is a key determinant in the developmental programming of maturation versus vivipary.
Low GA levels did not diminish vivipary in vp1 mutants. Vp1 function is absolutely required for suppressing precocious germination. In the absence of a functional Vp1 gene, vivipary occurs in spite of high ABA and independently of GA content. This suggests to us that GA and ABA signaling converges at or upstream from the site of Vp1 action. McCarty and colleagues have proposed that VP1 integrates the control of seed maturation and germination programs by acting in two modes: as a transcriptional activator of ABA-inducible gene expression and as an ABA-independent (and perhaps GA-independent) repressor of germination-specific gene expression (Hattori et al., 1992; Hoecker et al., 1995). Presumably, ABA-VP1-induced gene expression (and perhaps also VP1 repression of gene expression) are essential in suppressing vivipary. The integration of ABA and VP1 in regulating gene expression in seeds has been examined in detail for several promoters, identifying different classes of overlapping and non-overlapping cis control elements (Hattori et al., 1992; Vasil et al., 1995; Hill et al., 1996; Shen et al., 1996). No data have been published regarding possible GA-responsive negative control elements in these promoters, however, antagonistic ABA-responsive promoter elements have been identified in the GA-inducible alpha-amylase genes of other cereals (for review, see Jacobsen et al., 1995).
vp5-d1 embryos not only exhibited precocious germination, but they were also tolerant of desiccation and prolonged dry storage. The physiological characters required for desiccation tolerance and longevity of seeds are disputed (Ooms et al., 1993), but most research has emphasized the roles of carbohydrates and LEA (late embryogenesis abundant) proteins. Changes in carbohydrate composition that accompany seed dehydration are thought to afford protection by influencing the formation of a stable glass during desiccation (Koster, 1991). The diverse LEA proteins are amphipathic, highly hydrophilic proteins that have been proposed to provide desiccation protection (Dure et al., 1989). Both carbohydrate composition and LEA accumulation may be influenced by ABA during the maturation phase. ABA modulates the expression of LEA proteins (for review, see Skriver and Mundy, 1990). Through similar promoter motifs, it also regulates the expression of an aldose reductase-related gene from barley (AR-h) that is abundantly expressed during seed dehydration (Roncarati et al., 1995). A possible interpretation of our result is that maturation processes that confer desiccation tolerance are directly regulated by relative amounts of ABA and GAs. Some LEA genes and AR-h are down-regulated by exogenous GA in cultured embryos, but it remains to be seen whether this reflects normal developmental regulation or a superimposition of maturation and germination programs in excised embryos (Bartels et al., 1991; Hughes and Galau, 1991).
The inhibition of GA synthesis in ABA-deficient seeds may have influenced desiccation tolerance indirectly. By inhibiting precocious germination, the double hormone deficiency may have allowed the vp5-d1 embryos to complete the maturation phase and acquire desiccation tolerance by pathways unrelated to ABA. In maize, only ABA and VP1 have been clearly identified as factors required for desiccation tolerance, although in Arabidopsis, a larger variety of mutations are known that affect desiccation tolerance, including some that do not obviously perturb hormone synthesis or sensitivity. Single- and double-mutant studies indicate that a network of interacting regulators acts synergistically with ABA to contribute to a variety of aspects of late embryo development, including survival of desiccation (Ooms et al., 1993; Keith et al., 1994; Leon-Kloosterziel et al., 1996; Parcy et al., 1997). Further studies of the GA-deficient -vp5 seeds should also be helpful in identifying the regulatory factors that control seed carbohydrates and proteins, and in resolving the roles they play in providing dehydration protection in maize.
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of two outstanding undergraduate students: Fawn Tranh, who helped to make the hormone extractions, and Felicity Martini, who provided valuable assistance in the preparation of the manuscript.
Footnotes
This work was supported by the National Science Foundation (grant nos. DCB9007481 and IBN–9318447 to C.J.R.). This is Oregon Agricultural Experiment Station Technical Paper no. 11,606.
LITERATURE CITED
- Abbe EC, Stein OL. The growth of the shoot apex in maize: embryogeny. Am J Bot. 1954;41:285–293. [Google Scholar]
- Bartels D, Engelhardt K, Roncarati R, Schneider K, Rotter M, Salamini F. An ABA and GA modulated gene expressed in the barley embryo encodes an aldose reductase related protein. EMBO J. 1991;10:1037–1043. doi: 10.1002/j.1460-2075.1991.tb08042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley DJ, Black M. Seeds: Physiology of Development and Germination. New York: Plenum Press; 1994. [Google Scholar]
- Coolbaugh RC, Hirano SS, West CA. Studies on the specificity and site of action of α-cyclopropyl-α-(p-methoxyphenyl)-5-pyrimidine methyl alcohol (anycmidol), a plant growth regulator. Plant Physiol. 1978;62:571–576. doi: 10.1104/pp.62.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dure L, III, Crouch M, Harada J, Ho THD, Mundy J, Quatrano RS, Thomas T, Sung ZR. Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol. 1989;12:475–486. doi: 10.1007/BF00036962. [DOI] [PubMed] [Google Scholar]
- Fernandez DE. Developmental basis of homeosis in precociously germinating Brassica napus embryos: phase change at the shoot apex. Development. 1997;124:1149–1157. doi: 10.1242/dev.124.6.1149. [DOI] [PubMed] [Google Scholar]
- Fong F, Smith JD, Koehler DE. Early events in maize seed development: 1-methyl-3-phenyl-5-(3-(trifluormethyl)phenyl)-4-(1H)-pyridinone induction of vivipary. Plant Physiol. 1983;73:899–901. doi: 10.1104/pp.73.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hable WE, Schumaker KS, Oishi KK. Viviparous-5 encodes phytoene desaturase, an enzyme essential for abscisic acid (ABA) accumulation and seed development in maize. Mol Gen Genet. 1998;257:167–176. doi: 10.1007/s004380050636. [DOI] [PubMed] [Google Scholar]
- Hattori T, Vasil V, Rosenkrans L, Hannah C, McCarty DR, Vasil IK. The Viviparous-1 gene and abscisic acid activate the C1 regulatory gene for anthocyanin biosynthesis during seed maturation in maize. Genes Dev. 1992;6:609–618. doi: 10.1101/gad.6.4.609. [DOI] [PubMed] [Google Scholar]
- Hedden P, Graebe JE. Inhibition of gibberellin biosynthesis by paclobutrazol in cell-free homogenates of Cucurbita maxima endosperm and Malus pumila embryos. J Plant Growth Regul. 1985;4:111–122. [Google Scholar]
- Hill A, Nantel A, Rock CD, Quatrano RS. A conserved domain of the viviparous-1 gene product enhances the DNA binding activity of the bZIP protein EmBP-1 and other transcription factors. J Biol Chem. 1996;271:3366–3374. doi: 10.1074/jbc.271.7.3366. [DOI] [PubMed] [Google Scholar]
- Hoecker U, Vasil IK, McCarty DR. Integrated control of seed maturation and germination programs by activator and repressor functions of Viviparous-1 of maize. Genes Dev. 1995;9:2459–2469. doi: 10.1101/gad.9.20.2459. [DOI] [PubMed] [Google Scholar]
- Hughes DW, Galau GA. Developmental and environmental induction of Lea and LeaA mRNAs and the postabscission program during embryo culture. Plant Cell. 1991;3:605–618. doi: 10.1105/tpc.3.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JV, Chandler PM. Gibberellin and abscisic acid in germinating cereals. In: Davies PJ, editor. Plant Hormones and Their Role in Plant Growth and Development. Boston: Martinus Nijhoff; 1987. pp. 164–193. [Google Scholar]
- Jacobsen JV, Gubler F, Chandler PM. Gibberellin action in germinated cereals. In: Davies PJ, editor. Plant Hormones. Ed 2. Boston: Martinus Nijhoff; 1995. pp. 246–271. [Google Scholar]
- Keith K, Kraml M, Dengler NG, McCourt P. fusca3: a heterochronic mutation affecting late embryo development in Arabidopsis. Plant Cell. 1994;6:589–600. doi: 10.1105/tpc.6.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermode AR. Regulatory mechanisms involved in the transition from seed development to germination. Crit Rev Plant Sci. 1990;9:155–195. [Google Scholar]
- Koornneef M, Jorna ML, van der Swan DLC, Karssen CM. The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1982;61:385–395. doi: 10.1007/BF00272861. [DOI] [PubMed] [Google Scholar]
- Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- Koster KL. Glass formation and desiccation tolerance in seeds. Plant Physiol. 1991;96:302–304. doi: 10.1104/pp.96.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz AR, Wallace MS, Paiva R. Globulin gene expression in embryos of maize viviparous mutants: evidence for regulation of Glb1 gene by ABA. Plant Physiol. 1990;92:538–542. doi: 10.1104/pp.92.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Kloosterziel KM, van-de-Bunt GA, Zeevaart JA, Koornneef M. Arabidopsis mutants with a reduced seed dormancy. Plant Physiol. 1996;110:233–240. doi: 10.1104/pp.110.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YQ, Bergervoet JHW, deVos CHR, Hilhorst HWM, Kraak HL, Karssen CM, Bino RJ. Nuclear replication activities during imbibition of abscisic acid and gibberellin-deficient tomato (Lycopersicon esculentum Mill.) seeds. Planta. 1994;194:368–373. [Google Scholar]
- McCarty DR, Hattori T, Carson CB, Vasil V, Lazar M, Vasil IK. The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell. 1991;66:895–905. doi: 10.1016/0092-8674(91)90436-3. [DOI] [PubMed] [Google Scholar]
- Murofushi N, Honda I, Hirasawa R, Yamaguchi I, Takahashi N, Phinney BO. Gibberellins from the seed, tassel, cob and silk of maize. Agric Biol Chem. 1991;55:435–439. [Google Scholar]
- Neill SJ, Horgan R, Parry AD. The carotenoid and abscisic acid content of viviparous kernels and seedlings of Zea mays L. Planta. 1986;169:87–96. doi: 10.1007/BF01369779. [DOI] [PubMed] [Google Scholar]
- Ooms JJJ, Leon-Kloosterziel KM, Bartels D, Koornneef M, Karssen CM. Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana: a comparative study using abscisic acid-insensitive abi3 mutants. Plant Physiol. 1993;102:1185–1191. doi: 10.1104/pp.102.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F, Valon C, Atuko K, Misera S, Giraudat J. The ABSCISIC ACID-INSENSTITIVE3, FUSCA3, and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell. 1997;9:1265–1277. doi: 10.1105/tpc.9.8.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J. Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell. 1994;6:1567–1582. doi: 10.1105/tpc.6.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proebsting WM, Hedden P, Lewis MJ, Croker SJ, Proebsting LN. Gibberellin concentration and transport in genetic lines of pea: effect of grafting. Plant Physiol. 1992;100:1354–1360. doi: 10.1104/pp.100.3.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley M. The role of gibberellin, abscisic acid, and auxin in the regulation of developing wheat grains. J Exp Bot. 1979;30:381–389. [Google Scholar]
- Reeve DR, Crozier A. Gibberellin bioassays. In: Krishnamoorthy HN, editor. Gibberellins and Plant Growth. New Delhi, India: Wiley Eastern Limited; 1973. pp. 35–64. [Google Scholar]
- Reid JB. Gibberellin mutants. In: Blonstein AD, King PJ, editors. Plant Gene Research, a Genetic Approach to Plant Biochemistry. New York: Springer-Verlag; 1986. pp. 1–34. [Google Scholar]
- Rivin CJ, Grudt T. Abscisic acid and the developmental regulation of embryo storage proteins in maize. Plant Physiol. 1991;95:358–365. doi: 10.1104/pp.95.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DS. The genetics of vivipary in maize. Genetics. 1955;40:745–760. doi: 10.1093/genetics/40.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarati R, Salamini F, Bartels D. An aldose reductase homologous gene from barley: regulation and function. Plant J. 1995;7:809–822. doi: 10.1046/j.1365-313x.1995.07050809.x. [DOI] [PubMed] [Google Scholar]
- Shen Q, Zhang P, Ho TH. Modular nature of abscisic acid (ABA) response complexes: composite promoter units that are necessary and sufficient for ABA induction of gene expression in barley. Plant Cell. 1996;8:1107–1119. doi: 10.1105/tpc.8.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K, Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990;2:503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray CR, Kobayashi M, Suzuki Y, Phinney BO, Gaskin P, MacMillan J. The dwarf-1 (d1) mutant of Zea mays blocks three steps in the gibberellin-biosynthetic pathway. Proc Natl Acad Sci USA. 1996;93:10515–10518. doi: 10.1073/pnas.93.19.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber CM, Cooney SE, McCourt P. Isolation of the GA-response mutant sly1 as a suppressor of ABI1–1 in Arabidopsis thaliana. Genetics. 1998;149:509–521. doi: 10.1093/genetics/149.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil V, Marcotte WR, Jr, Rosenkrans L, Cocciolone SM, Vasil IK, Quatrano RS, McCarty DR. Overlap of Viviparous1 (VP1) and abscisic acid response elements in the Em promoter: G-box elements are sufficient but not necessary for VP1 transactivation. Plant Cell. 1995;7:1511–1518. doi: 10.1105/tpc.7.9.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CN, Rivin CJ. Characterization and expression of a cDNA encoding a seed specific metallothionein in maize. Plant Physiol. 1995a;108:831–832. doi: 10.1104/pp.108.2.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CN, Rivin CJ. Sequence and regulation of a late embryogenesis abundant group 3 protein of maize (Zea mays L.) Plant Physiol. 1995b;108:1337–1338. doi: 10.1104/pp.108.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]