Abstract
Objective:
Determination of the amount of parenchymal damage in nonalcoholic fatty liver disease (NAFLD) is crucial to choose the best treatment and management.
Aim:
Here, the associations between laboratory data and severity of steatosis and fibrosis plus hepatic vessel Doppler indices in NAFLD patients were investigated.
Patients and Methods:
Fifty patients (20 males and 30 females) with NAFLD criteria were enrolled. Fatty liver was graded by sonography (SGFL) and FibroScan (FGFL). In addition, liver fibrosis was graded through FGLF. Damages to the portal, hepatic, and splenic veins were evaluated by color Doppler/dopplex. Serum liver enzymes and C-reactive protein (CRP) were also measured.
Results:
Significant association existed between SGFL and FGFL (P = 0.006). Portal vein pulsatility index (PI) and phasicity plus the triphasic and monophasic pattern of hepatic veins significantly associated with fatty liver grade evaluated by sonography. Splenic vein Peak systolic velocity and PI showed significant association with FGFL. Eventually, elevated liver enzymes and CRP significantly correlated with FGLF.
Conclusion:
We found that the severity of fatty liver is correlated with hepatic and portal veins damages; however, the degree of parenchymal fibrosis was independent to these indices and can be directly evaluated by FGFL. In addition, elevated liver enzymes and CRP correlated with the degree of fibrosis.
Keywords: Color Doppler, fibroScan, nonalcoholic fatty liver disease, sonography

INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of the elevated liver enzyme in adult and one of the main etiologies of chronic liver disease.[1,2] Pathophysiology of NAFLD is related to the fat infiltration in the liver parenchyma and can be ranged from simple hepatic steatosis to cirrhosis and hepatocellular carcinoma.[3] Progression from simple hepatic steatosis to advanced stages of NAFLD has not clear risk factors or incidence. However, the progression of simple hepatic steatosis to cirrhosis through the development of nonalcoholic steatohepatitis (NASH) and fibrosis has been established. NASH is a clear risk factor for progression to cirrhosis, and such progression has been reported in up to 25% of patients.[4] NAFLD affects 15%–30% of the general population and up to 90% of obese and diabetic patients.[5,6] Diagnosis of NAFLD is very important because it can damage the hepatocytes and lead to liver failure (3%), fibrosis (50%), and cirrhosis (15%).[7]
There are different methods for the diagnosis of NAFLD and its progress to fibrosis which include liver biopsy, serum markers, sonography, transient elastography (also called FibroScan [FGFL]), computerized tomography scanning, and magnetic resonance imaging.[8] Color Doppler sonography is a noninvasive method for the assessment of liver blood supply and some of liver parenchyma's disorders.[9] In addition, FGFL is another noninvasive test to quantify liver fibrosis based on using ultrasound waves. Indeed, measuring of liver stiffness by this method is a noninvasive trusty assessment of liver fibrosis level.[7] In obese patients, results of FGFL are controversial due to the interfering effects of subcutaneous fat on the elastic wave.[10] However, the effects of fat infiltration in the liver parenchyma on the hepatic artery resistance and changes of the Doppler wave shape of the hepatic vein are not completely clear. In addition, there are no studies about the comparison of findings provided by different applicable techniques in NAFLD patients.
Therefore, this study was designed to evaluate and compare the findings of color Doppler sonography, FGFL, and laboratory data in grayscale sonography confirmed NAFLD patients.
PATIENTS AND METHODS
Ethical statement
Institutional Review Board approved the protocol of this study, and all investigations have been carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. In addition, all patients were informed about the procedures and possible outcomes of the study, and informed consent was obtained from all of them.
Patients
In a cross-sectional study, all patients who had grayscale sonography confirmed NAFLD during 1 year from March 2014 to March 2015 were included in this study. NAFLD was confirmed based on the amount of liver fat infiltration and categorized according to the related changes in the liver echogenicity. Patients with previous hepatic or cardiac diseases, cirrhosis, ascites, splenomegaly, morbid obesity, other liver disorders, rather than NAFLD, nonceliac trunk originated of hepatic arterial disorders; nonuniform hepatic fat infiltration, steroid and vasoactive drug consumption, and use of alcohol were excluded from the study.
Demographic information and laboratory analysis
Gender, age, weight, height, and waist circumference (WC) were recorded in all patients and body mass index (BMI) was calculated. Fasting blood samples were taken from cubical veins, and serum was obtained after centrifugation (3500 × g, 15 min). Serum concentration of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), ferritin, and C-reactive protein (CRP) plus platelet count were measured using routine laboratory methods. These data were categorized as normal, less and >1.5 fold increases in AST and ALT and as normal and increase for ALP, ferritin, CRP, and platelet count.
Grayscale sonography
During gray scale sonography with deep probe, diameter of portal and splenic veins plus hepatic fat infiltration as normal (normal hepatic echo), mild (small increase in hepatic echo), moderate (increase in hepatic echo which deface hepatic artery and portal vein margins), and severe (increase in hepatic echo which deface diaphragm margins) were evaluated by expert radiologist.
Color Doppler sonography
Color Doppler sonography was performed using 3.5 MHz probe (Sonix Co.). Evaluation of middle hepatic vein during deep inhalation as subcostal or right intercostal approaches was performed, and angles were corrected based on vessels direction. Automatic correction of the parameters was performed by apparatus and wave analysis was performed for 2–3 wave period. Hepatic waves were divided into three categories. Normal three phasic waveforms, biphasic waveforms without reverse flow and monophasic or flat waveforms. Spectral waves of splenic and portal veins were evaluated manually and frigid. Velocity and resistance index of splenic and portal veins were calculated by apparatus software.
FibroScan
Liver stiffness was evaluated using FGFL (502 touch, Echosense, France).[11] Patients were in the supine position, and the probe was placed on the right lobe of liver in the intercostal position. The unit of liver stiffness was kilopascal (KPa) which achieved in 10 replication with success of 60% and interquartile range of <30%. The results were expressed as F0 and F1 (normal), F2 (mild fibrosis), F3 (moderate fibrosis), and F4 (severe fibrosis). Cutoff levels of 7.1, 9.5, and 14.5 KPa were set as levels higher than F2, F3, and F4, respectively. Levels higher than F2 were considered as the existence of fibrosis. In addition, steatosis severity was graded as S0, S1, S2, and S3 based on the extent of fat in the liver as S0: steatosis lower than 11%, S1: steatosis 11%–33%, S2: steatosis 34%–66%, and S3: steatosis >66%.
Statistical analysis
Data were analyzed using IBM SPSS Statistics version 23 (IBM, Armonk, New York, United States). Kolmogorov–Smirnov test was used to check the data normality. Association between qualitative data was evaluated by Chi-square or Fisher exact tests. Comparison of quantitative variables between different categories was performed using Student's t-test or one-way analysis of variance. P < 0.05 was considered as significant difference.
RESULTS
Totally, 50 patients (20 males and 30 females) with age of 45.18 ± 10.92 years (range 22–70 years) were included in this study. Distribution of patients based on age, BMI, and WC are presented in Figure 1. None of the patients had normal weight. Overweight was detected in 26% of patients, and other 74% was obese.
Figure 1.
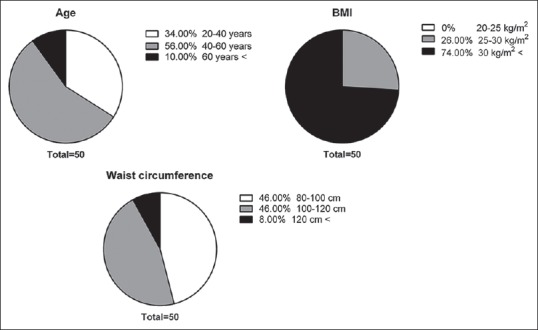
Age, body mass index, and waist circumference-related distribution of included patients.
Categorized laboratory data are presented in Figure 2. Increased AST and ALT concentrations were seen in 11 (22%) and 17 (34%) of patients, respectively. In addition, increase in ALP and CRP concentrations and platelet count was detected in 10 (20%), 5 (10%) and 1 (2%) of patients, respectively. None of the patients showed increased serum ferritin.
Figure 2.
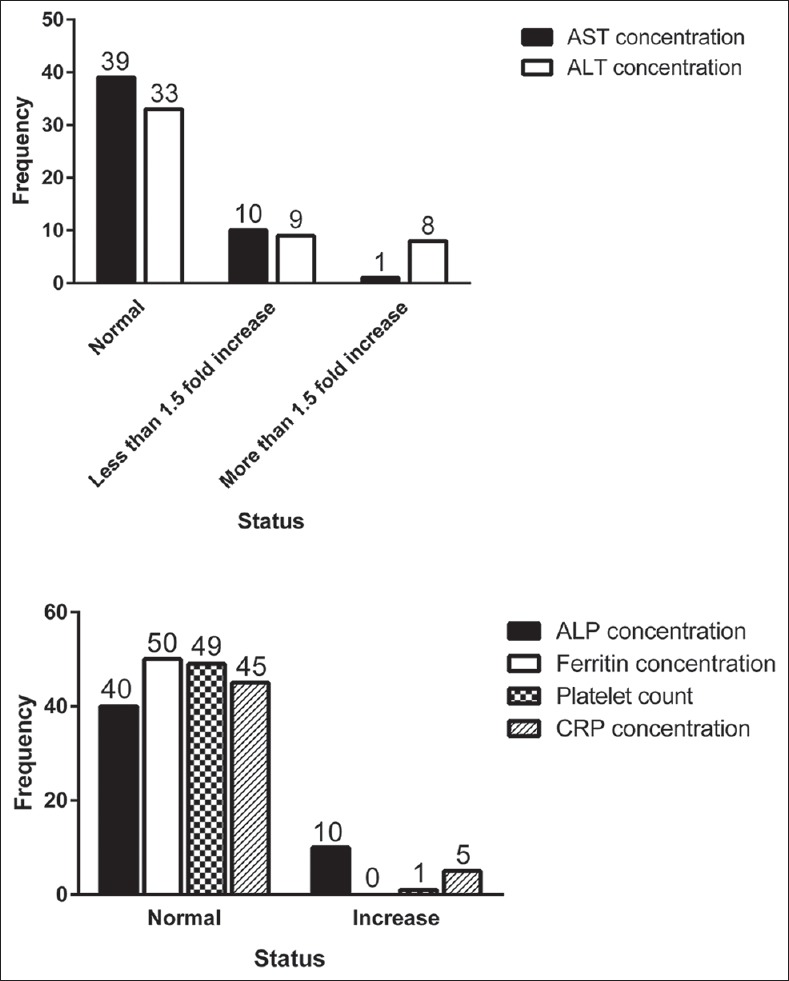
Categorized laboratory data in nonalcoholic fatty liver disease patients. Aspartate aminotransferase concentration (normal: 12–40 U/L, <1.5 fold increase: 40-60 U/L, more than 1.5 fold increase: more than 60 U/L); Alanine aminotransferase concentration (normal: 7–40 U/L, <1.5 fold increase: 40–60 U/L, more than 1.5 fold increase: more than 60 U/L); ALP (normal: 7–130 U/L, increase: more than 130 U/L); Ferritin (normal in female: 10–150 ng/ml, in male: 30–400 ng/ml, increase in female: more than 150 ng/ml, in male: more than 400 ng/ml); Platelet (normal: 1.5–4.5 × 105/mm3, increase: more than 4.5 × 105/mm3); CRP (normal: 0–10 mg/L, increase: more than 10 mg/L).
Sonographic and FGFL findings plus distribution of laboratory data in different stages of fatty liver, steatosis, and fibrosis in NAFLD patients are demonstrated in Table 1. As shown moderate fatty liver based on grayscale sonography and color Doppler was the most common grade of fatty liver. In addition, steatosis grade S3 and fibrosis grade F2 was the most frequent grades of FGFL study. In laboratory data, only associations between AST disturbance and increased CRP with fibrosis stage were significant (P = 0.025 and P = 0.037, respectively).
Table 1.
Frequency (%) of different statuses of liver disorders based on sonography, color Doppler, and FibroScan plus laboratory and demographic findings in each subsets
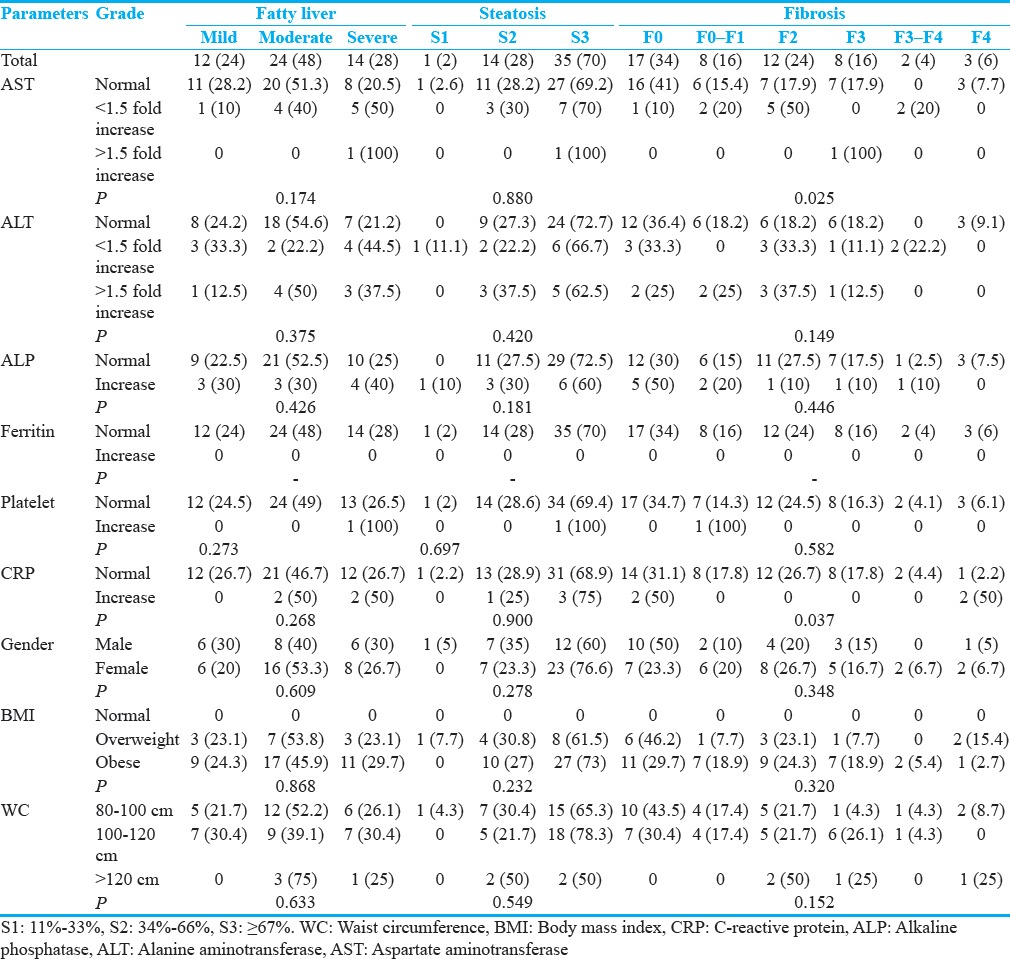
No significant associations were detected between severity of fatty liver based on sonography and FGFL with the severity of fibrosis based on FGFL [Table 2]. However, the significant positive association was seen between fatty liver grade based on FGFL and sonography [Table 3], (P = 0.006).
Table 2.
Associations between severity of fatty liver based on sonography and FibroScan with severity of fibrosis based on FibroScan
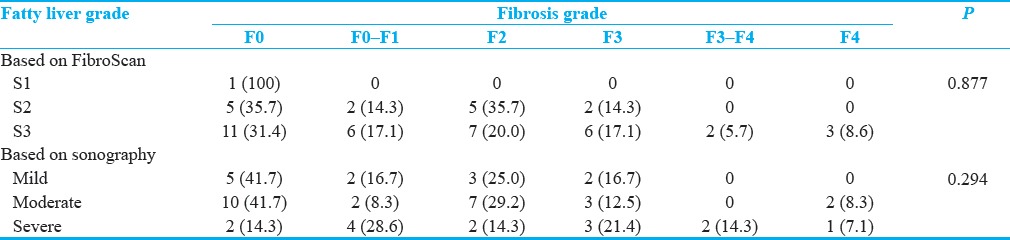
Table 3.
Associations between severity of fatty liver based on sonography and FibroScan
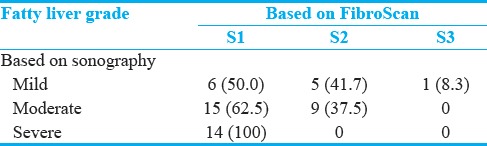
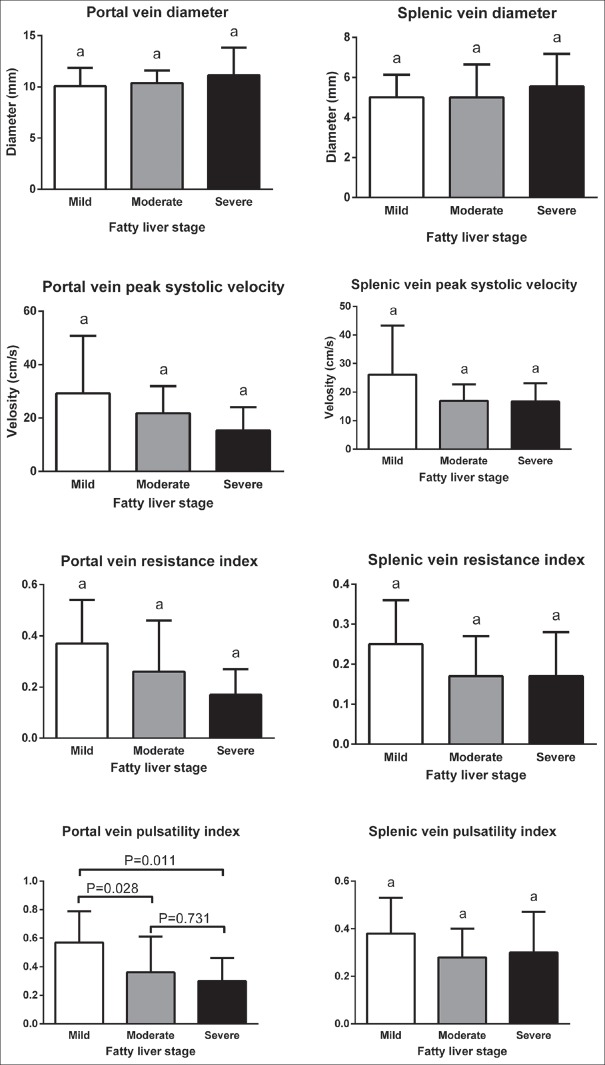
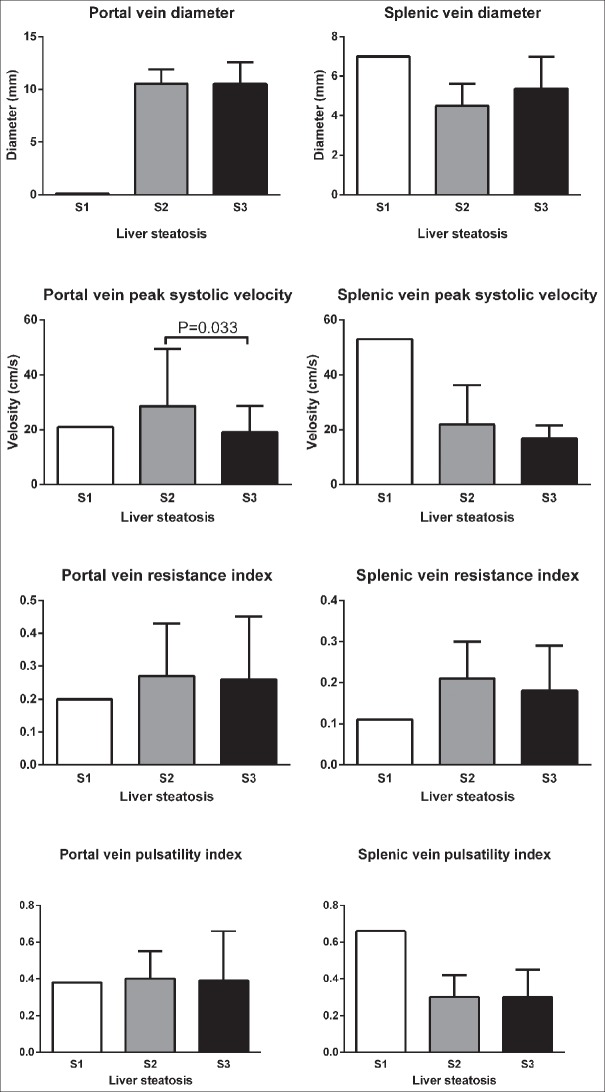
Mean ± standard deviation of color Doppler findings of the portal and splenic veins in different stages of fatty liver based on sonography, steatosis, and fibrosis based on FGFL are shown in Figures 3–5, respectively. Totally, the portal vein had a diameter, resistance index, and pulsatility index (PI) of 10.5 ± 1.9 mm, 0.3 ± 0.1, and 0.4 ± 0.2, respectively. These values were 5.2 ± 1.5 mm, 0.2 ± 0.1, and 0.3 ± 0.1, for a splenic vein, respectively. Significant differences were detected in portal vein pulsatility between mild with moderate and severe sonography-based fatty liver stages (P = 0.028 and P = 0.011, respectively), in portal vein peak systolic velocity between S2 and S3 stages of liver steatosis (P = 0.033) and in splenic vein diameter between F0 and F4 stages of liver fibrosis (P = 0.015).
Figure 3.
Comparison of mean ± standard deviation of color Doppler findings of portal and splenic veins in different stages of fatty liver based on sonography.
Figure 5.
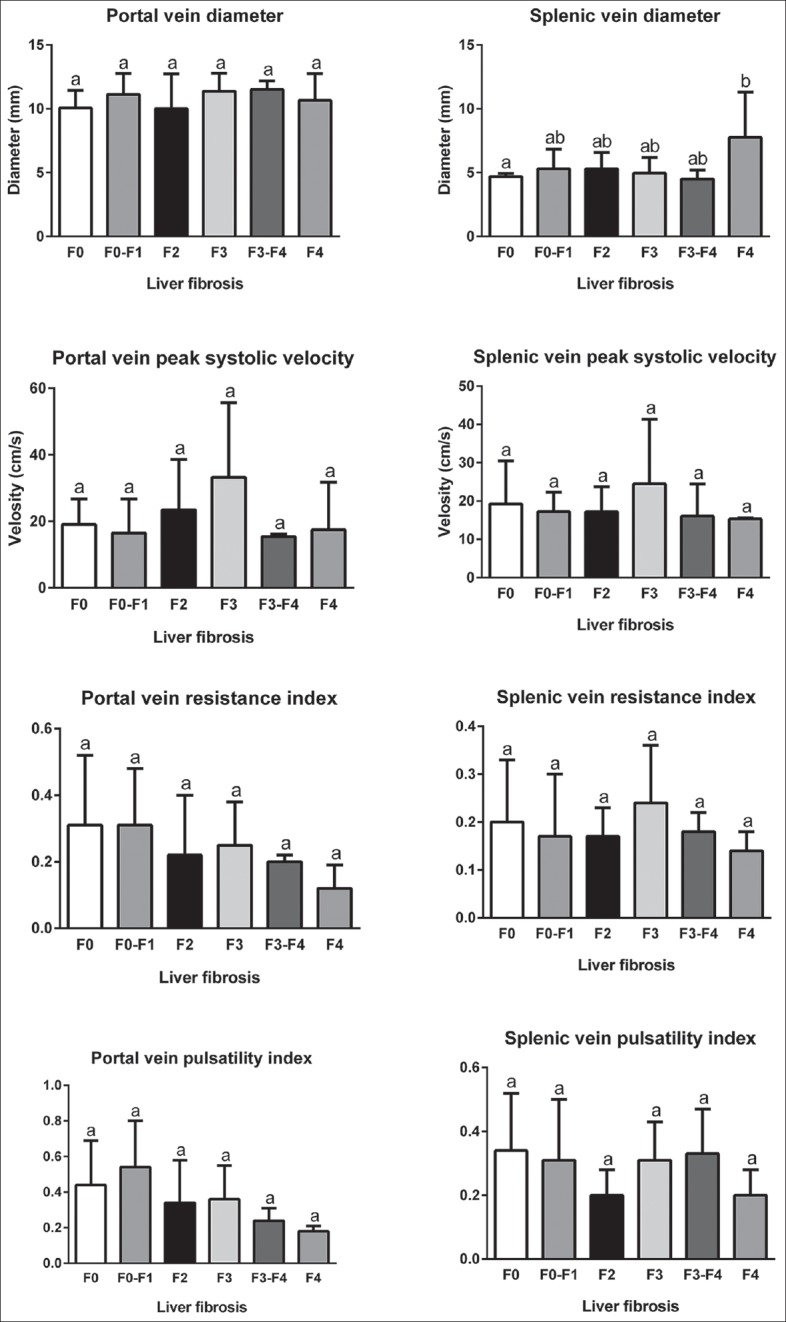
Comparison of mean ± standard deviation of color Doppler findings of portal and splenic veins in different stages of liver fibrosis based on FibroScan evaluation.
Figure 4.
Comparison of mean ± standard deviation of color Doppler findings of portal and splenic veins in different stages of liver steatosis based on FibroScan evaluation.
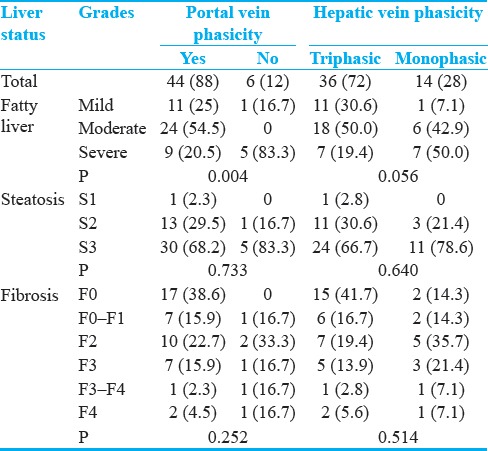
Portal and hepatic veins phasicity based on stages of fatty liver, steatosis, and fibrosis are presented in Table 4. As shown, only significant associations were detected between sonography-based fatty liver stage and portal vein phasicity (P = 0.004).
Table 4.
Frequency (%) of portal and hepatic veins phasicity in different stages of fatty liver, liver steatosis, and fibrosis
DISCUSSION
In the present study, laboratory findings and Doppler indices of hepatic, portal, and splenic veins based on fatty liver status calculated in sonography and steatosis and fibrosis stages calculated by FGFL were investigated. We found that FGFL based fibrosis grades were not associated with FGFL-based steatosis status and sonography-based fatty liver states which is in disagreement with previous reports.[12,13,14,15] However, it is confirmed that FGFL has high diagnostic accuracy in detection of fibrosis despite the underlying causes.[16] Sonography and FGFL can diagnose the severity of fat infiltration with different sensitivity and specificity. Fibrosis is the collagen displacement between hepatocytes, which can be detected as liver stiffness by FGFL. It cannot be evaluated by sonography directly but its secondary effects can be seen as changes in indices of liver vessels in color Doppler sonography. A significant positive association was between the frequency of monophasic waves of hepatic vein as abnormal waves and increase of sonography based fatty liver stage. In line with this finding, Mohammadinia et al. reported that the incidence of monophasic and biphasic hepatic vein waveform was 10%, 55%, and 80% for mild, moderate, and severe fatty live, respectively, and as the severity of fatty liver increased, the incidence of abnormal hepatic vein waveforms was also increased.[17] In addition, Oguzkurta and collaborator reported that 43% of fatty liver patients and just 2% of healthy ones had an abnormal HV Doppler waveform.[18] In the study of von Herbay et al., monophasic waveform was seen in 27% of NAFLD patients while all control participants had normal triphasic waveform.[19] Finally, Uzun et al. reported that triphasic, biphasic, and monophasic waveforms were seen in 47.5, 47.5, and 3% of fatty liver patients.[20] Higher incidence of abnormal waveforms of the hepatic vein in fatty liver patients may be due to hepatocyte swelling and a decrease of intracellular volume in response to fat infiltration. These can decrease the elasticity of liver capsule and can put pressure on the hepatic veins. All of them lead to a distribution of normal triphasic waveform and decrease compliance and undulation.[17]
In NAFLD patients, a significant negative association between PI of portal vein and stages of fatty liver in sonography was detected. This negative association was also reported in previous studies.[21,22] In addition, normal phasicity of the portal vein was lost when the grade of fatty liver was increased. Both of these observations are due to the fat infiltration related damages to the wall of the portal vein. About splenic veins, we found PSV decrease and PI increase in association with an increase in the severity of FGFL-based steatosis. These findings are in agreement with those reported by Dudanova and Belavina which used liver biopsy as a diagnostic test and expressed that by an increase of severity of fatty liver, PSV of the splenic vein was decreased.[23] Totally, with increases in the liver fat infiltration in NAFLD patients, decreasing in the hemodynamic indices of splenoportal are seen. Therefore, the color Doppler can be used as a noninvasive method for the assessment of portal hypertension and progress to the hepatic cirrhosis.[23]
No significant associations were detected between portosplenic indices and hepatic phasicity with a grade of liver fibrosis as six separate grades and as mild (F0, F0–F1, and F2) and severe (F3, F3–F4, and F4) categories of fibrosis. These findings may be due to this fact that most of the patients had a lower grade of fibrosis and collagen deposition was low. Therefore, the hepatic vessels were not destroyed extremely to induced significant changes in the Doppler indices.
A relationship between elevated AST and positive CRP with a higher grade of fibrosis was seen in this study as reported in all previous studies. However, such relationship was not seen for ALT, ALP, ferritin, and platelet count. AST elevation occurs in liver cell damage, and CRP is the marker of inflammation. However, lack of changes in the other laboratory parameters may be due to either the small sample size or patients with a lower grade of fibrosis. Finally, no significant relationship was detected between gender, BMI, and WC with the severity of fatty liver, steatosis, and fibrosis based on sonography and FGFL. None of the previously published studies reported a significant gender difference in fatty liver, steatosis, and fibrosis. However, lack of any association between these stages with BMI and/or WC may be due to the small sample size.
We found no significant relationship between portal vein PSV with sonography-based fatty liver stages and FGFL-based steatosis and fibrosis grades. Similar findings were also reported by Solhjoo et al. that no correlation was existed between the degree of fat infiltration and portal vein PI or hepatic vein waveform pattern.[24] However, a negative association between portal vein velocity and fatty liver severity was reported by Erdogmus et al., among 60 NAFLD patients and 20 healthy volunteers[22] and by Balci et al., among 105 NAFLD patients and 35 healthy volunteers.[21]
Limitations
Despite our findings, this study has three limitations. First, the low sample size of enrolled patients due to overinclusion criteria and 1 year duration of the study. Second is lack of obtaining of liver biopsy as a gold standard in the diagnosis of NAFLD. However, liver biopsy is an invasive method, and most of the patients do not agree to do it. Finally, we did not include healthy volunteers as comparison arm. This may be explained by the aims of the study, which just enrolled NAFLD patients to compare all of the things in them.
CONCLUSION
The findings of NAFLD patients based on different diagnostic techniques were compared in the present study. We found that the severity of fatty liver evaluated by FGFL and sonography is associated with vascular injuries which are measured by color Doppler. In addition, significant positive associations were detected between FGFL and sonography in fatty liver grade and between fatty liver stage and portal vein phasicity. Significant differences were detected in portal vein pulsatility between mild with moderate and severe fatty liver stages, in portal vein peak systolic velocity between S2 and S3 stages of liver steatosis and in splenic vein diameter between F0 and F4 stages of liver fibrosis. Although conventional Doppler can be helpful to detect NAFLD patients with the risk of fibrous tissue accumulation, the severity of liver fibrosis is independent of the above-mentioned topics while its associated with elevated liver enzyme and concentration of inflammatory markers. On the other hand, each technique evaluate a specific feature in the liver and therefore combining their findings together in high sample size study and creating a new predictive score are highly suggested.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2018/8/1/12/229168.
REFERENCES
- 1.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 2.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–61. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 3.Fattahi MR, Niknam R, Safarpour A, Sepehrimanesh M, Lotfi M. The prevalence of metabolic syndrome in non-alcoholic fatty liver disease; A population-based study. Middle East J Dig Dis. 2016;8:131–7. doi: 10.15171/mejdd.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7392–402. doi: 10.3748/wjg.v20.i23.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: The plot thickens. Diabet Med. 2007;24:1–6. doi: 10.1111/j.1464-5491.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 7.Magalotti D, Marchesini G, Ramilli S, Berzigotti A, Bianchi G, Zoli M, et al. Splanchnic haemodynamics in non-alcoholic fatty liver disease: Effect of a dietary/pharmacological treatment. A pilot study. Dig Liver Dis. 2004;36:406–11. doi: 10.1016/j.dld.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Festi D, Schiumerini R, Marzi L, Di Biase AR, Mandolesi D, Montrone L, et al. Review article: The diagnosis of non-alcoholic fatty liver disease – Availability and accuracy of non-invasive methods. Aliment Pharmacol Ther. 2013;37:392–400. doi: 10.1111/apt.12186. [DOI] [PubMed] [Google Scholar]
- 9.Liu LP, Dong BW, Yu XL, Zhang DK, Kang CS, Zhao XH, et al. Evaluation of focal fatty infiltration of the liver using color Doppler and contrast-enhanced sonography. J Clin Ultrasound. 2008;36:560–6. doi: 10.1002/jcu.20507. [DOI] [PubMed] [Google Scholar]
- 10.Conti F, Vukotic R, Foschi FG, Domenicali M, Giacomoni P, Savini S, et al. Transient elastography in healthy subjects and factors influencing liver stiffness in non-alcoholic fatty liver disease: An Italian community-based population study. Dig Liver Dis. 2016;48:1357–63. doi: 10.1016/j.dld.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Rastogi A, Sharma MK, Bhatia V, Tyagi P, Sharma P, et al. Liver stiffness measurements in patients with different stages of nonalcoholic fatty liver disease: Diagnostic performance and clinicopathological correlation. Dig Dis Sci. 2013;58:265–74. doi: 10.1007/s10620-012-2306-1. [DOI] [PubMed] [Google Scholar]
- 13.Lupsor M, Badea R, Stefanescu H, Grigorescu M, Serban A, Radu C, et al. Performance of unidimensional transient elastography in staging non-alcoholic steatohepatitis. J Gastrointestin Liver Dis. 2010;19:53–60. [PubMed] [Google Scholar]
- 14.Wong GL, Wong VW, Choi PC, Chan AW, Chum RH, Chan HK, et al. Assessment of fibrosis by transient elastography compared with liver biopsy and morphometry in chronic liver diseases. Clin Gastroenterol Hepatol. 2008;6:1027–35. doi: 10.1016/j.cgh.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD) Dig Liver Dis. 2008;40:371–8. doi: 10.1016/j.dld.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of transient elastography for the staging of liver fibrosis: A meta-analysis. Gastroenterology. 2008;134:960–74. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadinia AR, Bakhtavar K, Ebrahimi-Daryani N, Habibollahi P, Keramati MR, Fereshtehnejad SM, et al. Correlation of hepatic vein Doppler waveform and hepatic artery resistance index with the severity of nonalcoholic fatty liver disease. J Clin Ultrasound. 2010;38:346–52. doi: 10.1002/jcu.20696. [DOI] [PubMed] [Google Scholar]
- 18.Oguzkurt L, Yildirim T, Torun D, Tercan F, Kizilkilic O, Niron EA, et al. Hepatic vein Doppler waveform in patients with diffuse fatty infiltration of the liver. Eur J Radiol. 2005;54:253–7. doi: 10.1016/j.ejrad.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 19.von Herbay A, Frieling T, Häussinger D. Association between duplex Doppler sonographic flow pattern in right hepatic vein and various liver diseases. J Clin Ultrasound. 2001;29:25–30. doi: 10.1002/1097-0096(200101)29:1<25::aid-jcu4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Uzun H, Yazici B, Erdogmus B, Kocabay K, Buyukkaya R, Buyukkaya A, et al. Doppler waveforms of the hepatic veins in children with diffuse fatty infiltration of the liver. Eur J Radiol. 2009;71:552–6. doi: 10.1016/j.ejrad.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Balci A, Karazincir S, Sumbas H, Oter Y, Egilmez E, Inandi T, et al. Effects of diffuse fatty infiltration of the liver on portal vein flow hemodynamics. J Clin Ultrasound. 2008;36:134–40. doi: 10.1002/jcu.20440. [DOI] [PubMed] [Google Scholar]
- 22.Erdogmus B, Tamer A, Buyukkaya R, Yazici B, Buyukkaya A, Korkut E, et al. Portal vein hemodynamics in patients with non-alcoholic fatty liver disease. Tohoku J Exp Med. 2008;215:89–93. doi: 10.1620/tjem.215.89. [DOI] [PubMed] [Google Scholar]
- 23.Dudanova OP, Belavina IA. Splenoportal blood flow with nonalcoholic fatty liver disease. Eksp Klin Gastroenterol. 2010;5:14–8. [PubMed] [Google Scholar]
- 24.Solhjoo E, Mansour-Ghanaei F, Moulaei-Langorudi R, Joukar F. Comparison of portal vein Doppler indices and hepatic vein Doppler waveform in patients with nonalcoholic fatty liver disease with healthy control. Hepat Mon. 2011;11:740–4. doi: 10.5812/kowsar.1735143X.729. [DOI] [PMC free article] [PubMed] [Google Scholar]