Abstract
Background:
Oral lichen planus (OLP) is a common mucocutaneous disease with malignant transformation potential. Several etiologies such as humoral, autoimmunity, and viral infections might play a role, but still there is no definite etiology for this disease. The aim of this study was to investigate the presence of Epstein–Barr virus (EBV) genome in Iranian patients with OLP as compared to people with normal mucosa.
Materials and Methods:
The study was carried out on a case group including 38 tissue specimens of patients with histopathological confirmation of OLP and a control group including 38 samples of healthy mucosa. All samples were examined by nested polymerase chain reaction (PCR) method to determine the DNA of EBV.
Results:
Twenty-two (57.9%) female samples and 16 (42.1%) male samples with OLP were randomly selected as the case group, and 20 (52.6%) female samples and 18 (47.4%) male samples with healthy mucosa as the control group. There was a statistically significant difference in the percentage of EBV positivity between the case (15.8%) and the control groups (P < 0.05); in the case group, three female samples (13.6%) and three male samples (18.8%) were infected with EBV; the difference between the genders was not statistically significant (P = 0.50).
Conclusion:
Results emphasized that EBV genome was significantly higher among Iranian patients with OLP so antiviral therapy might be helpful.
Keywords: Epstein–Barr virus infections, oral lichen planus, polymerase chain reaction
INTRODUCTION
Oral lichen planus (OLP) is a common mucocutaneous disease with malignant transformation potential. It is a chronic mucocutaneous disease without a definite etiology but humoral, viral, environmental, and genetic factors might be important. OLP is relatively prevalent, affecting 0.5%–2.2% of the general population. The mean age of patients is about 55 years, and its prevalence is higher among women.[1]
OLP is a white lace-like pattern that occurs predominantly in cheeks and has different clinical types, including reticular, papular, plaque, bullous, erosive, and sometimes it is wounded.[2]
Epstein–Barr virus (EBV) is a human herpes virus that can affect anyone from childhood. The virus is an opportunistic pathogen in immune-compromised patients.[3] Infection with EBV is normally transmitted from one person to another through contact with infected body fluids including infectious saliva, sexual contact, and breastfeeding.[4,5] In the past years, Pederson[6] examined EBV DNA in some samples of OLP and showed that EBV might be involved in the pathogenesis of some oral lesions. In another study, EBV was found in 26.1% of OLP patients as well as in 7.3% of the control group.[7]
Several prospective studies[8,9] have reported that there is a risk of malignancy in OLP. Three studies, one of which conducted in Sweden, showed clear diagnostic criteria of the risk of OLP becoming the SCC.[10,11] Therefore, if OLP, especially the ulcerative type, has a potential to become malignant, it can be considered a major source of oral cancer in many regions of the world.[12]
Recent reports say that immunological factors are involved in the etiology of this disease. However, the etiology of lichen planus is still generally unknown.[13] The antigen responsible for lichen planus has not been found yet, but viral factors are proposed as its etiologic factors.[6] For example, EBV is associated with benign and malignant diseases of the head and neck, but OLP sometimes shows malignant transformation, too.[14,15]
Oral malignancies diagnosis in comparison to malignancies of other parts of the body is very critical due to rapid metastasis and early death of patients. There are certain oral conditions known as premalignant condition including lichen planus, and an early diagnosis of such conditions and follow-ups of these lesions is highly crucial. Because of the tendency to malignancy and the risks involved in such changes, OLP has a crucial role in oral and dentistry diseases.[3,16]
EBV is associated with a wide range of human infective diseases including infectious mononucleosis, Hodgkin's lymphoma, Burkitt's lymphoma, and nasopharyngeal carcinoma.[17,18] The progress of EBV-related neoplasms is strongly associated with environmental factors and genetic disruption for regulating patient immune system.[19] The virus is a type of herpes virus with double-stranded DNA and is prevalent among the normal population. About 90% of adults have EBV Antibodies. In fact, EBV infection targets two types of cells: (1) oropharyngeal epithelium or salivary glands and (2) B-lymphocytes.[20]
The objectives of this study include determination of the relative frequency of EBV genotype in Iranian patients with OLP as well as non-OLP cases and comparing the two groups. Moreover, the association between EBV frequency and the patients' age will be studied in OLP patients. Furthermore, the relative frequency of EBV in OLP cases will be determined and compared between two genders. If such correlations are found, in addition to epidemiological value, antiviral medicine can be used as a treatment for lichen planus and even as prophylaxis for the prevention of premalignant lesions in high-risk cases.
MATERIALS AND METHODS
This study is a case–control descriptive analysis that was carried out on the patients of Al-Zahra hospital in the city of Isfahan from 2006 to 2016. Participants were selected using randomized simple sampling method, and there was no limitation for the patients' age and gender. All the selected patients in the study were suffering from oral lesions with OLP criteria. The minimum sample size was calculated as 38 using the formula 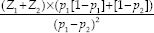 where Z1 was 95% or 1.64 and Z2 was 75% or 0.67. P1 and P2, which were estimates of the relative frequency of EBV genotype in Iranian OLP patients and non-OLP individuals, were about 26% and 7%, respectively.[5] The control group had the same size as the case group. In total, there were 78 participants in the study.
where Z1 was 95% or 1.64 and Z2 was 75% or 0.67. P1 and P2, which were estimates of the relative frequency of EBV genotype in Iranian OLP patients and non-OLP individuals, were about 26% and 7%, respectively.[5] The control group had the same size as the case group. In total, there were 78 participants in the study.
The clinical history of the selected samples was recorded using their medical profiles in hospital archives to collect information about their clinical history of oral lesions confirmed by biopsy. Furthermore, the demographic data of the selected samples were recorded. All the samples with confirmed OLP were selected as the case group. Samples that had received some treatment during the last 3 months before collection of the mucosa sample, including topical, oral, or IV treatments for EBV were excluded. Also having any simultaneous diseases or malignancies and the small size of the paraffin block which would not be appropriate for DNA extraction were other exclusion criteria. Moreover, the healthy oral mucosa was the criterion for the control group. The exclusion criteria for the control group were having simultaneous diseases or malignancies and the small size of the paraffin block which would not be appropriate for DNA extraction.
Once OLP and healthy oral mucosa were diagnosed in the stabilized blocks of the tissue samples for both groups, again by the pathologist researcher, the samples were examined with formalin using polymerase chain reaction (PCR) method to determine the DNA of EBV. In this study, PCR method was used to determine the presence of virus genotypes, which are among the most sensitive and specific methods of virus diagnosis. To carry out this study, a CORBETT device made in Australia and a CINNAGEN kit made in Iran with a sensitivity of up to 30 copy/mg were used. The PCR method was based on the procedure described by SinaClon BioScience Company. According to the description:
CinnaGen EBV PCR detection kit is destined for the qualitative detection of EBV DNA in infected samples by the method of PCR. CinnaGen EBV PCR detection kit may be used in clinical medicine to detect EBV DNA. The reagent of ready to use mix is an optimized 1X PCR mixture of Taq DNA Polymerase (recombinant), PCR buffer, MgCl2, dNTPs, and primers. Primer set is specified to the highly specific repetitive region of BLLF1 gene. This primer set allows for detection of 30 copies of EBV.
The mix contains all the components for PCR expect DNA. In addition, sterile water, PCR grade mineral oil, and positive control have supplied. Positive control tube contains a plasmid with cloned PCR fragment which indicates a successfully performed reaction. Blue and ready to load mix does not need any loading dye for electrophoresis. About 256 bp PCR products indicate a positive reaction.
This kit is sufficient for 50 amplification reactions of 25 μl volume each (http://www.sinaclon.com/product-114-Epstein --Barr-virus-PCR-Detection-kit-EBV-Kit).
Based on the guidelines of the company, the kit was taken out, and the tubes were unfrozen and put on ice. The new PCR tubes were labeled for amplification reaction for test, positive and negative control. Then, PCR MIX 20 μl and Taq DNA polymerase 0.2 μl was added to each tube on ice. Furthermore, one drop (20–25 μl) of mineral oil was added to each tube when needed. The reactions' tubes were capped, and the tube tray was placed in a resealable plastic bag, and the bag was securely sealed. The next steps were done at preamplification 1, specimen and control preparation area by adding 5 μl DNA, using a specified pipette for DNA sampling. The tubes were closed, and the mixtures were microfuged for 3–5 s. Then, the tubes were transferred to preheated thermocycler and the program was started.
Data were analyzed using Chi-square, Independent t-test, Fisher's exact test, and Mann–Whitney U-test. The collected data were analyzed using the SPSS-20 software (Armonk, NY:IBM Corp). The significant level for all the statistical tests was set at 0.05.
RESULTS
The case group (patients with OLP) included 22 (57.9%) female samples and 16 (42.1%) male samples (n = 38) and the control group included 20 (52.6%) female samples and 18 (47.4%) male samples (n = 38) [Table 1]. Chi-square test showed that the frequency distribution of gender was not significantly different between both groups (P = 0.64). All the demographic characteristic of the samples are shown in [Table 1]. In the case group, 6 samples (15.8%) had EBV infection, but none of the samples in the control group had EBV infection, and Fisher's exact test showed that EBV infection in the case group was significantly higher than the control group (P = 0.01) [Table 2]. In the case group, 3 female samples (13.6%) and 3 male samples (18.8%) were infected with EBV, and Fisher's exact test showed that EBV infection among women and men in the case group was not significantly different (P = 0.50) [Table 3]. The mean age of the EBV infection negative patients in the case group was 46.5, and of the EBV infection positive patients was 44.8 years, and independent t-test showed no significant difference between them (P = 0.61) [Table 4].
Table 1.
Average age and gender distribution in the two groups
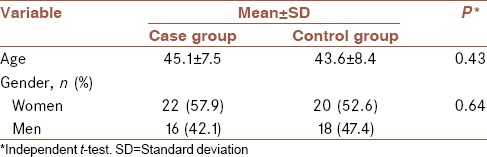
Table 2.
The frequency of Epstein–Barr virus in oral lichen planus patients and the control group

Table 3.
The frequency of Epstein–Barr virus infection in the case group by gender

Table 4.
Mean age of the case group based on Epstein – Barr virus infection

DISCUSSION
Early discovery of malignant and dysplastic lesions has always been an important aim. Early diagnosis and treatment can immune patients from irreparable damages. Since lichen planus is a lesion with occasionally malignant changes, and more importantly, it might be considered premalignant and malignant in the differential diagnosis of the lesions,[1,3] on one hand, and the infection with EBV exists in all societies,[21] even among people living in forlorn and isolated places such as Melanesia and Amazonian plateau,[22] on the other hand, this study was carried out to investigate the relationship between EBV and OLP.
A study in Esfahan Dentistry School found an increase in the number of patients with OLP in recent years. This increase might be related to the increase in population or the change in people's and dentists' attitudes toward the disease and its high tendency to malignant changes. The study also showed the average age of patients with OLP had been lowered.[23]
There are few studies on the frequency and epidemic changes of OLP in Iran. Esmaily et al.[24] study in Tehran that showed the prevalence of OLP in men, especially in their 30s. Khalili and Shojaee[25] study, also in Tehran, reported the average age for the emergence of the disease is 42 years in an age range of 5–83 years old. Pakfetrat et al. study[26] in Mashhad also reported an average age of 41.16 for OLP patients, 64.9% of whom were females. In 2010, a study in Isfahan[23] showed that the most prevalence of OLP is at the age of 41–50 years of age and it is more prevalent among women (64.7%).
Some authors say that OLP is a malignant transformation, but this is a questionable statement.[14,27,28] In 2002, a study showed the presence of EBV in oral malignant lesions and OLP.[6] In 2011, a study in Turkey[29] investigated the prevalence of EBV, herpes simplex virus (HSV), and human papillomaviruses (HPV) 16 in 65 OLP cases and found that the risk for EBV and HPV among OLP patients is very high, but not the risk for HSV. The study concluded that OLP patients must be checked for being infected with both EBV and HPV because these two viruses have potential oncogenicity.
It is also useful to mention that studies in the past years have reported the association between the degree of dysplasia in leukoplakia and prevalence of EBV.[30,31,32] On the other hand, some studies used PRC method on OSCC patients but found no significant difference in the prevalence of EBV between OLP patients and control groups.[33,34]
Few studies have been conducted in Iran about the relation between EBV virus and OLP and conducted studies in other countries have been performed on small samples;[35] they have eventually recommended performing further studies on larger sample sizes. Furthermore, some of the previous studies have not used the modern methods of PCR, and they have performed their studies using serologic or immunohistochemistry methods.[29] Therefore, the present study was conducted to evaluate this matter in Isfahan, Iran, using the modern method of DNA detect (PCR).
The purpose of this study was to examine the presence of EBV in patients with OLP in comparison with healthy mucosa individuals. Considering the fact that various viruses can be found in the lichen planus, this study discussed the presence of common EBV in OLP. Once such correlation is found, in addition to epidemiological value, antiviral medicine can be used as a treatment for lichen planus and even as prophylaxis for the prevention of premalignant lesions in high-risk cases.
The PCR method was used in the present study to determine the presence of virus genotypes is one of the most sensitive and specific methods of virus diagnosis. Although other studies[35] found no significant difference in the EBV prevalence between OLP and controls using the same method; this study showed that EBV infection in OLP group was significantly higher than the control group. In fact, our results suggested that EBV may be involved in the pathogenesis of OLP.
We recommend further studies in the future on the EBV oncogenic potential, to detect this virus in OLP cases. Furthermore, long-term follow-ups are recommended on the lesions that are found to be EBV-positive.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Burket LW, Greenberg MS, Glick M, Ship JA. Burket's Oral Medicine. 11th ed. New York: PMPH-USA; 2008. pp. 89–95. [Google Scholar]
- 2.Katta R. Lichen planus. Am Fam Physician. 2000;61:3319. [PubMed] [Google Scholar]
- 3.de Sousa FA, Paradella HC. Malignant potential of oral lichen planus: A meta-analysis. J Dent Sci. 2009;24:194–7. [Google Scholar]
- 4.Walling DM. Oral hairy leukoplakia: An Epstein-Barr virus-associated disease of patients with HIV. Res Initiat Treat Action. 2000;6:10–5. [PubMed] [Google Scholar]
- 5.Milagres A, Dias EP, Tavares Ddos S, Cavalcante RM, Dantas VA, de Oliveira SP, et al. Prevalence of oral hairy leukoplakia and epithelial infection by Epstein-Barr virus in pregnant women and diabetes mellitus patients – Cytopathologic and molecular study. Mem Inst Oswaldo Cruz. 2007;102:159–64. doi: 10.1590/s0074-02762007005000017. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen A. Abnormal EBV immune status in oral lichen planus. Oral Dis. 1996;2:125–8. doi: 10.1111/j.1601-0825.1996.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 7.Sand LP, Jalouli J, Larsson PA, Hirsch JM. Prevalence of Epstein-Barr virus in oral squamous cell carcinoma, oral lichen planus, and normal oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:586–92. doi: 10.1067/moe.2002.124462. [DOI] [PubMed] [Google Scholar]
- 8.Camisa C, Hamaty FG, Gay JD. Squamous cell carcinoma of the tongue arising in lichen planus: A case report and review of the literature. Cutis. 1998;62:175–8. [PubMed] [Google Scholar]
- 9.Chainani-Wu N, Silverman S, Jr, Lozada-Nur F, Mayer P, Watson JJ. Oral lichen planus: Patient profile, disease progression and treatment responses. J Am Dent Assoc. 2001;132:901–9. doi: 10.14219/jada.archive.2001.0302. [DOI] [PubMed] [Google Scholar]
- 10.Gandolfo S, Richiardi L, Carrozzo M, Broccoletti R, Carbone M, Pagano M, et al. Risk of oral squamous cell carcinoma in 402 patients with oral lichen planus: A follow-up study in an Italian population. Oral Oncol. 2004;40:77–83. doi: 10.1016/s1368-8375(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 11.Rödström PO, Jontell M, Mattsson U, Holmberg E. Cancer and oral lichen planus in a Swedish population. Oral Oncol. 2004;40:131–8. doi: 10.1016/s1368-8375(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan I, Ventura-Sharabi Y, Gal G, Calderon S, Anavi Y. The dynamics of oral lichen planus: A retrospective clinicopathological study. Head Neck Pathol. 2012;6:178–83. doi: 10.1007/s12105-011-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: A comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi: 10.1155/2014/742826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markopoulos AK, Antoniades D, Papanayotou P, Trigonidis G. Malignant potential of oral lichen planus; a follow-up study of 326 patients. Oral Oncol. 1997;33:263–9. doi: 10.1016/s0964-1955(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 15.Mignogna MD, Lo Muzio L, Lo Russo L, Fedele S, Ruoppo E, Bucci E, et al. Clinical guidelines in early detection of oral squamous cell carcinoma arising in oral lichen planus: A 5-year experience. Oral Oncol. 2001;37:262–7. doi: 10.1016/s1368-8375(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 16.Bokor-Bratic M, Picuric I. The prevalence of precancerous oral lesion: Oral lichen planus. Arch Oncol. 2001;9:107–9. [Google Scholar]
- 17.Goldenberg D, Benoit NE, Begum S, Westra WH, Cohen Y, Koch WM, et al. Epstein-Barr virus in head and neck cancer assessed by quantitative polymerase chain reaction. Laryngoscope. 2004;114:1027–31. doi: 10.1097/00005537-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Pietersma F, Piriou E, van Baarle D. Immune surveillance of EBV-infected B cells and the development of non-Hodgkin lymphomas in immunocompromised patients. Leuk Lymphoma. 2008;49:1028–41. doi: 10.1080/10428190801911662. [DOI] [PubMed] [Google Scholar]
- 19.Kushekhar K, van den Berg A, Nolte I, Hepkema B, Visser L, Diepstra A, et al. Genetic associations in classical Hodgkin lymphoma: A systematic review and insights into susceptibility mechanisms. Cancer Epidemiol Biomarkers Prev. 2014;23:2737–47. doi: 10.1158/1055-9965.EPI-14-0683. [DOI] [PubMed] [Google Scholar]
- 20.Syrjänen S. Viral infections in oral mucosa. Scand J Dent Res. 1992;100:17–31. doi: 10.1111/j.1600-0722.1992.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 21.Tischendorf P, Shramek GJ, Balagtas RC, Deinhardt F, Knospe WH, Noble GR, et al. Development and persistence of immunity to Epstein-Barr virus in man. J Infect Dis. 1970;122:401–9. doi: 10.1093/infdis/122.5.401. [DOI] [PubMed] [Google Scholar]
- 22.Lang DJ, Garruto RM, Gajdusek DC. Early acquisition of cytomegalovirus and Epstein-Barr virus antibody in several isolated Melanesian populations. Am J Epidemiol. 1977;105:480–7. doi: 10.1093/oxfordjournals.aje.a112407. [DOI] [PubMed] [Google Scholar]
- 23.Shirani A, Razavi S, Khazaei S, Akhavan Khaleghi M, Haerian A. Evaluation of oral lichen planus frequency in patients referred to the Oral Pathology Department of Isfahan School of Dentistry during the last two decades (1988-2008) J Isfahan Dent Sch. 2010;6:276–82. [Google Scholar]
- 24.Esmaily N, Barzegarri M, Rezaee M. Prevalence a clinical manifestation at lichen planus: Case reports 120 cases. J Skin Dis. 2003;8:110–4. [Google Scholar]
- 25.Khalili M, Shojaee M. A retrospective study of oral lichen planus in oral pathology department Tehran University of Medical Science (1968-2002) J Dent Tehran Univ Med Sci. 2006;19:45–52. [Google Scholar]
- 26.Pakfetrat A, Basir Shabestari S, Falaki F. Five year clinical and epidemiologic findings of oral lichen planus patients referred to oral medicine department of Mashhad dental school – Iran. J Mashhad Dent Sch. 2008;32:195–8. [Google Scholar]
- 27.van der Meij EH, Schepman KP, Smeele LE, van der Wal JE, Bezemer PD, van der Waal I, et al. Areview of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:307–10. doi: 10.1016/s1079-2104(99)70033-8. [DOI] [PubMed] [Google Scholar]
- 28.Lozada-Nur F, Miranda C. Oral lichen planus: Epidemiology, clinical characteristics, and associated diseases. Semin Cutan Med Surg. 1997;16:273–7. doi: 10.1016/s1085-5629(97)80016-8. [DOI] [PubMed] [Google Scholar]
- 29.Yildirim B, Sengüven B, Demir C. Prevalence of herpes simplex, Epstein Barr and human papilloma viruses in oral lichen planus. Med Oral Patol Oral Cir Bucal. 2011;16:e170–4. doi: 10.4317/medoral.16.e170. [DOI] [PubMed] [Google Scholar]
- 30.Mao EJ, Smith CJ. Detection of Epstein-Barr virus (EBV) DNA by the polymerase chain reaction (PCR) in oral smears from healthy individuals and patients with squamous cell carcinoma. J Oral Pathol Med. 1993;22:12–7. doi: 10.1111/j.1600-0714.1993.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 31.D'Costa J, Saranath D, Sanghvi V, Mehta AR. Epstein-Barr virus in tobacco-induced oral cancers and oral lesions in patients from India. J Oral Pathol Med. 1998;27:78–82. doi: 10.1111/j.1600-0714.1998.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 32.Horiuchi K, Mishima K, Ichijima K, Sugimura M, Ishida T, Kirita T, et al. Epstein-Barr virus in the proliferative diseases of squamous epithelium in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:57–63. doi: 10.1016/s1079-2104(05)80075-7. [DOI] [PubMed] [Google Scholar]
- 33.van Heerden WE, van Rensburg EJ, Engelbrecht S, Raubenheimer EJ. Prevalence of EBV in oral squamous cell carcinomas in young patients. Anticancer Res. 1995;15:2335–9. [PubMed] [Google Scholar]
- 34.Mizugaki Y, Sugawara Y, Shinozaki F, Takada K. Detection of Epstein-Barr virus in oral papilloma. Jpn J Cancer Res. 1998;89:604–7. doi: 10.1111/j.1349-7006.1998.tb03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahebjamee M, Eslami M, Jahanzad I, Babaee M, Kharazani Tafreshi N. Presence of Epstein-Barr virus in oral lichen planus and normal oral mucosa. Iran J Public Health. 2007;36:92–8. [Google Scholar]


